Volatile Constituents, Inorganic Elements and Primary Screening of Bioactivity of Black Coral Cigarette Holders
Abstract
:1. Introduction
2. Results and Discussion
2.1. Supercritical Fluid Extraction and Gas Chromatography-Mass Spectrometry (GC-MS)
2.2. Element Analysis
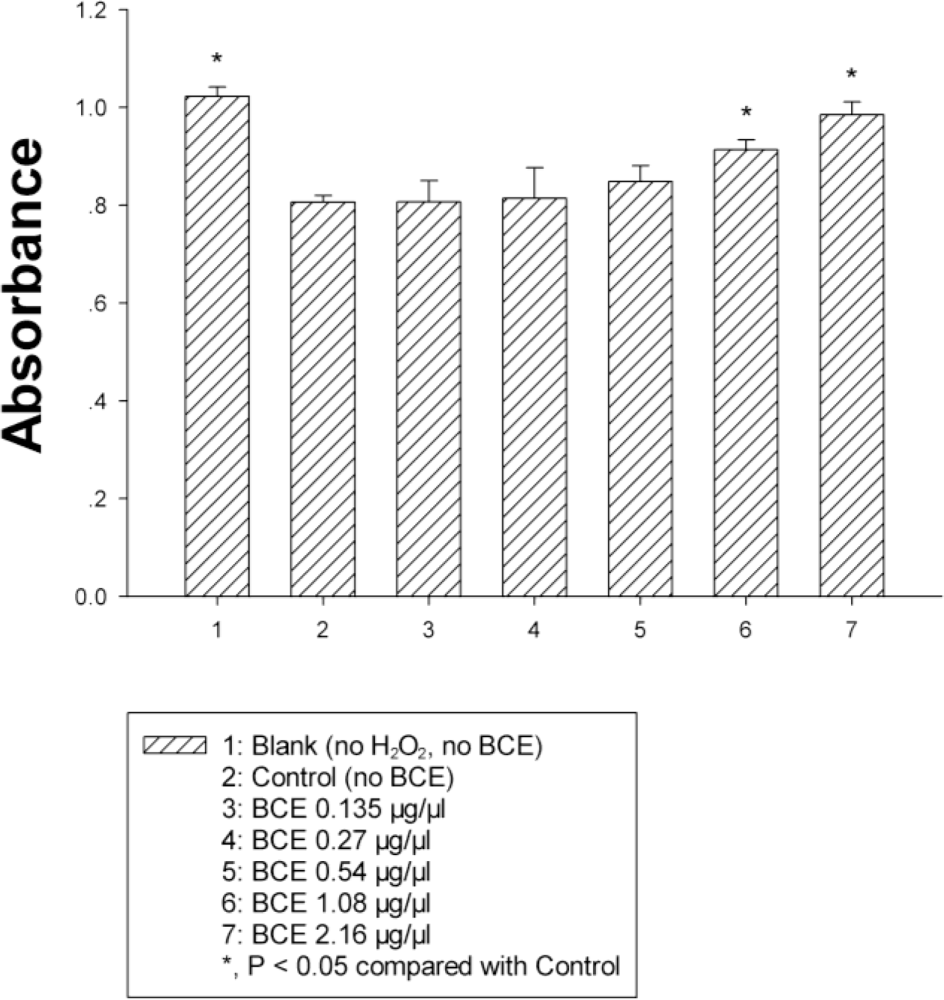
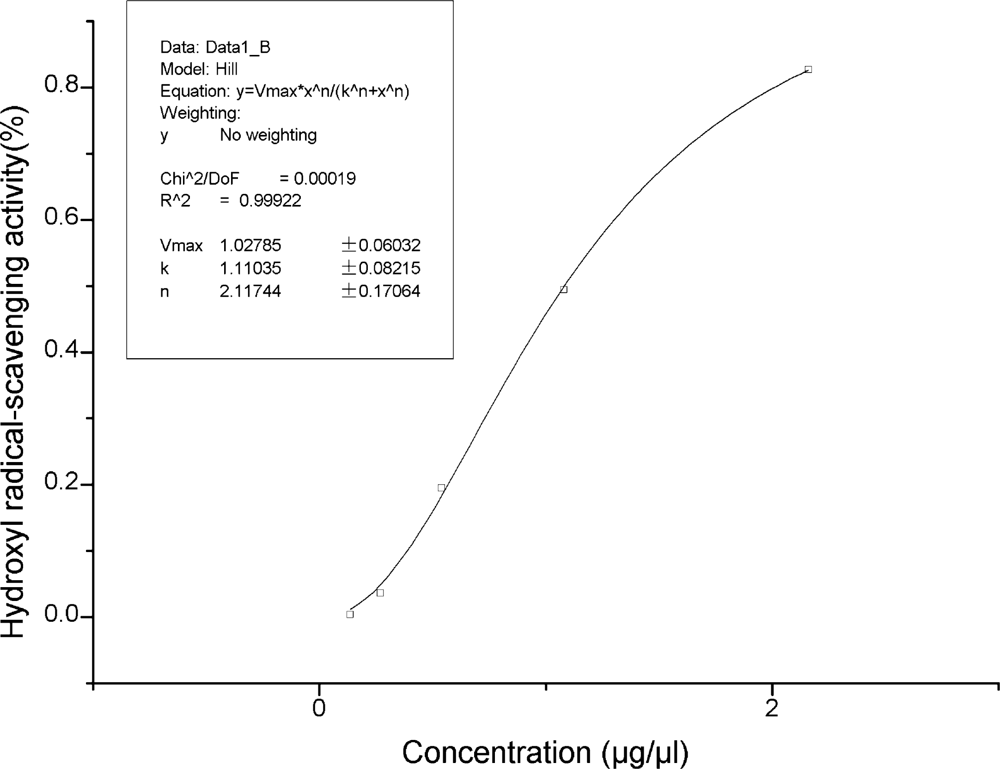
2.3. Antioxidant Property Assays of Extract
2.4. Antimicrobial Susceptibility Testing
3. Experimental Section
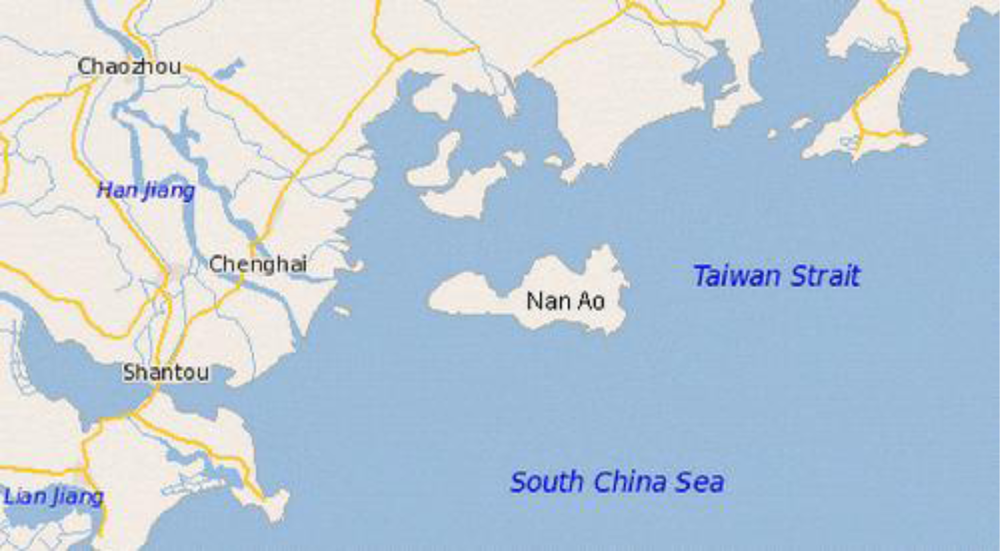
3.1. Sample Collection
3.2. Chemicals and Reagents
3.3. Supercritical Fluid Extraction with Carbon Dioxide
3.4. Gas Chromatography-Mass Spectrometry Analysis
3.5. Element Analysis
3.6. Antioxidant Property Assays of Extracts
3.7. Antimicrobial Susceptibility Testing and Determination of Minimum Inhibitory Concentration (MIC)
3.8. Statistical Analyses
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- WoRMS. Myriopathes japonica; Brook, 1889. World Register of Marine Species. 2010. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=290434 (accessed on 16 May 2011).
- Guan, HS; Wang, SG. Marine invertebrates. In Chinese Marine Materia Medica; Guan, HS, Wang, SG, Eds.; Science and Technology Publishing: Shanghai, China, 2009; Volume 3; pp. 46–47. [Google Scholar]
- Goldberg, WM. Chemistry and structure of skeletal growth rings in the black coral Antipathes fiordensis (Cnidaria, Antipatharia). Hydrobiologia 1991, 216–217, 403–409. [Google Scholar]
- California Environmental Protection Agency, Health Effects of Exposure to Environmental Tobacco Smoke; OEHHA: Sacramento, CA, USA, 1997.
- Pryor, WS; Stone, K. Oxidants in cigarette smoke: Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci 1993, 686, 12–27. [Google Scholar]
- Church, DF; Pryor, WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 1985, 64, 111–126. [Google Scholar]
- Bagaitkar, J; Demuth, DR; Scott, DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis 2008, 4, 12. [Google Scholar]
- Nuorti, JP; Butler, JC; Farley, MM; Harrison, LH; McGeer, A; Kolczak, MS; Breiman, RF. Cigarette smoking and invasive pneumococcal disease. Active bacterial core surveillance team. N Engl J Med 2000, 342, 681–689. [Google Scholar]
- Gold, R. Epidemiology of bacterial meningitis. Infect Dis Clin North Am 1999, 13, 515–525. [Google Scholar]
- Sethi, S; Murphy, TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008, 359, 2355–2365. [Google Scholar]
- Aiello, A; Fattorusso, E; Menna, M. Five new polar sterols from the black coral. Antipathes subpinnata Steroids 1991, 56, 513–517. [Google Scholar]
- Aiello, A; Fattorusso, E; Menna, M. Four new bioactive polyhydroxylated sterols from the black coral Antipathes subpinnata. J Nat Prod 1992, 55, 321–325. [Google Scholar]
- Qi, SH; Su, GC; Wang, YF; Liu, QY; Gao, CH. Alkaloids from the South China sea black coral antipathes dichotoma. Chem. Pharm. Bull. (Tokyo) 2009, 57, 87–88. [Google Scholar]
- Zeng, YX; Zhao, CX; Liang, YZ; Yang, H; Fang, HZ; Yi, LZ; Zeng, ZD. Comparative analysis of volatile components from Clematis species growing in China. Anal Chim Acta 2007, 595, 328–39. [Google Scholar]
- Zhao, CX; Liang, YZ; Fang, HZ; Li, XN. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils. J Chromatogr A 2005, 1096, 76–85. [Google Scholar]
- Pourmortazavi, SM; Hajimirsadeghi, SS. Supercritical fluid extraction in plant essential and volatile oil analysis. J Chromatogr A 2007, 1163, 2–24. [Google Scholar]
- Gumbmann, MR; Williams, SN. Anticholinesterase activity of triethyl phosphate resulting from an impurity. J Agric Food Chem 1970, 18, 76–77. [Google Scholar]
- Reddy, LH; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv Drug Deliv Rev 2009, 61, 1412–1426. [Google Scholar]
- Williams, GM; Iatropoulos, MJ; Whysner, J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol 1999, 37, 1027–1038. [Google Scholar]
- Arfa, AB; Combes, S; Preziosi-Belloy, L; Gontard, N; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett Appl Microbiol 2006, 43, 149–154. [Google Scholar]
- Dayawansa, S; Umeno, K; Takakura, H; Hori, E; Tabuchi, E; Nagashima, Y; Oosu, H; Yada, Y; Suzuki, T; Ono, T; et al. Autonomic responses during inhalation of natural fragrance of Cedrol in humans. Auton Neurosci 2003, 108, 79–86. [Google Scholar]
- Pourmortazavi, SM; Hajimirsadeghi, SS. Supercritical fluid extraction in plant essential and volatile oil analysis. J Chromatogr A 2007, 1163, 2–24. [Google Scholar]
- LaVigne, M; Matthewsb, AK; Grottoli, AG; Cobb, KM; Anagnostou, E; Cabioch, G; Sherrell, RM. Coral skeleton P/Ca proxy for seawater phosphate: Multi-colony calibration with a contemporaneous seawater phosphate record. Geochim Cosmochim Acta 2009, 74, 1282–1293. [Google Scholar]
- Fred, Y. Leung trace elements that act as antioxidants in parenteral micronutrition. J Nutr Biochem 1998, 9, 304–307. [Google Scholar]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med J 2004, 45, 776–788. [Google Scholar]
- Choudhury, RP; Kumar, A; Garg, AN. Analysis of Indian mint (Mentha spicata) for essential trace and toxic elements and its antioxidant behaviour. J Pharm Biomed Anal 2006, 41, 825–832. [Google Scholar]
- Berger, MM; Baines, M; Raffoul, W; Benathan, M; Chiolero, RL; Reeves, C; Revelly, JP; Cayeux, MC; Sénéchaud, I; Shenkin, A. Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. Am J Clin Nutr 2007, 85, 1293–1300. [Google Scholar]
- Koleva, II; van Beek, TA; Linssen, JP; de Groot, A; Evstatieva, LN. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem Anal 2002, 13, 8–17. [Google Scholar]
- Aruoma, OI; Grootveld, M; Bahorun, T. Free radicals in biology and medicine: From inflammation to biotechnology. Biofactors 2006, 27, 1–3. [Google Scholar]
- Kosecik, M; Erel, O; Sevinc, E; Selek, S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol 2005, 100, 61–64. [Google Scholar]
- Harats, D; Ben-Naim, M; Dabach, Y; Hollander, G; Havivi, E; Stein, O; Stein, Y. Effect of vitamin C and E supplementation on susceptibility of plasma lipoproteins to peroxidation induced by acute smoking. Atherosclerosis 1990, 85, 47–54. [Google Scholar]
- van Antwerpen, VL; Theron, AJ; Richards, GA; Steenkamp, KJ; van der Merwe, CA; van der Walt, R; Anderson, R. Vitamin E, pulmonary functions, and phagocyte-mediated oxidative stress in smokers and nonsmokers. Free Radic Biol Med 1995, 18, 935–941. [Google Scholar]
- Banerjee, S; Chattopadhyay, R; Ghosh, A; Koley, H; Panda, K; Roy, S; Chattopadhyay, D; Chatterjee, IB. Cellular and molecular mechanisms of cigarette smoke-induced lung damage and prevention by vitamin C. J Inflamm 2008, 5, 21. [Google Scholar]
- Panda, K; Chattopadhyay, R; Chattopadhyay, DJ; Chatterjee, IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol 2000, 29, 115–124. [Google Scholar]
- Silva Bezerra, F; Valenca, SS; Lanzetti, M; Pimenta, WA; Castro, P; Gonçalves Koatz, VL; Porto, LC. Alpha-tocopherol and ascorbic acid supplementation reduced acute lung inflammatory response by cigarette smoke in mouse. Nutrition 2006, 22, 1192–1201. [Google Scholar]
- Graziano, MJ; Gairola, C; Dorough, HW. Effects of cigarette smoke and dietary vitamin E levels on selected lung and hepatic biotransformation enzymes in mice. Drug Nutr Interact 1985, 3, 213–222. [Google Scholar]
- Uneri, C; Sari, M; Baglam, T; Polat, S; Yüksel, M. Effects of vitamin E on cigarette smoke induced oxidative damage in larynx and lung. Laryngoscope 2006, 116, 97–100. [Google Scholar]
- Banerjee, S; Maity, P; Mukherjee, S; Sil, AK; Panda, K; Chattopadhyay, D; Chatterjee, IB. Black tea prevents cigarette smoke-induced apoptosis and lung damage. J Inflamm 2007, 4, 3. [Google Scholar]
- Lanzetti, M; Bezerra, FS; Romana-Souza, B; Brando-Lima, AC; Koatz, VL; Porto, LC; Valenca, SS. Mate tea reduced acute lung inflammation in mice exposed to cigarette smoke. Nutrition 2008, 24, 375–381. [Google Scholar]
- de Moura, RS; Pires, KMP; Ferreira, TS; Lopes, AA; Nesi, RT; Resende, AC; Sousa, PJC; da Silva, AJR; Porto, LC; Valenca, SS. Addition of açaí (Euterpe oleracea) to cigarettes has a protective effect against emphysema in mice. Food Chem Toxicol 2011, 49, 855–863. [Google Scholar]
- Jiao, GL; Yu, GL; Zhang, JZ; Ewart, HS. Review: Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 2011, 9, 196–223. [Google Scholar]
- Tiwari, OP; Tripathi, YB. Antioxidant properties of different fractions of vitex negundo linn. Food Chem 2007, 100, 1170–1176. [Google Scholar]
- Brown, HW. Organic Chemistry; Saunders College Publishing: New York, NY, USA, 1995; p. 521. [Google Scholar]
- Burton, GW; Ingold, KU. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J Am Chem Soc 1981, 103, 6472–6477. [Google Scholar]
- Kohno, Y; Egawa, Y; Itoh, S; Naganka, S; Takahashi, M; Mukai, K. Kinetik study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim Biophys Acta 1995, 1256, 52–56. [Google Scholar]
- Das, B; Yeger, H; Baruchel, H; Freedman, MH; Koren, G; Baruchel, S. In vitro cytoprotective activity of squalene on a bone marrow versus neuroblastoma model of cisplatin-induced toxicity: Implications in cancer chemotherapy. Eur J Cancer 2003, 39, 2556–2565. [Google Scholar]
- MacNee, W. Oxidants/antioxidants and COPD. Chest 2000, 117, 303S–317S. [Google Scholar]
- Rahman, I; MacNee, W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med 1996, 21, 669–681. [Google Scholar]
- Zang, LY; Stone, K; Pryor, WA. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic Biol Med 1995, 19, 161–167. [Google Scholar]
- Pryor, WA; Church, DF; Evans, MD; Rice, WY, Jr; Hayes, JR. A comparison of the free radical chemistry of tobacco-burning cigarettes and cigarettes that only heat tobacco. Free Radic Biol Med 1990, 8, 275–279. [Google Scholar]
- Smith-Palmer, A; Stewart, J; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol 1998, 26, 118–122. [Google Scholar]
- Drake, DR; Brogden, KA; Dawson, DV; Wertz, PW. Thematic review series: Skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res 2008, 49, 4–11. [Google Scholar]
- Mann, CM; Cox, SD; Markham, JL. The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil). Lett Appl Microbiol 2000, 30, 294–297. [Google Scholar]
- Georgel, P; Crozat, K; Lauth, X; Makrantonaki, E; Seltmann, H; Sovath, S; Hoebe, K; Du, X; Rutschmann, S; Jiang, ZF; et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun 2005, 73, 4512–4521. [Google Scholar]
- Skrivanova, E; Marounek, M; Dlouha, G; Kanka, J. Susceptibility of Clostridium perfringens to C-C fatty acids. Lett Appl Microbiol 2005, 41, 77–81. [Google Scholar]
- Banday, MR; Farshori, NN; Ahmad, A; Khan, AU; Rauf, A. Synthesis and characterization of novel fatty acid analogs of cholesterol: In vitro antimicrobial activity. Eur J Med Chem 2010, 45, 1459–1464. [Google Scholar]
- Huang, CM; Chen, CH; Pornpattananangkul, D; Zhang, L; Chan, M; Hsieh, MF; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar]
- Hashem, FA; Saleh, MM. Antimicrobial components of some cruciferae plants (Diplotaxis harra Forsk. and Erucaria microcarpa Boiss.). Phytother Res 1999, 13, 329–332. [Google Scholar]
- Marino, M; Bersani, C; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from lamiaceae and compositae. Int J Food Microbiol 2001, 67, 187–195. [Google Scholar]
- Wade, JC; Schimpff, SC; Newman, KA; Wiernik, PH. Staphylococcus epidermidis: An increasing cause of infection in patients with granulocytopenia. Ann Intern Med 1982, 97, 503–508. [Google Scholar]
- Kimrua, T; Chang, FD; Pae, S; Hui, H; Ang, PO; Je, JG; Choi, CLS. Status of coral reefs in east and north Asia: China, Hong Kong, Taiwan, Korea and Japan. In Status of Coral Reefs of the World; Wilkinson, C, Ed.; Australian Institute of Marine Science: Townsville, Australia, 2004; p. 278. [Google Scholar]
- Vandendool, H; Kratz, PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr A 1963, 11, 463–471. [Google Scholar]
- Blois, MS. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar]
- Goldstein, S; Meyerstein, D; Czapski, G. The fenton reagents. Free Radic Biol Med 1993, 15, 435–445. [Google Scholar]
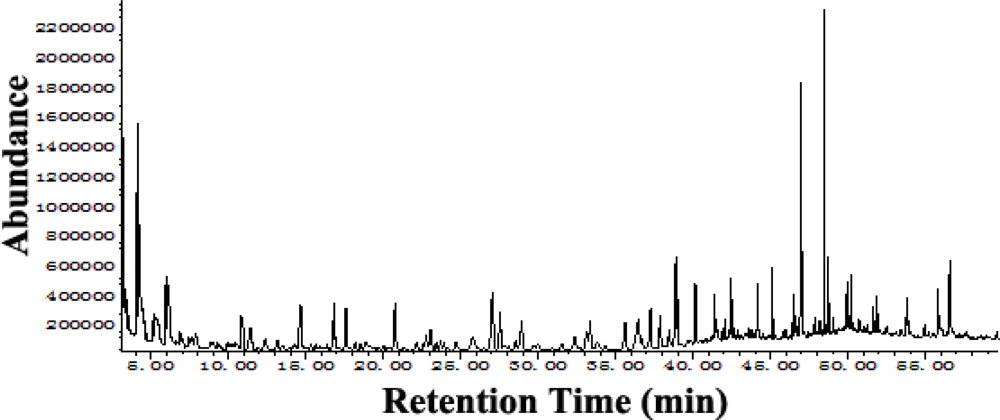

| No. | RT a | Constituents | RI b | % c |
|---|---|---|---|---|
| 1 | 4.014 | Triethyl phosphate | 1121.3 | 3.97 |
| 2 | 9.293 | Thujopsene | 1436.8 | 0.19 |
| 3 | 11.407 | Butylated hydroxytoluene | 1514 | 1.90 |
| 4 | 14.604 | Cedrol | 1605.3 | 1.21 |
| 5 | 22.571 | Tetradecanoic acid | 1762.1 | 0.19 |
| 6 | 28.923 | 1,2-Benzenedicarboxylic acid bis(2-methylpropyl) ester | 1861.5 | 2.24 |
| 7 | 33.340 | Hexadecanoic acid, methyl ester | 1927.0 | 1.65 |
| 8 | 36.452 | n-Hexadecanoic acid | 1973.4 | 3.15 |
| 9 | 41.396 | 11-Octadecenoic acid, methyl ester | 2097.8 | 1.16 |
| 10 | 42.903 | Oleic Acid | 2167.5 | 0.25 |
| 11 | 45.234 | Tricosane | 2298.6 | 0.32 |
| 12 | 46.929 | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl- | 2420.9 | 4.91 |
| 13 | 48.483 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 2550.9 | 5.00 |
| 14 | 51.638 | Squalene | 2830.8 | 0.54 |
| 15 | 55.800 | Cholesterol | 3077.6 | 1.92 |
| Elements determined | Concentration (μg/g) |
|---|---|
| P | 21401.48 ± 2194.83 |
| Ca | 13738.4 ± 476.42 |
| Na | 3916.90 ± 574.11 |
| S | 3691.77 ± 159.52 |
| Mg | 1776.84 ± 193.78 |
| Fe | 781.80 ± 344.78 |
| Cu | 597.60 ± 32.26 |
| Zn | 296.19 ± 37.76 |
| K | 268.25 ± 137.38 |
| Al | 184.47 ± 32.89 |
| Si | 71.96 ± 16.09 |
| B | 42.16 ± 20.34 |
| Ba | 5.81 ± 2.91 |
| Sample | Mean ± SD |
|---|---|
| Blank | 1.247 ± 0.004 |
| VC, 0.01 mM (1.7 mg/mL) | 0.045 ± 0.003 * |
| BCE, 2.5 μL (875 μg/μL) | 0.668 ± 0.016 * |
| BCE, 5 μL (875 μg/μL) | 0.368 ± 0.018 * |
| BCE, 10 μL (875 μg/μL) | 0.171 ± 0.008 * |
| BCE, 20 μL (875 μg/μL) | 0.102 ± 0.007 * |
| BCE, 40 μL (875 μg/μL) | 0.085 ± 0.004 * |
| BCE, 80 μL (875 μg/μL) | 0.074 ± 0.003 * |
| Bacterial strains | BCE (875 μg/μL) | |||
|---|---|---|---|---|
| 20 μL | 40 μL | 80 μL | 160 μL | |
| Staphylococcus aureus | 6.9 ± 0.4 | 10.1 ± 0.6 | 13.8 ± 0.5 | 17.9 ± 0.3 |
| Staphylococcus epidermidis | 7.8 ± 0.3 | 10.0 ± 0.3 | 11.9 ± 0.2 | 14.0 ± 0.3 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bai, X.; Chen, Y.; Chen, W.; Lei, H.; Shi, G. Volatile Constituents, Inorganic Elements and Primary Screening of Bioactivity of Black Coral Cigarette Holders. Mar. Drugs 2011, 9, 863-878. https://doi.org/10.3390/md9050863
Bai X, Chen Y, Chen W, Lei H, Shi G. Volatile Constituents, Inorganic Elements and Primary Screening of Bioactivity of Black Coral Cigarette Holders. Marine Drugs. 2011; 9(5):863-878. https://doi.org/10.3390/md9050863
Chicago/Turabian StyleBai, Xueting, Yicun Chen, Weizhou Chen, Huaping Lei, and Ganggang Shi. 2011. "Volatile Constituents, Inorganic Elements and Primary Screening of Bioactivity of Black Coral Cigarette Holders" Marine Drugs 9, no. 5: 863-878. https://doi.org/10.3390/md9050863
APA StyleBai, X., Chen, Y., Chen, W., Lei, H., & Shi, G. (2011). Volatile Constituents, Inorganic Elements and Primary Screening of Bioactivity of Black Coral Cigarette Holders. Marine Drugs, 9(5), 863-878. https://doi.org/10.3390/md9050863





