Assessing the Mobility of Lead, Copper and Cadmium in a Calcareous Soil of Port-au-Prince, Haiti †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Characterization
 ).
).
2.2. Experimental Set-up
2.2.1. Adsorption Kinetics
2.2.2. Monometal Adsorption
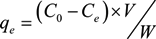
2.2.3. Competitive Adsorption
2.3. Theory
2.3.1. Kinetics Models
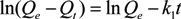
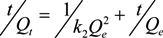
2.3.2. Isotherms Adsorption Models
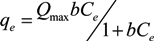
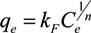
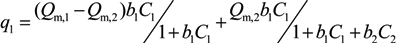
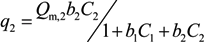
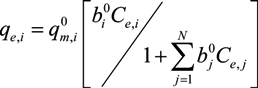
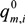 ,
, 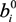 and
and 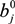 are Langmuir extended parameters obtained from Equation (4) in monometal batch tests and Ce,i and Ce,j are respectively the concentrations of metals i and j from tri-metal batch testsafter equilibrium.
are Langmuir extended parameters obtained from Equation (4) in monometal batch tests and Ce,i and Ce,j are respectively the concentrations of metals i and j from tri-metal batch testsafter equilibrium.3. Results and Discussion
3.1. Soil Characteristics
| Parameters | Concentration | Standards and analysis methods |
|---|---|---|
| pH-H2O | 8.26 | AFNOR X31-104 |
| pH-KCl | 7.46 | AFNOR X31-104 |
| CaCO3 (g·kg−1) | 343.00 | AFNOR X31-105 |
| Organic carbon (g·kg−1) | 100.00 | AFNOR X31-106 |
| Organic matter (g·kg−1) | 57.85 | Calcination at 550 °C |
| Clay (g·kg−1) | 17.00 | AFNOR X31-107 |
| CEC (meq·kg−1) | 135.00 | Metson Method AFNOR X31-130 |
| Surface area (m2·g−1) | 9.48 | B.E.T Method |
| Total Ca (g·kg−1) | 9.67 | AFNOR X31-108 |
| Total Mg (g·kg−1) | 0.45 | AFNOR X31-108 |
| Total K (g·kg−1) | 0.051 | AFNOR X31-108 |
| Total Cr (mg·kg−1) | 17.40 | NF ISO 11885 |
| Total Cu (mg·kg−1) | 61.40 | NF ISO 11885 |
| Total Ni (mg·kg−1) | 24.10 | NF ISO 11885 |
| Total Zn (mg·kg−1) | 28.10 | NF ISO 11885 |
| Total Cd (mg·kg−1) | Ud * | NF ISO 11885 |
| Total Pb (mg·kg−1) | Ud | NF ISO 11885 |
| Total Hg (mg·kg−1) | Ud | NF ISO 11885 |
| Total Se (mg·kg−1) | Ud | NF ISO 11885 |
3.2. Kinetics
| Metal ions | Pseudo-first order | Pseudo-second order | |||
|---|---|---|---|---|---|
| K1 (min−1) | R12 | Qe (mg·g−1) | K2 (g·mg−1·min) | R22 | |
| Pb2+ | 0.00139 | 0.66 | 2.50 | 0.25 | 1.00 |
| Cu2+ | 0.00147 | 0.68 | 0.79 | 0.77 | 1.00 |
| Cd2+ | 0.00010 | 0.83 | 1.24 | 0.01 | 0.99 |
| Pb2+ (Pb2+–Cu2+–Cd2+) | 0.00047 | 0.48 | 2.61 | 0.075 | 1.00 |
| Cu2+ (Cu2+–Pb2+–Cd2+) | 0.0012 | 0.71 | 0.86 | 0.055 | 0.99 |
| Cd2+ (Cd2+–Pb2+–Cu2+) | 0.00073 | 0.91 | 1.58 | 0.002 | 0.94 |
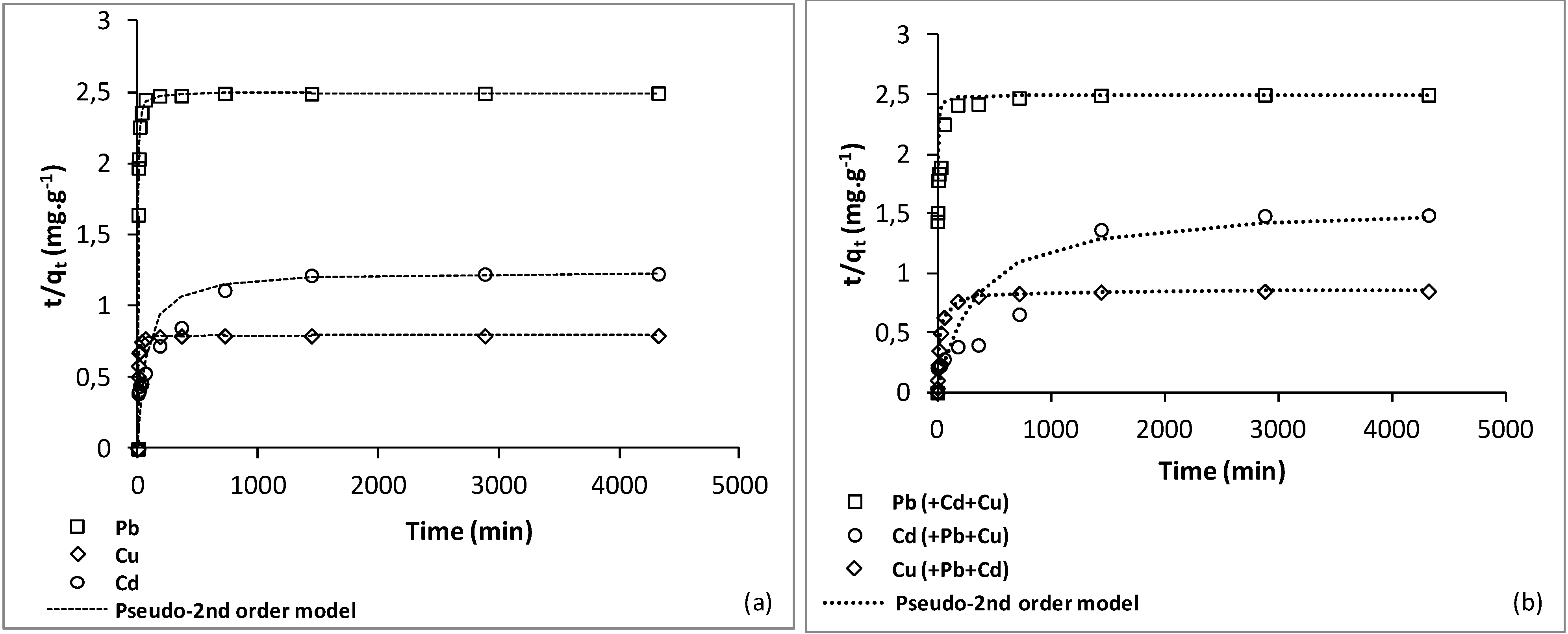
3.3. Monometal Adsorption
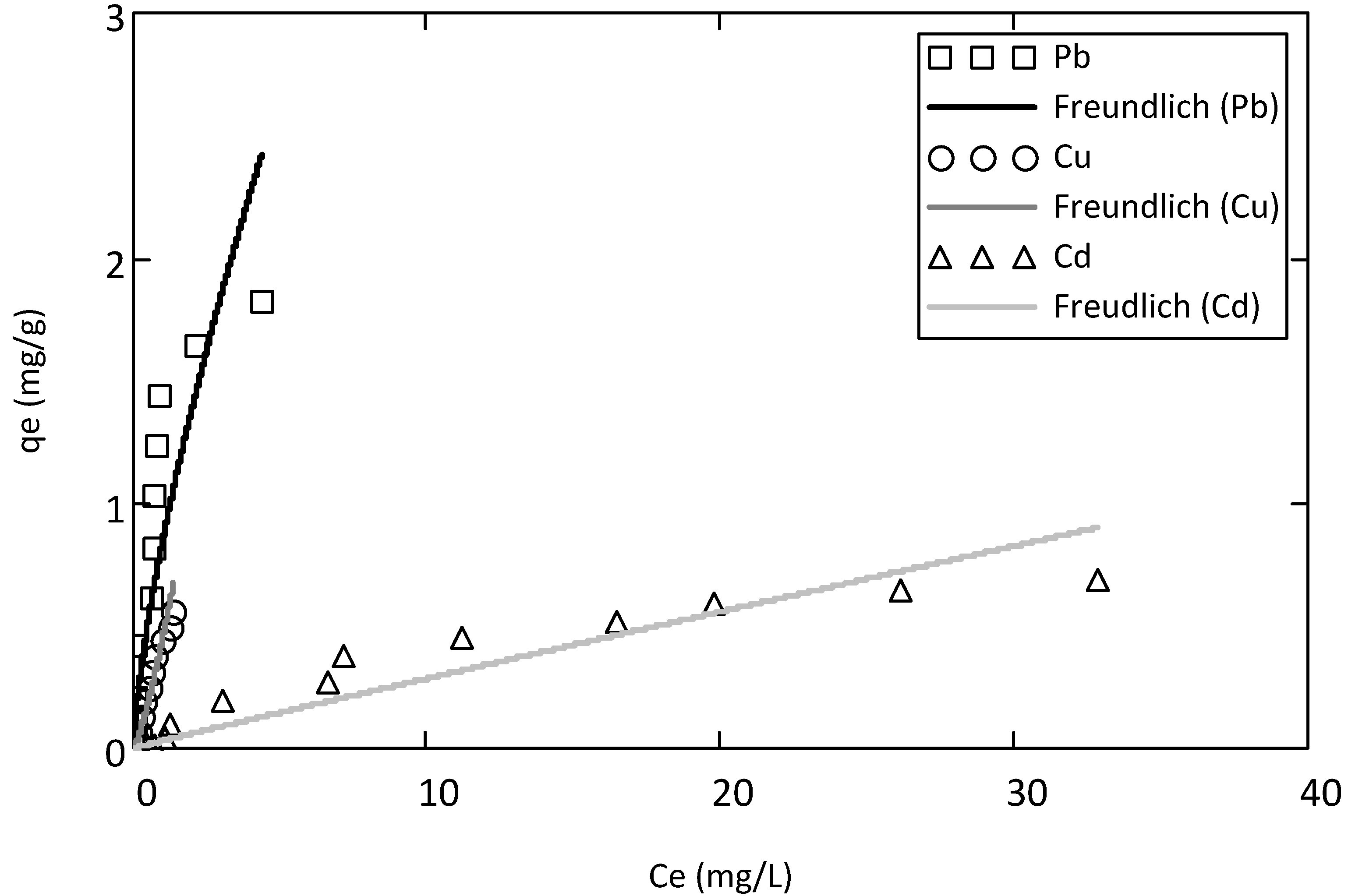
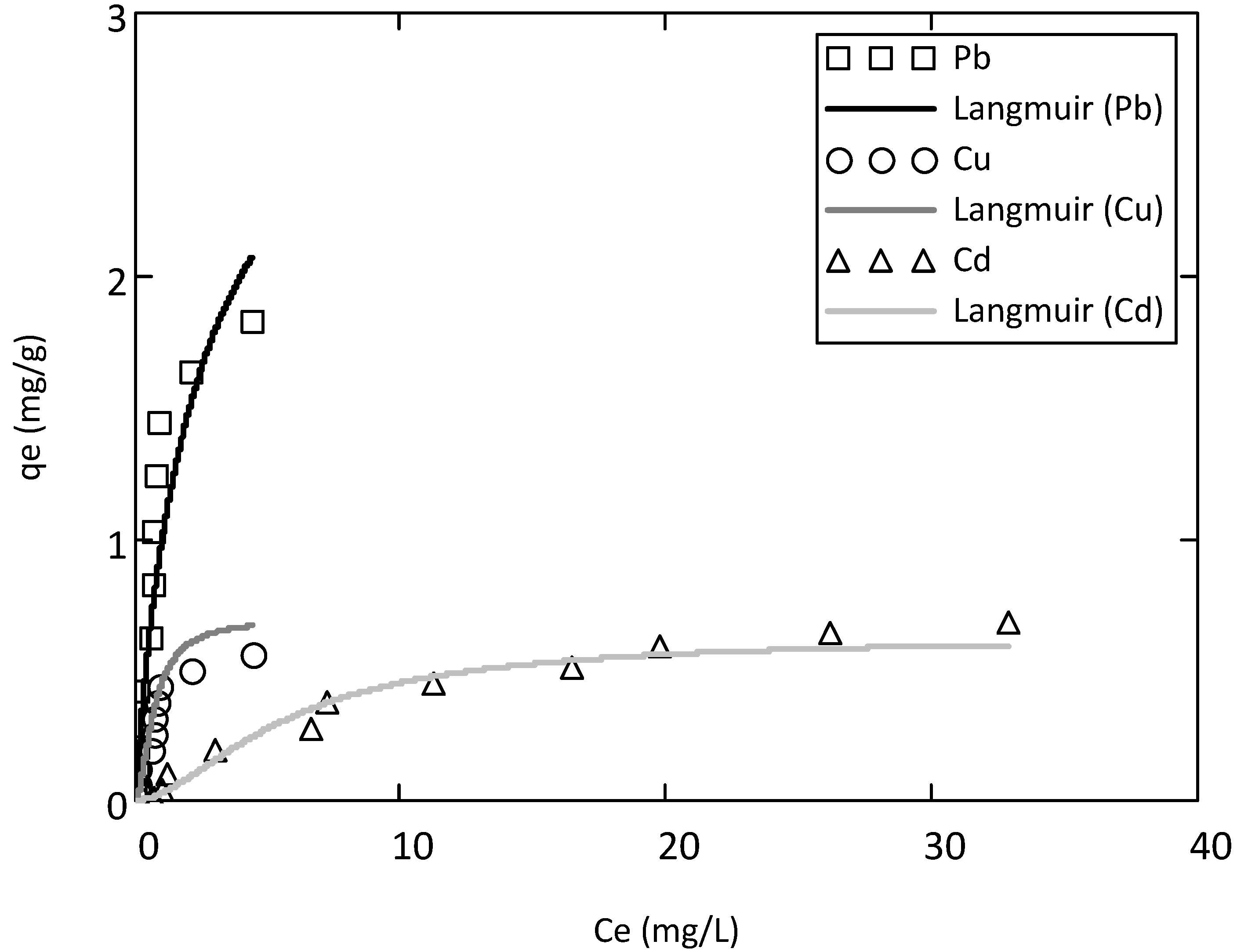
3.4. Competitive Adsorption
| Metals | Adsorption batch tests | Langmuir parameters | Freundlich parameters | ||||
|---|---|---|---|---|---|---|---|
| Monometal | qmaxL | bL | RL2 | 1/n | KF | RF2 | |
| Pb | Cd2+ | 3.64 | 0.37 | 0.91 | 1.41 | 0.85 | 0.91 |
| Cu | Cu2+ | 0.70 | 1.81 | 0.91 | 0.78 | 0.45 | 0.92 |
| Cd | Cd2+ | 0.63 | 0.05 | 0.90 | 1.01 | 0.03 | 0.89 |
| Bi-metal | Jain and Snoeyink parameters | ||||||
| qmaxJS | bJS | RJS2 | ΔqJS (%) | rJS | |||
| Pb | (Pb2+–Cd2+) | 3.09 | 0.36 | 0.99 | 15.11 | 0.85 | |
| (Pb2+–Cu2+) | 2.95 | 1.40 | 0.97 | 18.95 | 0.81 | ||
| Cu | (Cu2+–Cd2+) | 0.59 | 2.07 | 0.98 | 15.71 | 0.84 | |
| (Cu2+–Pb2+) | 0.45 | 1.63 | 0.94 | 35.71 | 0.64 | ||
| Cd | (Cd2+–Pb2+) | 0.46 | 0.09 | 0.87 | 26.98 | 0.73 | |
| (Cd2+–Cu2+) | 0.10 | 0.44 | 0.75 | 84.13 | 0.16 | ||
| Tri-metal | Extended Langmuir parameters | ||||||
| qmaxLE | bLE | RLE2 | ΔqLE (%) | rLE | |||
| Pb | (Pb2+–Cu2+–Cd2+) | 0.77 | 1.56 | 0.95 | 78.86 | 0.21 | |
| Cu | (Cu2+–Pb2+–Cd2+) | 0.43 | 1.79 | 0.98 | 38.57 | 0.61 | |
| Cd | (Cd2+–Pb2+–Cu2+) | 0.10 | 0.85 | 0.91 | 84.13 | 0.16 | |
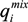 and
and 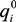 the single-solute system, , following this equation:
the single-solute system, , following this equation:
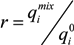
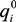 and
and 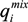 are the maximum amount sorbed according to monometal or multi-metal batch tests, respectively and r is the adsorption capacity ratio. If r > 1, metal i enhanced the adsorption of the others ions. If r = 1, metals had no effects on each other. If r < 1, metal i completed for with other metals for the adsorption sites of adsorbents. As showed in Table 3, all the values of r are lower than 1 which indicates the mutual competitive effect of each metal in all the experiments. The r values obtained from tri-metal batch tests are lower than those from bi-metal adsorption systems. These results indicated that the competitive adsorption processes depend on the quantity of metals ions from solid and liquid phases.
are the maximum amount sorbed according to monometal or multi-metal batch tests, respectively and r is the adsorption capacity ratio. If r > 1, metal i enhanced the adsorption of the others ions. If r = 1, metals had no effects on each other. If r < 1, metal i completed for with other metals for the adsorption sites of adsorbents. As showed in Table 3, all the values of r are lower than 1 which indicates the mutual competitive effect of each metal in all the experiments. The r values obtained from tri-metal batch tests are lower than those from bi-metal adsorption systems. These results indicated that the competitive adsorption processes depend on the quantity of metals ions from solid and liquid phases.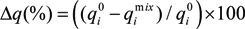
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Siegel, F.R. Environmental Geochemistry of Potentially Toxic Heavy Metals; Springer: Berlin, Germany, 2002. [Google Scholar]
- Usman, A.R.A. The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley, Egypt. Geoderma 2008, 144, 334–343. [Google Scholar] [CrossRef]
- Qin, F.; Wen, B.; Shan, X.-Q.; Xie, Y.-N.; Liu, T.; Zhang, S.-Z.; Khan, S.U. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ. Pollut. 2006, 144, 669–680. [Google Scholar] [CrossRef]
- Antoniadis, V.; Tsadilas, C.D.; Ashworth, D.J. Monometal and competitive adsorption of heavy metals by sewage sludge-amended soil. Chemosphere 2007, 68, 489–494. [Google Scholar] [CrossRef]
- Krishnamurti, G.S.R.; Naidu, R. Solid-solution equilibria of cadmium in soils. Geoderma 2003, 113, 17–30. [Google Scholar] [CrossRef]
- Adhikari, T.; Singh, M.V. Sorption characteristics of lead and cadmium in some soils of India. Geoderma 2003, 114, 81–92. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J. Environ. Qual. 2002, 31, 581–589. [Google Scholar] [CrossRef]
- Hooda, P.S.; Alloway, B.J. Cadmium and lead sorption behavior of selected English and Indian soils. Geoderma 1998, 84, 121–134. [Google Scholar] [CrossRef]
- Jalali, M.; Moharrami, S. Competitive adsorption of trace elements in calcareous soils of western Iran. Geoderma 2007, 140, 156–163. [Google Scholar] [CrossRef]
- Kuo, S.; Baker, A.S. Sorption of copper, zinc, and cadmium by some acid soils. Soil Sci. Soc. Am. J. 1980, 44, 969–974. [Google Scholar] [CrossRef]
- Martinez, C.E.; McBride, M.B. Solubility of Cd2+, Cu2+, Pb2+, and Zn2+ in aged coprecipitates with morphous iron hydroxides. Environ. Sci. Technol. 1998, 32, 743–748. [Google Scholar] [CrossRef]
- Plassard, F.; Winiarski, T.; Petit-Ramel, M. Retention and distribution of three heavy metals in a carbonated soil: Comparison between batch and unsaturated column studies. J. Contam. Hydrol. 2000, 42, 99–111. [Google Scholar] [CrossRef]
- Sauvé, S.; Hendershot, W.; Allen, H.E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Serrano, S.; Garrido, F.; Campbell, C.G.; Garcia-Gonzalez, M.T. Competitive sorption of cadmium and lead in acid soils of Central Spain. Geoderma 2005, 124, 91–104. [Google Scholar] [CrossRef]
- Trivedi, P.; Dyer, J.A.; Sparks, D.L. Lead sorption onto ferrihydrite. 1. A macroscopic and spectroscopic assessment. Environ. Sci. Technol. 2003, 37, 908–914. [Google Scholar] [CrossRef]
- Ponizovsky, A.A.; Allen, H.E.; Ackerman, A.J. Copper activity in soil solutions of calcareous soils. Environ. Pollut. 2007, 145, 1–6. [Google Scholar] [CrossRef]
- Fontes, M.P.F.; de Matos, A.T.; da Costa, L.M.; Neves, J.C.L. Compititive adsorption of Zn, Cd, Cu and Pb in three highly weathered Brazilian soils. Commun. Soil Sci. Plant Anal. 2000, 31, 2939–2958. [Google Scholar] [CrossRef]
- Assessment of Human Exposures to Lead in Drinking Water. Available online: http://www.bvsde.paho.org/bvsAIDIS/PuertoRico29/ruth.pdf (accessed on 29 October 2013).
- Emmanuel, E.; Angerville, R.; Joseph, O.; Perrodin, Y. Human health risk assessment of lead in drinking water : A case study from Port-au-Prince Haiti. Int. J. Environ. Pollut. 2007, 31, 280–291. [Google Scholar] [CrossRef]
- Emmanuel, E.; Pierre, M.G.; Perrodin, Y. Groundwater contamination by microbiological and chemical substances released from hospital wastewater: Health risk assessment for drinking water consumers. Environ. Int. 2009, 35, 718–726. [Google Scholar] [CrossRef]
- Metson, A.J. Methods of Chemical Analysis for Soil Survey Samples; New Zealand Department of Scientific and Industrial Research, Soil Bureau Bulletin: New Zealand, Wellington, 1956.
- Equilibrium Sorption of Pb(II), Cd(II) and Cu(II) into Soil of Port-au-Prince: Single-Element System Studies. Available online: http://theses.insa-lyon.fr/publication/2010ISAL0122/these.pdf (accessed on 29 October 2013).
- Jang, A.; Lee, S.-W.; Seo, Y.; Kim, K.-W.; Kim, I.S.; Bishop, P.L. Application of mulch for treating metals in urban runoff: Batch and column test. Water Sci. Technol. 2007, 55, 95–103. [Google Scholar]
- Ho, Y.-S. Citation review of Lagergreen kinetic rate equation on adsorption reaction. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Achak, M.; Hafidi, A.; Ouazzani, N.; Sayadi, S.; Mandi, L. Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies. J. Hazard. Mater. 2009, 166, 117–125. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Utilization of raw and activated date pits for the removal of phenol from aqueous solutions. Chem. Eng. Technol. 2004, 27, 80–86. [Google Scholar] [CrossRef]
- Goud, V.V.; Mohanty, K.; Rao, M.S.; Jayakumar, N.S. Phenol removal fromaqueous solutions using tamarind nut shell activated carbon: Batch and column study. Chem. Engin. Technol. 2005, 28, 814–821. [Google Scholar] [CrossRef]
- Fifi, U.; Winiarski, T.; Emmanuel, E. Groundwater Vulnerability towards Pollutants from Urban Stormwater in Developing Countries—Study of Heavy Metals Adsorption on a Representative Soil of Port-au-Prince, Haiti; (in French). GRAIE: Lyon, France, 2010. [Google Scholar]
- Jain, J.S.; Snoeyink, V.L. Adsorption from bisolute systems on active carbon. Water Pollut. Control Federation 1973, 45, 2463–2479. [Google Scholar]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Kanellopoulos, N.K. Prediction of binary adsorption isotherms of Cu2+, Cd2+ and Pb2+ on calcium alginate beads from single adsorption data. J. Hazard. Mater. 2009, 162, 1347–1354. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, C.F.; McKay, G. Sorption kinetics for the removal of copper and zinc from effluents using bone char. Sep. Purif. Technol. 2000, 19, 55–64. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.Z.L.; Basibuyuk, M.; Forster, C.F. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour. Technol. 2004, 92, 197–200. [Google Scholar] [CrossRef]
- Arias, M.; Pérez-Novo, C.; Lopez, E.; Soto, B. Competitive adsorption and desorption of copper and zinc in acids soils. Geoderma 2006, 133, 151–159. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley-Interscience Publication: Hoboken, NJ, USA, 1981. [Google Scholar]
- Frimmel, F.H.; Huber, L. Influence of humic substances on the aquatic sorption of heavy metals on defined minerals phases. Environ. Int. 1996, 22, 507–517. [Google Scholar] [CrossRef]
- Nagernaik, P.B.; Bhole, A.G.; Natarajan, G.S. Arsenic (II) removal by Adsorption rice husks carbon. Int. J. Environ. Stud. 2002, 5, 1097–1164. [Google Scholar]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. The removal of color from effluent using various adsorbents—IV silica: Equilibria and column studies. Water Res. 1980, 14, 21–27. [Google Scholar] [CrossRef]
- Tellan, A.C.; Owalude, S.O. Some Langmuir and Freundlich parameters of adsorption studies of chlorpheniramine maleate. Res. J. Appl. Sci. 2007, 2, 875–878. [Google Scholar]
- Elliott, H.A.; Liberat, M.R.; Huang, C.P. Competitive adsorption of heavy metals by soils. J. Environ. Qual. 1986, 15, 214–219. [Google Scholar] [CrossRef]
- Gomes, P.C.; Fontes, M.P.F.; da Silva, A.G.; de S. Mendonça, E.; Netto, A.R. Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils. Soil Sci. Soc. Am. J. 2001, 65, 1115–1121. [Google Scholar] [CrossRef]
- Yong, R.N.; Mohamed, A.M.O.; Warkentin, B.P. Principles of Contaminant Transport in Soils; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Berti, W.R.; Jacobs, L.W. Distribution of trace elements in soil from repeated sewage sludge application. J. Environ. Qual. 1998, 27, 1280–1286. [Google Scholar] [CrossRef]
- Morera, M.T.; Echeverría, J.C.; Mazkiarán, C.; Garrido, J.J. Isotherms and sequential extraction procedures for evaluating sorption and distribution of heavy metals in soils. Environ. Pollut. 2001, 113, 135–144. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—a biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fifi, U.; Winiarski, T.; Emmanuel, E. Assessing the Mobility of Lead, Copper and Cadmium in a Calcareous Soil of Port-au-Prince, Haiti. Int. J. Environ. Res. Public Health 2013, 10, 5830-5843. https://doi.org/10.3390/ijerph10115830
Fifi U, Winiarski T, Emmanuel E. Assessing the Mobility of Lead, Copper and Cadmium in a Calcareous Soil of Port-au-Prince, Haiti. International Journal of Environmental Research and Public Health. 2013; 10(11):5830-5843. https://doi.org/10.3390/ijerph10115830
Chicago/Turabian StyleFifi, Urbain, Thierry Winiarski, and Evens Emmanuel. 2013. "Assessing the Mobility of Lead, Copper and Cadmium in a Calcareous Soil of Port-au-Prince, Haiti" International Journal of Environmental Research and Public Health 10, no. 11: 5830-5843. https://doi.org/10.3390/ijerph10115830




