Flow Injection Analysis with Electrochemical Detection for Rapid Identification of Platinum-Based Cytostatics and Platinum Chlorides in Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and pH Measurement
2.2. River Water Sample
2.3. FIA-ED System
2.4. Descriptive Statistics
3. Results and Discussion

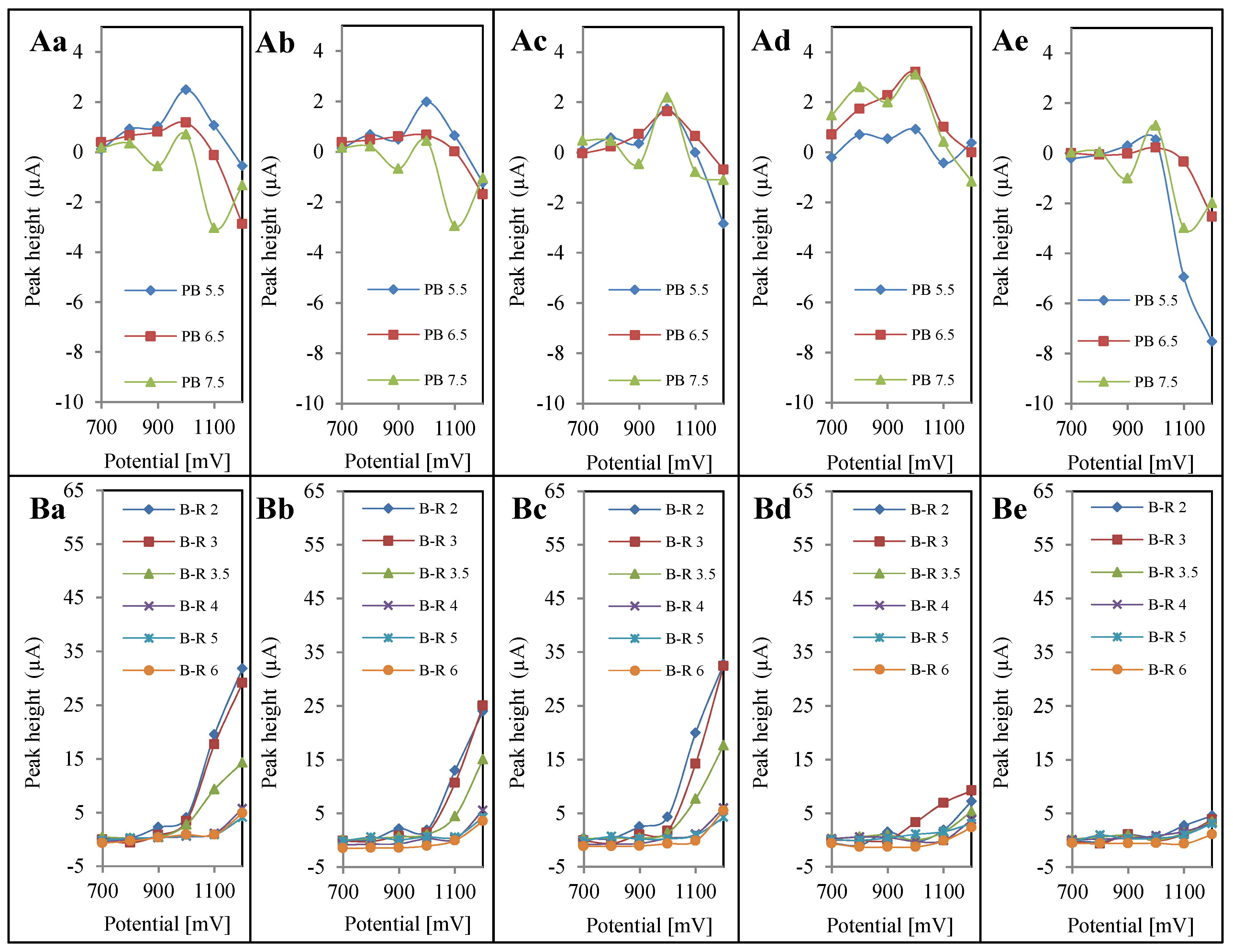
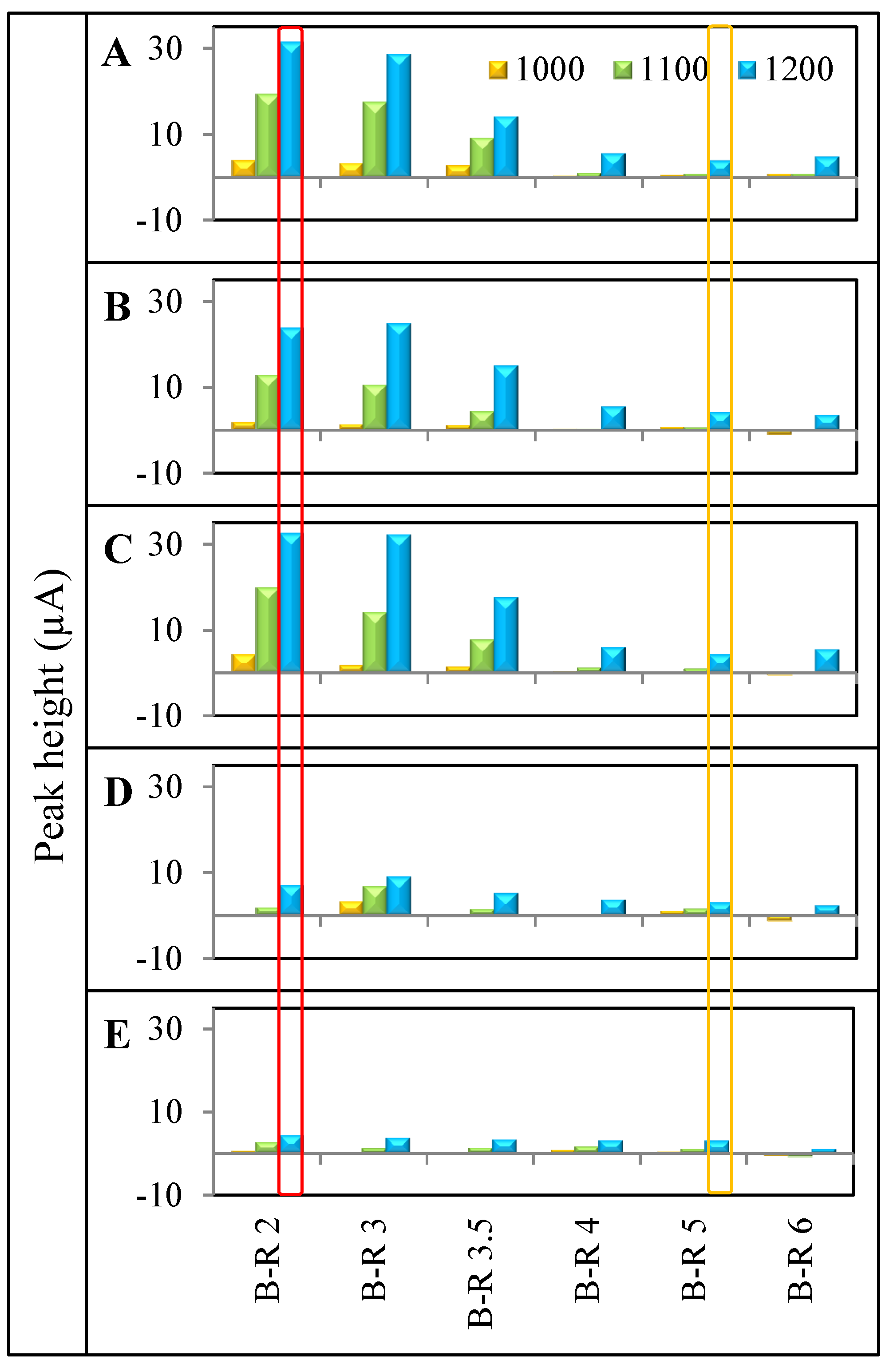
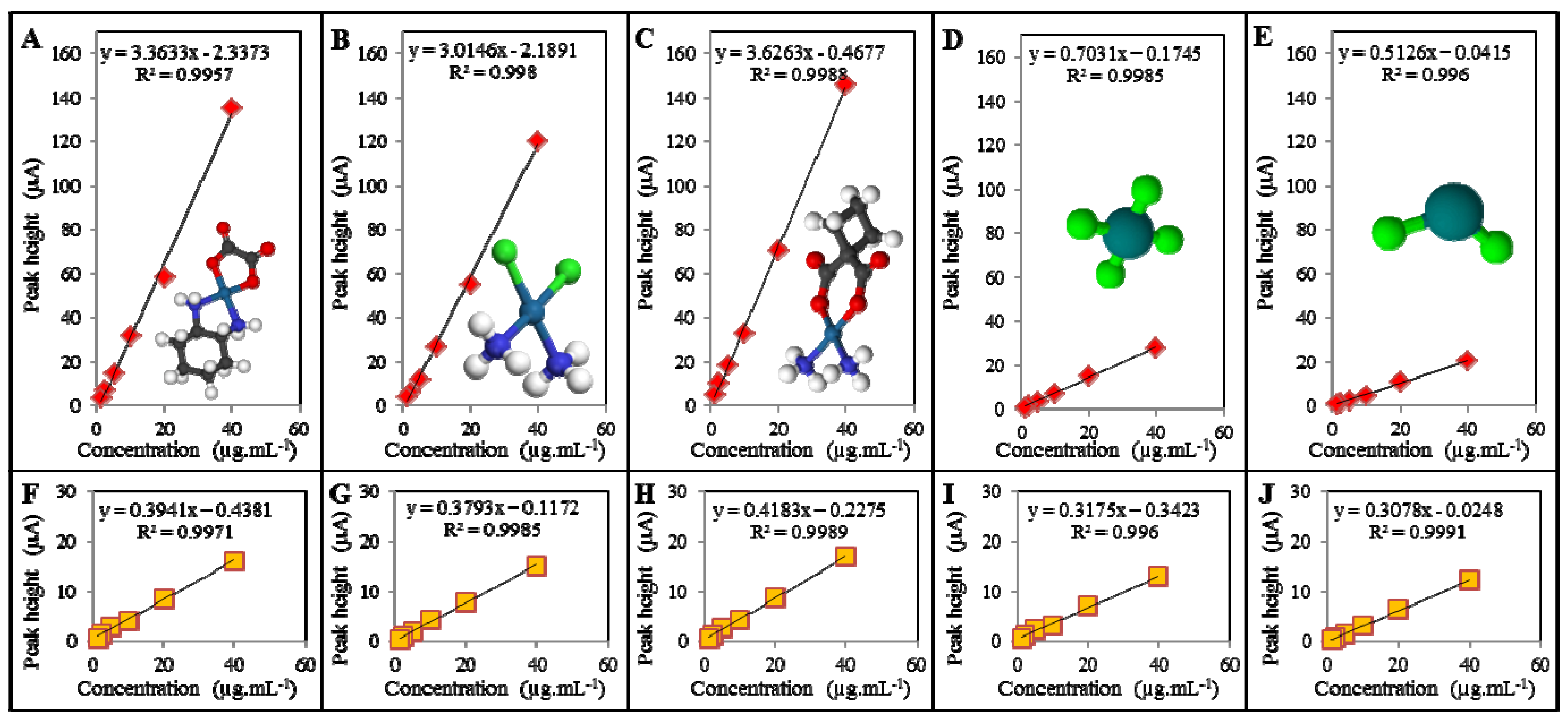

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Seriani, N.; Mittendorfer, F. Platinum-group and noble metals under oxidizing conditions. J. Phys. Condens. Matter 2008, 20, 1–6. [Google Scholar]
- Sobrova, P.; Zehnalek, J.; Adam, V.; Beklova, M.; Kizek, R. The effects on soil/water/plant/animal systems by platinum group elements. Cent. Eur. J. Chem. 2012, 10, 1369–1382. [Google Scholar] [CrossRef]
- Iavicoli, I.; Bocca, B.; Carelli, G.; Caroli, S.; Caimi, S.; Alimonti, A.; Fontana, L. Biomonitoring of tram drivers exposed to airborne platinum, rhodium and palladium. Int. Arch. Occup. Environ. Health 2007, 81, 109–114. [Google Scholar] [CrossRef]
- Sato, K.; Kusaka, Y.; Zhang, Q.W.; Zhu, X.Q.; Okada, K. Effect of platinum coordination complex (PtCx) on citrate uptake by rat renal brush border membrane vesicles (BBMV): Direct effect of cisplatin. Ind. Health 2000, 38, 327–329. [Google Scholar] [CrossRef]
- Ravindra, K.; Bencs, L.; van Grieken, R. Platinum group elements in the environment and their health risk. Sci. Total Environ. 2004, 318, 1–43. [Google Scholar] [CrossRef]
- Ek, K.H.; Morrison, G.M.; Rauch, S. Environmental routes for platinum group elements to biological materials—A review. Sci. Total Environ. 2004, 334, 21–38. [Google Scholar]
- Pan, S.H.; Zhang, G.; Sun, Y.L.; Chakraborty, P. Accumulating characteristics of platinum group elements (PGE) in urban environments, China. Sci. Total Environ. 2009, 407, 4248–4252. [Google Scholar] [CrossRef]
- Easton, C.; Turner, A.; Sewell, G. An evaluation of the toxicity and bioaccumulation of cisplatin in the marine environment using the macroalga, Ulva lactuca. Environ. Pollut. 2011, 159, 3504–3508. [Google Scholar] [CrossRef]
- Johnson, A.C.; Oldenkamp, R.; Dumont, E.; Sumpter, J.P. Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of europe. Environ. Toxicol. Chem. 2013, 32, 1954–1961. [Google Scholar] [CrossRef]
- Supalkova, V.; Beklova, M.; Baloun, J.; Singer, C.; Sures, B.; Adam, V.; Huska, D.; Pikula, J.; Rauscherova, L.; Havel, L.; et al. Affecting of aquatic vascular plant Lemna minor by cisplatin revealed by voltammetry. Bioelectrochemistry 2008, 72, 59–65. [Google Scholar] [CrossRef]
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152. [Google Scholar] [CrossRef]
- Yoon, H.; Yoon, C.; Park, C.S.; Ko, T.; Kim, N.S.; Han, K.N. Quantitative determination of PGM using ICP-MS, ICP-AES, AAS and XRF. Miner. Metall. Process. 2005, 22, 59–64. [Google Scholar]
- Herincs, E.; Puschenreiter, M.; Wenzel, W.; Limbeck, A. A novel flow-injection method for simultaneous measurement of platinum (Pt), palladium (Pd) and rhodium (Rh) in aqueous soil extracts of contaminated soil by ICP-OES. J. Anal. At. Spectrom. 2013, 28, 354–363. [Google Scholar] [CrossRef]
- Hann, S.; Stefanka, Z.; Lenz, K.; Stingeder, G. Novel separation method for highly sensitive speciation of cancerostatic platinum compounds by HPLC-ICP-MS. Anal. Bioanal. Chem. 2005, 381, 405–412. [Google Scholar] [CrossRef]
- Svancara, I.; Galik, M.; Vytras, K. Stripping voltammetric determination of platinum metals at a carbon paste electrode modified with cationic surfactants. Talanta 2007, 72, 512–518. [Google Scholar] [CrossRef]
- Locatelli, C. Ultratrace osmium, ruthenium and lead in airborne particulate matter: Peak area as instrumental datum to improve their simultaneous voltammetric determination. Electroanalysis 2012, 24, 2273–2282. [Google Scholar] [CrossRef]
- Van der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Stripping voltammetric determination of palladium, platinum and rhodium in freshwater and sediment samples from South African water resources. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 2084–2093. [Google Scholar] [CrossRef]
- Zitka, O.; Huska, D.; Krizkova, S.; Adam, V.; Chavis, G.J.; Trnkova, L.; Horna, A.; Hubalek, J.; Kizek, R. An investigation of glutathione-platinum(II) interactions by means of the flow injection analysis using glassy carbon electrode. Sensors 2007, 7, 1256–1270. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. Anal. Chem. 1983, 55, A712–A724. [Google Scholar]
- Allard, B. A comparative study on the chemical composition of humic acids from forest soil, agricultural soil and lignite deposit—Bound lipid, carbohydrate and amino acid distributions. Geoderma 2006, 130, 77–96. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Fast and accurate analysis of drugs using amperometry associated with flow injection analysis. J. Pharm. Sci. 2010, 99, 4784–4804. [Google Scholar] [CrossRef]
- Van Staden, J.F.; van Staden, R.I. S. Flow-injection analysis systems with different detection devices and other related techniques for the in vitro and in vivo determination of dopamine as neurotransmitter. A review. Talanta 2012, 102, 34–43. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kominkova, M.; Heger, Z.; Zitka, O.; Kynicky, J.; Pohanka, M.; Beklova, M.; Adam, V.; Kizek, R. Flow Injection Analysis with Electrochemical Detection for Rapid Identification of Platinum-Based Cytostatics and Platinum Chlorides in Water. Int. J. Environ. Res. Public Health 2014, 11, 1715-1724. https://doi.org/10.3390/ijerph110201715
Kominkova M, Heger Z, Zitka O, Kynicky J, Pohanka M, Beklova M, Adam V, Kizek R. Flow Injection Analysis with Electrochemical Detection for Rapid Identification of Platinum-Based Cytostatics and Platinum Chlorides in Water. International Journal of Environmental Research and Public Health. 2014; 11(2):1715-1724. https://doi.org/10.3390/ijerph110201715
Chicago/Turabian StyleKominkova, Marketa, Zbynek Heger, Ondrej Zitka, Jindrich Kynicky, Miroslav Pohanka, Miroslava Beklova, Vojtech Adam, and Rene Kizek. 2014. "Flow Injection Analysis with Electrochemical Detection for Rapid Identification of Platinum-Based Cytostatics and Platinum Chlorides in Water" International Journal of Environmental Research and Public Health 11, no. 2: 1715-1724. https://doi.org/10.3390/ijerph110201715








