Lack of Genomic Instability in Bone Marrow Cells of SCID Mice Exposed Whole-Body to Low-Dose Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Irradiation
2.3. Collection of BM Cells and Cytogenetic Assays
2.4. Fluorescence in situ Hybridization (FISH) Assay
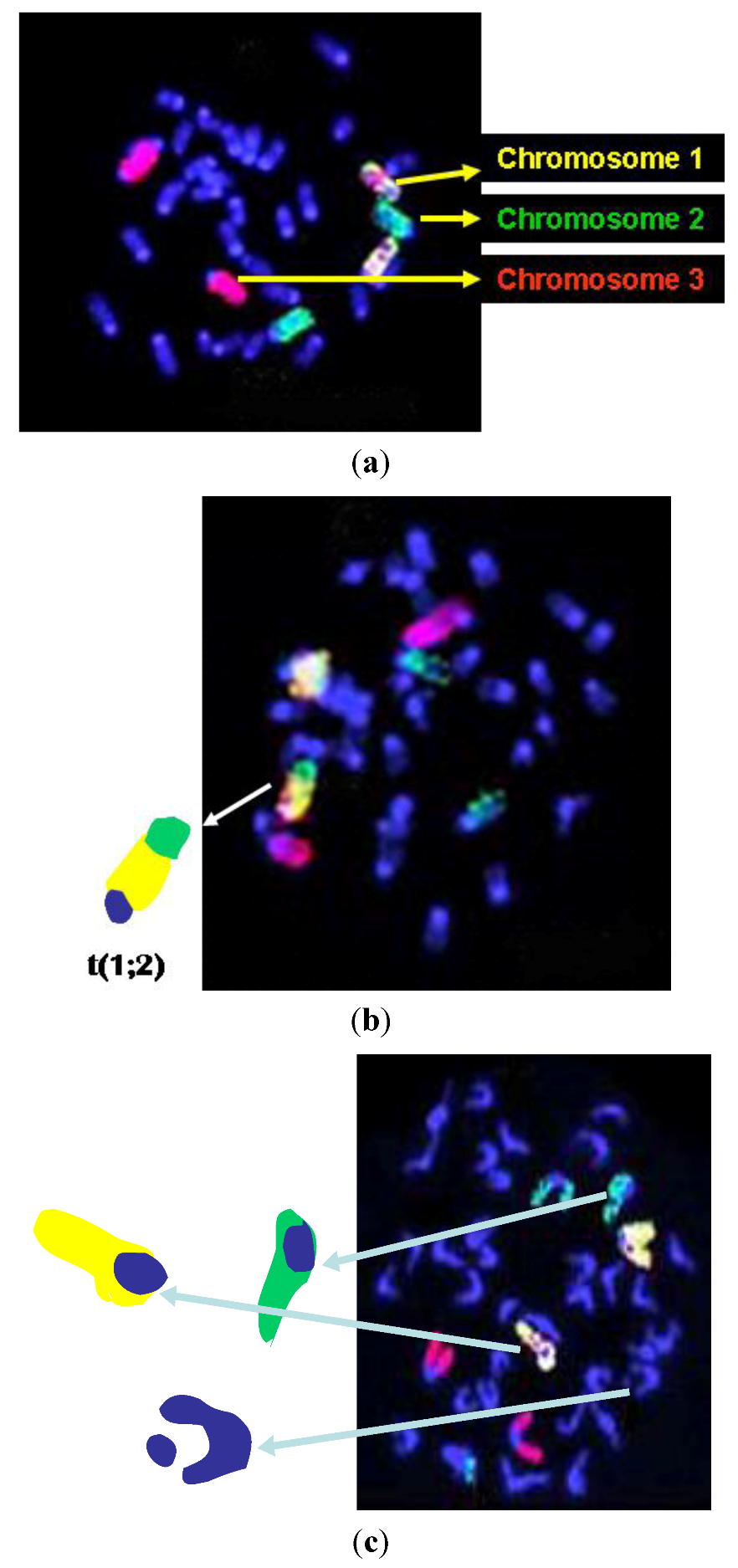
2.5. Chromosome Aberration Scoring
2.6. Statistical Analysis
3. Results and Discussion
3.1. Early Time-Points (1 and 4 h Post-Irradiation)
| Total | Total | Total | Chromatid breaks | Total | Iso-chromatid breaks | Total | Exchanges | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | number of | number of | number of | (Chromosome involved) | number of | (Chromosome involved) | number of | |||||||
| (Gy) | cells | abnormal | chromatid | Chromosome | Iso-chormatid | Chromosome | exchanges | |||||||
| scored | cells | breaks | (1) | (2) | (3) | (nP) | breaks | (1) | (2) | (3) | (nP) | |||
| 0 | 1,078 | 44 | 34 | 2 | 3 | 1 | 28 | 13 | 3 | 2 | 2 | 6 | 5 | t(nP;1),t(nP;1),t(nP;nP),t(nP;3), ins(nP;1;nP) |
| 0.05 | 483 | 37 | 39 | 3 | 4 | 0 | 49 | 27 | 6 | 9 | 5 | 7 | 3 | recipt(nP;3),t(np:1); t(nP;1) |
| 0.10 | 908 | 110 | 133 | 14 | 8 | 6 | 95 | 53 | 14 | 8 | 9 | 22 | 8 | t(nP;1),t(nP;3),t(2;nP),t(nP;1), t(nP;2),t(nP;3),dic(3;nP), dic(nP;nP) |
| 1.00 | 513 | 311 | 475 | 35 | 22 | 30 | 388 | 62 | 13 | 10 | 15 | 24 | 18 | t(1;2) two cells,t(2;3),t(nP;1)t(nP;1), t(3;2) two cells,t(3;nP), recip t(3;nP), t(nP;1),t(nP;1),t(nP;1),t(nP;2), t(nP;2),t(nP;3),t(nP;3),t(nP;3), dic(nP;nP) |
| Total | Total | Total | Chromatid breaks | Total | Iso-chromatid breaks | Total | Exchanges | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | number of | number of | number of | (Chromosome involved) | number of | (Chromosome involved) | number of | |||||||
| (Gy) | cells | abnormal | chromatid | Chromosome | Iso-chromatid | Chromosome | exchanges | |||||||
| scored | cells | breaks | (1) | (2) | (3) | (nP) | breaks | (1) | (2) | (3) | (nP) | |||
| 0 | 1,056 | 38 | 28 | 5 | 2 | 1 | 20 | 20 | 8 | 1 | 1 | 9 | 7 | t(nP;1),t(nP;2),t(nP;1),t(nP;1), ins(1;nP;1),ins(3;1) two cells |
| 0.05 | 383 | 19 | 18 | 3 | 2 | 1 | 12 | 8 | 1 | 1 | 0 | 6 | 1 | t(nP;1) |
| 0.10 | 935 | 125 | 81 | 11 | 5 | 7 | 58 | 48 | 17 | 4 | 7 | 20 | 11 | t(1;3),t(nP;3),t(nP;1),t(nP;3), ring(nP), dic(nP;nP), dic(nP;nP),dic (nP;nP), ins(nP;1;nP),RT(1;2),RT(nP;nP) |
| 1.00 | 256 | 137 | 242 | 25 | 11 | 13 | 193 | 73 | 16 | 8 | 11 | 38 | 14 | t(2;1),t(nP;1),t(nP;2),t(nP;2),t(nP;2),recip-t(nP;nP),t(3;1), t(nP;1),t(nP;2),t(nP;3), ins(nP;2;nP),ins(nP;3;nP), ins(nP;3;nP),RT(nP;nP) |
| Dose (Gy) | Total number of abnormal cells ± S.E. | Total number of chromatid breaks ± S.E. | Total number of iso-chromatid breaks ± S.E. | Total number of exchanges ± S.E. |
|---|---|---|---|---|
| 0 | 2.67 ± 0.52 | 2.33 ± 0.47 | 1.40 ± 0.41 | 1.04 ± 0.35 |
| 0.05 | 5.03 ± 0.68 | 5.40 ± 0.51 | 4.90 ± 1.02 | 2.03 ± 0.57 |
| 0.10 | 5.01 ± 0.61 | 5.55 ± 0.84 | 3.41 ± 0.41 | 1.21 ± 0.25 |
| 1.00 | 13.82 ± 1.76 | 16.49 ± 3.70 | 6.14 ± 1.16 | 3.54 ± 0.79 |
| Dose (Gy) | Total number of abnormal cells ± S.E. | Total number of chromatid breaks ± S.E. | Total number of iso-chromatid breaks ± S.E. | Total number of exchanges ± S.E. |
|---|---|---|---|---|
| 0 | 2.73 ± 0.25 | 2.44 ± 0.28 | 1.87 ± 0.15 | 1.07 ± 0.20 |
| 0.05 | 5.21 ± 1.05 | 4.99 ± 1.10 | 3.61 ± 0.73 | 1.73 ± 0.46 |
| 0.10 | 6.05 ± 0.79 | 4.73 ± 0.83 | 3.67 ± 0.59 | 1.52 ± 0.28 |
| 1.00 | 19.53 ± 2.03 | 26.40 ± 3.48 | 13.62 ± 1.69 | 7.02 ± 1.20 |

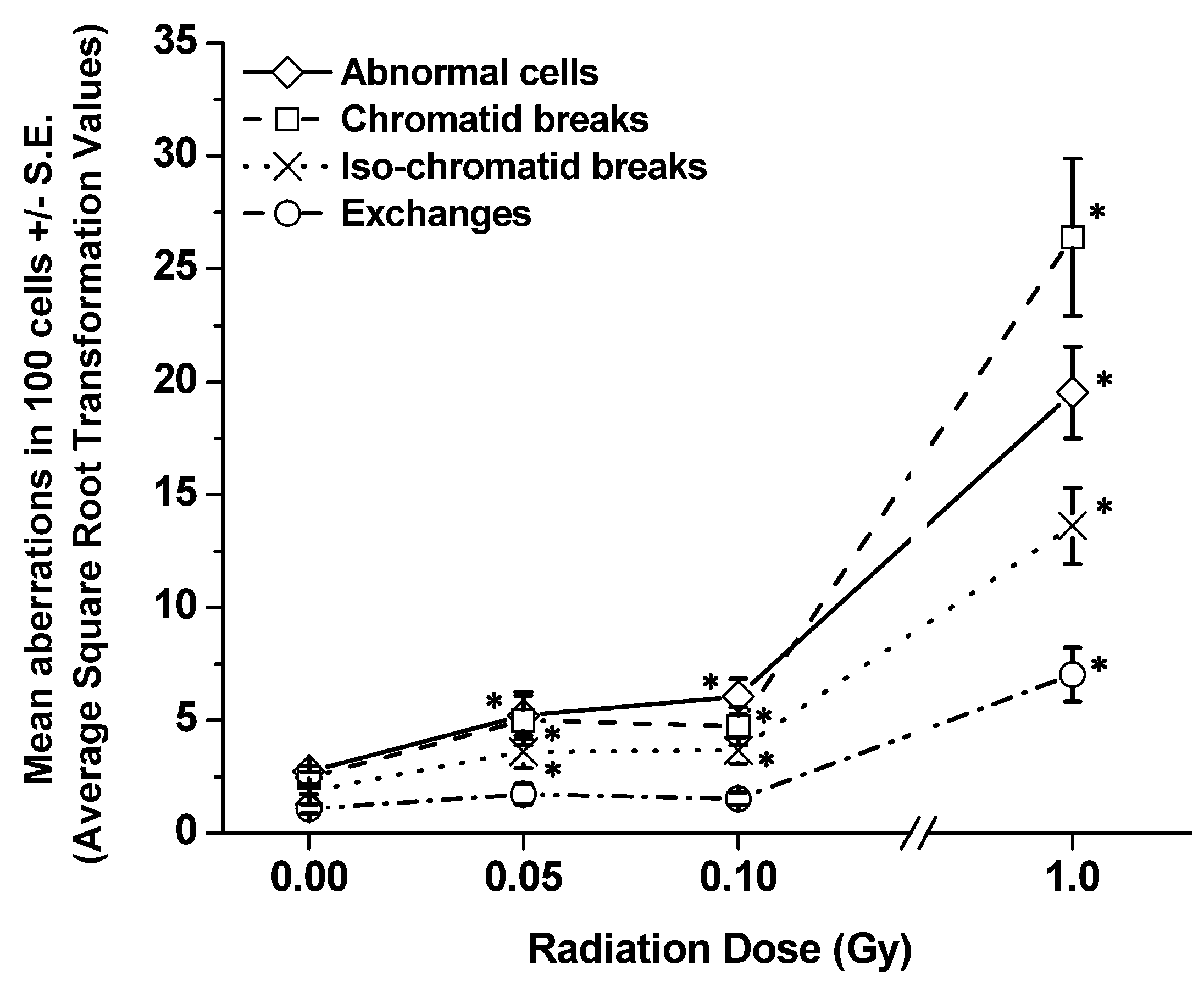
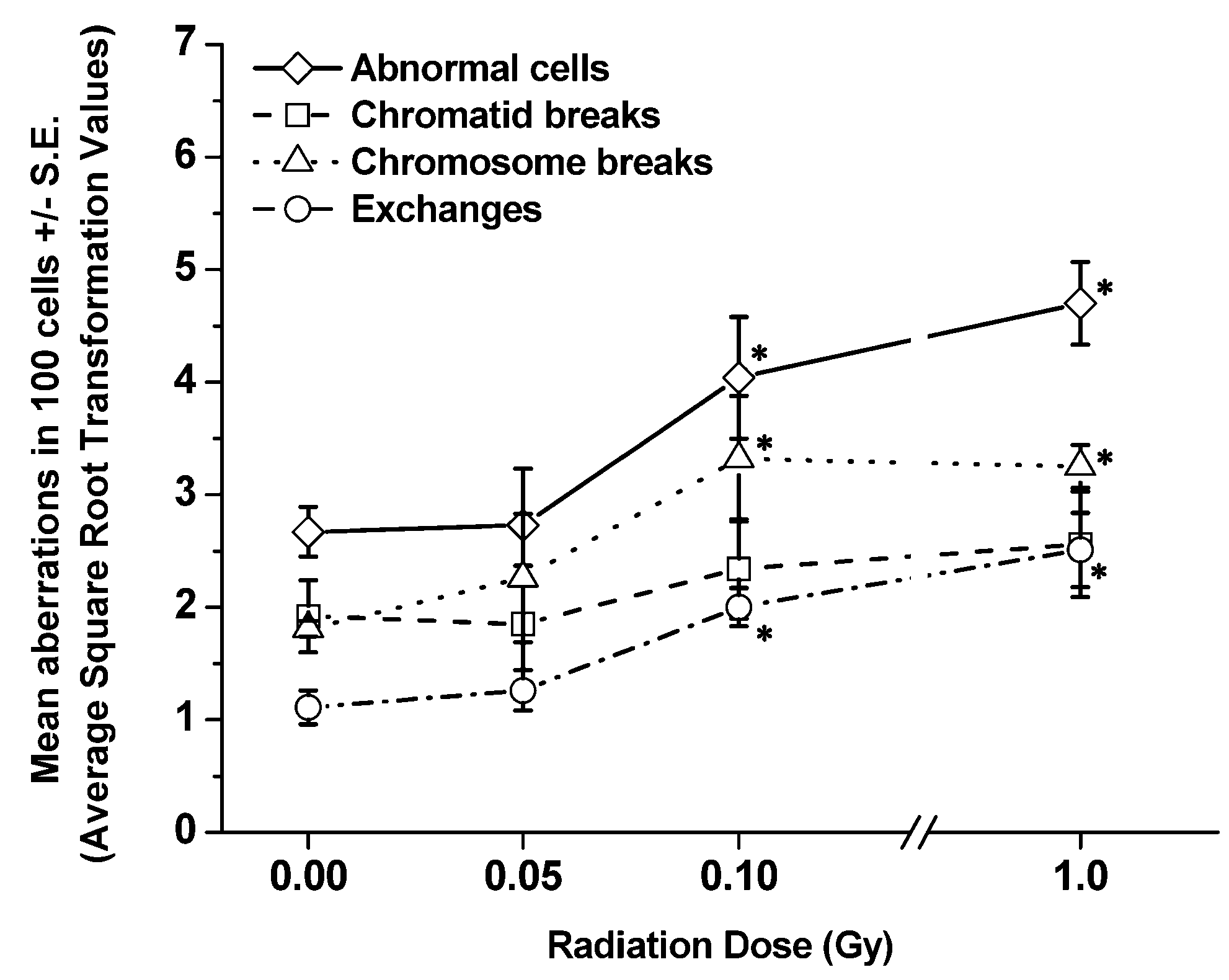
3.2. Late Time-Points (1 and 6 mo Post-Irradiation)

| Total | Total | Total | Chromatid breaks | Total | Chromosome breaks | Total | Exchanges | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | number of | number of | number of | (Chromosome involved) | number of | (Chromosome involved) | number of | (Both chromatid- and chromosome-types) | ||||||
| (Gy) | cells | abnormal | chromatid | Chromosome | Chromosome | Chromosome | exchanges | |||||||
| scored | cells | breaks | (1) | (2) | (3) | (nP) | breaks | (1) | (2) | (3) | (nP) | |||
| 0 | 941 | 37 | 30 | 3 | 0 | 0 | 27 | 16 | 4 | 0 | 0 | 12 | 4 | t(nP;1),t(nP;1),t(3;nP),t(nP;nP) |
| 0.05 | 393 | 10 | 4 | 1 | 0 | 1 | 2 | 5 | 0 | 0 | 0 | 5 | 1 | t*(nP;1) |
| 0.10 | 1,373 | 78 | 57 | 8 | 1 | 8 | 40 | 18 | 7 | 2 | 2 | 7 | 12 | t(1;3),recip t(1;nP),t(nP;1), t(nP;1),t(nP;1),t(nP;1), recip t(nP;2),t(nP;nP), dic(nP;nP),dic(nP;nP),ring(nP), RT(3;nP) |
| 1.00 | 518 | 49 | 27 | 7 aa | 4 | 1 | 15 | 25 | 3a | 2 | 1 | 19 | 7 | recip t(1;nP),t(2;nP),t(nP;1), t(nP;2),t(nP;2),t*(2;3), RT(1;nP) |
| Dose (Gy) | Total number of cells scored | Total number of abnormal cells | Total number of chromatid breaks | Chromatid breaks (Chromosome involved) Chromosome | Total number of Chromosome breaks | Chromosome breaks (Chromosome involved) Chromosome | Total number of exchanges | Exchanges (Both chromatid- and chromosome-types) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (nP) | (1) | (2) | (3) | (nP) | |||||||
| 0 | 1,693 | 95 | 43 | 10 | 2 | 1 | 30 | 30 | 7 | 0 | 5 | 18 | 17 | t(1;2),t(1;nP),t(1;nP),recip t(2;3),t(3;nP),t(nP;1),t(nP;1), t(nP;nP), t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;2), dic(nP;1),ins(nP;1),RT(nP;nP) |
| 0.05 | 948 | 32 | 19 | 2 | 4 | 2 | 11 | 21 | 5 | 7 | 1 | 8 | 8 | t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;2), t(nP;2),t(nP;2), t*(nP;2) |
| 0.10 | 1,030 | 93 | 22 | 7a | 9a | 3 | 3 | 63 | 25aa | 4 | 7 | 27 | 23 | t(1;nP),recip t(1;nP),t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;1), t(nP;1),t(nP;2),t(nP;2),t(nP;nP),t*(2;nP),t*(3;1),t*(nP;1), t*(nP;1),t*(nP;1),t*(nP;1),t*(nP;1),t*(nP;2),ins(nP;1), ins(nP;1), RT(nP;nP),RT(nP;nP) |
| 1.00 | 1,695 | 306 | 103 | 25 aa | 13 aa | 9 a | 56 | 153 | 52 aa | 14 a | 7 | 80 | 91 | t(1;2)a,t(1;3)a,t(1;nP),recip t(1;nP), recip t(1;nP),recip t(1;nP),recip t(1;nP), recip t(1;nP),t(2;1),t(2;nP),t(2;nP), recip t(2;nP),recip t(2;nP), recip t(2;nP),t(3;1),t(nP;1),t(nP;1), t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;1),t(nP;1), t(nP;1),t(nP;1),t(nP;1),recip t(nP;1),recip t(nP;1),t(nP;2), t(nP;2),t(nP;2),t(nP;2),t(nP;2),t(nP;2),t(nP;2),t(nP;2),t(nP;2), t(nP;2),t(nP;2), recip-t(nP;2),t(nP;3),t(nP;3),t(nP;3),t(nP;3), t(nP;3),recip-t(nP;3),t(nP1),t(nP1),t(nP1),t(nP1),t(nP1), t(nP1),t(nP1),t*(nP;1),t*(nP;1),t*(nP;1),t*(nP;1),t*(nP;1), t*(nP;1),t*(nP;1),t*(nP;2),t*(nP;2),t*(nP;2),t*(nP;2),t*(nP;3), t*(nP;3),t*(nP;3),ins(1;nP),ins(2;1),ins*(2;1),ins(2;3), ins(2;nP),ins(3;nP),ins(nP;1),ins(nP;1),ins(nP;1),ins(nP;1), ins(nP;1),ins(nP;2),ins(nP;2),ins(nP;2),ins(nP;2),ins(nP;3), ins(nP;3),ins(nP;3),ins(nP;3),RT(nP;nP),RT(nP;nP) |
| Dose (Gy) | Total number of abnormal cells ± S.E. | Total number of chromatid breaks ± S.E. | Total number of chromosome breaks ± S.E. | Total number of exchanges ± S.E. |
|---|---|---|---|---|
| 0 | 3.67 ± 0.69 | 3.37 ± 0.75 | 2.57 ± 0.92 | 1.06 ± 0.68 |
| 0.05 | 3.51 ± 0.68 | 2.24 ± 0.59 | 2.84 ± 0.36 | 1.61 ± 0.30 |
| 0.10 | 3.43 ± 0.75 | 3.04 ± 0.84 | 1.83 ± 0.50 | 1.18 ± 0.18 |
| 1.00 | 6.51 ± 0.89 | 5.40 ± 0.55 | 4.54 ± 0.03 | 2.94 ± 0.53 |
| Dose(Gy) | Total number of abnormalcells ± S.E. | Total numberof chromatidbreaks ± S.E. | Total numberof chromosome breaks ± S.E. | Total number of exchanges ± S.E. |
|---|---|---|---|---|
| 0 | 2.67 ± 0.22 | 1.92 ± 0.32 | 1.81 ± 0.07 | 1.11 ± 0.15 |
| 0.05 | 2.73 ± 0.50 | 1.85 ± 0.52 | 2.26 ± 0.57 | 1.26 ± 0.18 |
| 0.10 | 4.04 ± 0.54 | 2.34 ± 0.44 | 3.32 ± 0.56 | 2.00 ± 0.17 |
| 1.00 | 4.70 ± 0.37 | 2.56 ± 0.47 | 3.25 ± 0.19 | 2.51 ± 0.33 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rithidech, K.N.; Udomtanakunchai, C.; Honikel, L.M.; Whorton, E.B. No evidence for the in vivo induction of genomic instability by low doses of 137Cs gamma rays in bone marrow cells of BALB/CJ and C57BL/6J mice. Dose Response 2012, 10, 11–36. [Google Scholar] [CrossRef]
- Hooker, A.M.; Bhat, M.; Day, T.K.; Lane, J.M.; Swinburne, S.J.; Morley, A.A.; Sykes, P.J. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat. Res. 2004, 162, 447–452. [Google Scholar] [CrossRef]
- Rithidech, K.N.; Scott, B.R. Evidence for radiation hormesis after in vitro exposure of human lymphocytes to low doses of ionizing radiation. Dose Response 2008, 6, 252–271. [Google Scholar] [CrossRef]
- Okayasu, R.; Suetomi, K.; Yu, Y.; Silver, A.; Bedford, J.S.; Cox, R.; Ullrich, R.L. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Canc. Res. 2000, 60, 4342–4345. [Google Scholar]
- Ullrich, R.L.; Davis, C.M. Radiation-induced cytogenetic instability in vivo. Radiat. Res. 1999, 152, 170–173. [Google Scholar] [CrossRef]
- Slabbert, J.P.; Theron, T.; Serafin, A.; Jones, D.T.; Bohm, L.; Schmitt, G. Radiosensitivity variations in human tumor cell lines exposed in vitro to p(66)/Be neutrons or 60Co gamma-rays. Strahlenther. Onkol. 1996, 172, 567–572. [Google Scholar]
- Ponnaiya, B.; Cornforth, M.N.; Ullrich, R.L. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: The difference is as clear as black and white. Radiat. Res. 1997, 147, 121–125. [Google Scholar] [CrossRef]
- Shadley, J.D.; Whitlock, J.L.; Rotmensch, J.; Atcher, R.W.; Tang, J.; Schwartz, J.L. The effects of radon daughter alpha-particle irradiation in K1 and xrs-5 CHO cell lines. Mutat. Res. 1991, 248, 73–83. [Google Scholar] [CrossRef]
- Boulton, E.; Cleary, H.; Papworth, D.; Plumb, M. Susceptibility to radiation-induced leukaemia/lymphoma is genetically separable from sensitivity to radiation-induced genomic instability. Int. J. Radiat. Biol. 2001, 77, 21–29. [Google Scholar] [CrossRef]
- Kadhim, M.A. Role of genetic background in induced instability. Oncogene 2003, 22, 6994–6999. [Google Scholar] [CrossRef]
- Yu, Y.; Okayasu, R.; Weil, M.M.; Silver, A.; McCarthy, M.; Zabriskie, R.; Long, S.; Cox, R.; Ullrich, R.L. Elevated breast cancer risk in irradiated BALB/c mice associates with unique functional polymorphism of the Prkdc (DNA-dependent Protein Kinase Catalytic Subunit) gene. Canc. Res. 2001, 61, 1820–1824. [Google Scholar]
- Bauer, G. Low dose radiation and intercellular induction of apoptosis: Potential implications for the control of oncogenesis. Int. J. Radiat. Biol. 2007, 83, 873–888. [Google Scholar] [CrossRef]
- Portess, D.I.; Bauer, G.; Hill, M.A.; O’Neill, P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Canc. Res. 2007, 67, 1246–1253. [Google Scholar] [CrossRef]
- Blunt, T.; Gell, D.; Fox, M.; Taccioli, G.E.; Lehmann, A.R.; Jackson, S.P.; Jeggo, P.A. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc. Natl. Acad. Sci. USA 1996, 93, 10285–10290. [Google Scholar]
- Danska, J.S.; Holland, D.P.; Mariathasa, S.; Williams, K.M.; Guidos, C.J. Biochemical and genetic defects in the DNA-PK in murinescid lymphocytes. Mol. Cell Biol. 1996, 16, 5507–5517. [Google Scholar]
- Fulop, G.M.; Phillips, R.A. The scid mutation in mice causes a general defect in DNA repair. Nature 1990, 347, 479–482. [Google Scholar]
- Custer, R.; Bosma, G.C.; Bosma, M.J. Severe combined immunodeficiency (SCID) in the mouse. Am. J. Pathol. 1985, 464–477. [Google Scholar]
- Biedermann, K.A.; Sun, J.R.; Giaccia, A.J.; Tosto, L.M.; Brown, J.M. Scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 1991, 88, 1394–1397. [Google Scholar] [CrossRef]
- Budach, W.; Hartford, A.; Gioioso, D.; Freeman, J.; Taghian, A.; Suit, H.D. Tumors arising in SCID mice share enhanced radiation sensitivity of SCID normal tissues. Canc. Res. 1992, 52, 6292–6296. [Google Scholar]
- Barber, R.C.; Miccoli, L.; van Buul, P.P.W.; Burr, K.L.A.; van Duyn-Goedhart, A.; Angulo, J.F.; Dubrova, Y.E. Germline mutation rates at tandem repeat loci in DNA-repair deficient mice. Mutat. Res. 2004, 554, 287–295. [Google Scholar] [CrossRef]
- Grigorova, M.; Boei, J.J.; van Duyn-Goedhart, A.; Natarajan, A.T.; van Buul, P.P.W. X-ray induced translocations in bone marrow cells of scid and wild type mice detected by fluorescence in situ hybridization using mouse chromosome specific DNA libraries. Mutat. Res. 1995, 331, 39–45. [Google Scholar] [CrossRef]
- Van Buul, P.P.; Abramsson-Zetterberg, L.; Zandman, I.M.; van Duyn-Goedhart, A. Further characterization of the radiosensitivity of the scid mouse. Int. J. Radiat. Biol. 1998, 74, 35–41. [Google Scholar] [CrossRef]
- Disney, J.E.; Barth, A.L.; Shultz, L.D. Defective repair of radiation-induced chromosomal damage in scid/scid mice. Cytogenet. Cell Genet. 1992, 59, 39–44. [Google Scholar] [CrossRef]
- Van Buul, P.P.; De Rooij, D.G.; Zandman, I.M.; Grigorova, M.; van Duyn-Goedhart, A. X-ray-induced chromosomal aberrations and cell killing in somatic and germ cells of the scid mouse. Int. J. Radiat. Biol. 1995, 67, 549–555. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Lorimore, S.A.; Hepburn, M.D.; Goodhead, D.T.; Buckle, V.J.; Wright, E.G. Alpha-particle-induced chromosomal instability in human bone marrow cells. Lancet 1994, 344, 987–988. [Google Scholar]
- Kadhim, M.A.; Lorimore, S.A.; Townsend, K.M.; Goodhead, D.T.; Buckle, V.J.; Wright, E.G. Radiation-induced genomic instability: Delayed cytogenetic aberrations and apoptosis in primary human bone marrow cells. Int. J. Radiat. Biol. 1995, 67, 287–293. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Macdonald, D.A.; Goodhead, D.T.; Lorimore, S.A.; Marsden, S.J.; Wright, E.G. Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature 1992, 355, 738–740. [Google Scholar]
- Kadhim, M.A.; Marsden, S.J.; Goodhead, D.T.; Malcolmson, A.M.; Folkard, M.; Prise, K.M.; Michael, B.D. Long-term genomic instability in human lymphocytes induced by single-particle irradiation. Radiat. Res. 2001, 155, 122–126. [Google Scholar] [CrossRef]
- Watson, G.E.; Lorimore, S.A.; Wright, E.G. Long-term in vivo transmission of alpha-particle-induced chromosomal instability in murinehaemopoietic cells. Int. J. Radiat. Biol. 1996, 69, 175–182. [Google Scholar] [CrossRef]
- Watson, G.E.; Pocock, D.A.; Papworth, D.; Lorimore, S.A.; Wright, E.G. In vivo chromosomal instability and transmissible aberrations in the progeny of haemopoietic stem cells induced by high- and low-LET radiations. Int. J. Radiat. Biol. 2001, 77, 409–417. [Google Scholar] [CrossRef]
- Rithidech, K.N.; Honikel, L.; Whorton, E.B. mFISH analysis of chromosomal damage in bone marrow cells collected from CBA/CaJ mice following whole body exposure to heavy ions (56Fe ions). Radiat. Environ. Biophys. 2007, 46, 137–145. [Google Scholar] [CrossRef]
- Ware, J.H.; Sanzari, J.; Avery, S.; Sayers, C.; Krigsfeld, G.; Nuth, M.; Wan, X.S.; Rusek, A.; Kennedy, A.R. Effects of proton radiation dose, dose rate and dose fractionation on hematopoietic cells in mice. Radiat. Res. 2010, 174, 325–330. [Google Scholar] [CrossRef]
- Rithidech, K.; Bond, V.P.; Cronkite, E.P.; Thompson, M.H.; Bullis, J.E. Hypermutability of mouse chromosome 2 during the development of x-ray-induced murine myeloid leukemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1152–1156. [Google Scholar] [CrossRef]
- Spruill, M.D.; Ramsey, M.J.; Swiger, R.R.; Nath, J.; Tucker, J.D. The persistence of aberrations in mice induced by gamma radiation as measured by chromosome painting. Mutat. Res. 1996, 356, 135–145. [Google Scholar] [CrossRef]
- Bouffler, S.D.; Meijne, E.I.; Morris, D.J.; Papworth, D. Chromosome 2 hypersensitivity and clonal development in murine radiation acute myeloid leukaemia. Int. J. Radiat. Biol. 1997, 72, 181–189. [Google Scholar] [CrossRef]
- Hande, M.P.; Boei, J.J.; Granath, F.; Natarajan, A.T. Induction and persistence of cytogenetic damage in mouse splenocytes following whole-body X-irradiation analysed by fluorescence in situ hybridization. I. Dicentrics and translocations. Int. J. Radiat. Biol. 1996, 69, 437–446. [Google Scholar] [CrossRef]
- Xiao, Y.; Darroudi, F.; Grigorova, M.; Natarajan, A.T. Induction and persistence of chromosomal exchanges in mouse bone marrow cells following whole-body exposure to X rays. Int. J. Radiat. Biol. 1999, 75, 1119–1128. [Google Scholar] [CrossRef]
- Giver, C.R.; Moore, D.H., II; Pallavicini, M.G. Radiation-induced translocations in mice: Persistence, chromosome specificity, and influence of genetic background. Radiat. Res. 2000, 154, 283–292. [Google Scholar] [CrossRef]
- Tucker, J.D.; Breneman, J.W.; Briner, J.F.; Eveleth, G.G.; Langlois, R.G.; Moore, D.H. Persistence of radiation-induced translocations in rat peripheral blood determined by chromosome painting. Environ. Mol. Mutagen. 1997, 30, 264–272. [Google Scholar] [CrossRef]
- Rowley, J.D.; Potter, D. Chromosomal banding patterns in acute nonlymphocytic leukemia. Blood 1976, 47, 705–721. [Google Scholar]
- Whorton, E.B., Jr. Some experimental design and analysis considerations for cytogenetics studies. Environ. Mutagen. 1985, 7, 9–15. [Google Scholar] [CrossRef]
- Durante, M.; George, K.; Wu, H.; Cucinotta, F.A. Karyotypes of human lymphocytes exposed to high-energy iron ions. Radiat. Res. 2002, 158, 581–590. [Google Scholar] [CrossRef]
- Huumonen, K.; Immonen, H.-K.; Baverstock, K.; Hiltunen, M.; Korkalainen, M.; Lahtinen, T.; Parviainen, J.; Viluksela, M.; Wong, G.; Naarala, J.; et al. Radiation-induced genomic instability in Caenorhabditis elegans. Mutat. Res. 2012, 748, 36–41. [Google Scholar] [CrossRef]
- Huang, L.; Snyder, A.R.; Morgan, W.F. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene 2003, 22, 5848–5854. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C.B. Mechanisms and implications of genomic instability and other delayed effects of ionizing radiation exposure. Mutagenesis 1998, 13, 421–426. [Google Scholar] [CrossRef]
- Sabatier, L.; Dutrillaux, B.; Martin, M.B. Chromosomal instability. Nature 1992, 357, 548–548. [Google Scholar] [CrossRef]
- Martins, M.B.; Sabatier, L.; Ricoul, M.; Pinton, A.; Dutrillaux, B. Specific chromosome instability induced by heavy ions: A step towards transformation of human fibroblasts? Mutat. Res. 1993, 285, 229–237. [Google Scholar] [CrossRef]
- Holmberg, K.; Fält, S.; Johansson, A.; Lambert, B. Clonal chromosome aberrations and genomic instability in X-irradiated human T-lymphocyte cultures. Mutat. Res. Mutagen. 1993, 286, 321–330. [Google Scholar] [CrossRef]
- Marder, B.A.; Morgan, W.F. Delayed chromosomal instability induced by DNA damage. Mol. Cell Biol. 1993, 13, 6667–6677. [Google Scholar]
- Watson, G.E.; Lorimore, S.A.; Macdonald, D.A.; Wright, E.G. Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Canc. Res. 2000, 60, 5608–5611. [Google Scholar]
- Morgan, W.F.; Day, J.P.; Kaplan, M.I.; McGhee, E.M.; Limoli, C.L. Genomic instability induced by ionizing radiation. Radiat. Res. 1996, 146, 247–258. [Google Scholar] [CrossRef]
- Anderson, C.W. Protein kinases and the response to DNA damage. Semin. Cell Biol. 1994, 5, 427–436. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.K.; Dent, P.; Grant, S.; Mikkelsen, R.B.; Valerie, K. Signal transduction and cellular radiation responses. Radiat. Res. 2000, 153, 245–257. [Google Scholar] [CrossRef]
- Takahashi, A.; Kondo, N.; Inaba, H.; Uotani, K.; Kiyohara, Y.; Ohnishi, K.; Ohnishi, T. Radiation-induced apoptosis in SCID mice spleen after low dose irradiation. Adv. Space Res. 2003, 31, 1569–1573. [Google Scholar] [CrossRef]
- Takahashi, A.; Asakawa, I.; Yuki, K.; Matsumoto, T.; Kumamoto, M.; Kondo, N.; Ohnishi, K.; Tachibana, A.; Ohnishi, T. Radiation-induced apoptosis in the scid mouse spleen after low dose-rate irradiation. Int. J. Radiat. Biol. 2002, 78, 689–693. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rithidech, K.N.; Udomtanakunchai, C.; Honikel, L.; Whorton, E. Lack of Genomic Instability in Bone Marrow Cells of SCID Mice Exposed Whole-Body to Low-Dose Radiation. Int. J. Environ. Res. Public Health 2013, 10, 1356-1377. https://doi.org/10.3390/ijerph10041356
Rithidech KN, Udomtanakunchai C, Honikel L, Whorton E. Lack of Genomic Instability in Bone Marrow Cells of SCID Mice Exposed Whole-Body to Low-Dose Radiation. International Journal of Environmental Research and Public Health. 2013; 10(4):1356-1377. https://doi.org/10.3390/ijerph10041356
Chicago/Turabian StyleRithidech, Kanokporn Noy, Chatchanok Udomtanakunchai, Louise Honikel, and Elbert Whorton. 2013. "Lack of Genomic Instability in Bone Marrow Cells of SCID Mice Exposed Whole-Body to Low-Dose Radiation" International Journal of Environmental Research and Public Health 10, no. 4: 1356-1377. https://doi.org/10.3390/ijerph10041356
APA StyleRithidech, K. N., Udomtanakunchai, C., Honikel, L., & Whorton, E. (2013). Lack of Genomic Instability in Bone Marrow Cells of SCID Mice Exposed Whole-Body to Low-Dose Radiation. International Journal of Environmental Research and Public Health, 10(4), 1356-1377. https://doi.org/10.3390/ijerph10041356




