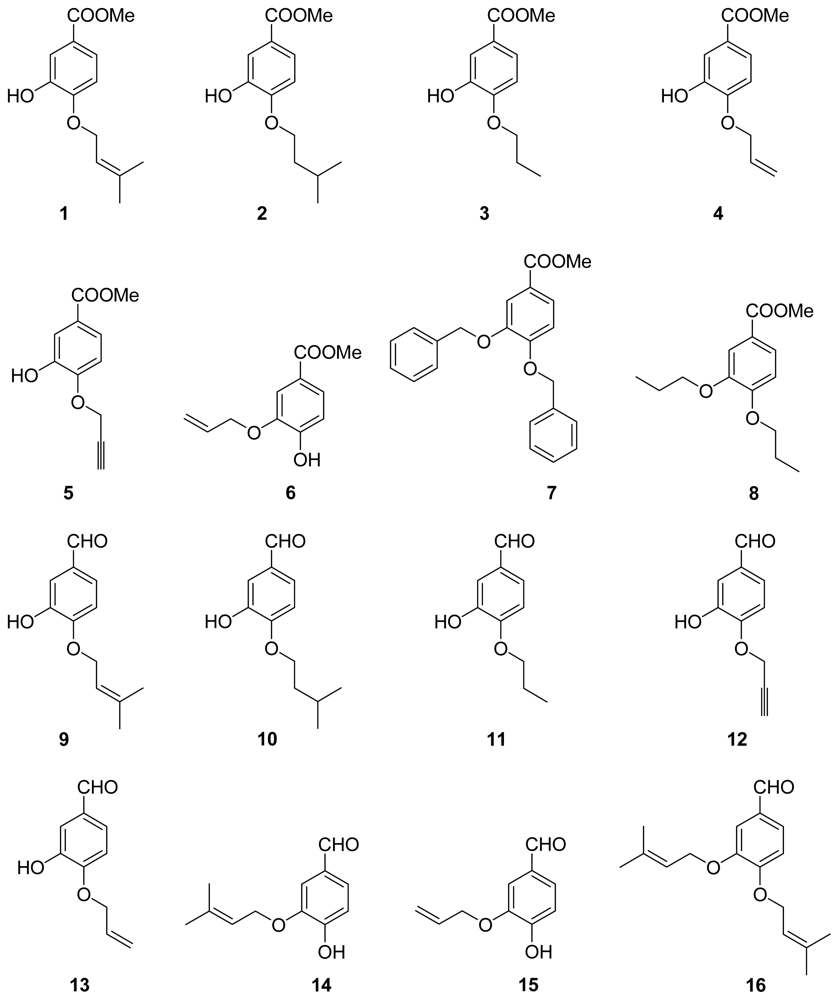
3.2.1. General Procedure 1: Synthesis of Compounds 1–5, 7–13, 16–25
The starting materials were dissolved in acetone and 5 equiv K2CO3 were added. The mixture was stirred for 24 h and then poured into water. The aqueous layer was extracted with ethyl acetate. The combined organic extracts were washed with saturated brine, dried over anhydrous MgSO4 and removed the solvent under reduced pressure. Purification by column-chromatography on silica gel:
methyl 3-hydroxy-4-((3-methylbut-2-en-1-yl)oxy)benzoate (1)
White solid. Mp 82–84 °C; 1H NMR (400 MHz, CDCl3) δ 7.60–7.58 (m, 1H), 7.58 (d, J = 2.9 Hz, 1H), 6.87 (d, J = 9.0 Hz, 1H), 5.69 (s, 1H), 5.47 (t, J = 6.8 Hz, 1H), 4.63 (d, J = 6.8 Hz, 2H), 3.87 (s, 3H), 1.81 (s, 3H), 1.75 (s, 3H). MS: m/z 236 (M+).
methyl 3-hydroxy-4-(isopentyloxy)benzoate (2)
White solid. Mp 68–70 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 1H), 7.58 (d, J = 0.8 Hz, 1H), 6.86 (d, J = 9.0 Hz, 1H), 5.68 (s, 1H), 4.12 (t, J = 6.6 Hz, 2H), 0.97 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 166.97, 149.96, 145.25, 123.36, 122.46, 115.53, 110.38, 67.46, 51.99, 37.94, 24.96, 22.72. MS: m/z 238 (M+); HRMS calcd for C13H18O4: 238.1200 (M+), found: 238.1201.
methyl 3-hydroxy-4-propoxybenzoate (3)
White solid. Mp 66–68 °C; 1H NMR (400 MHz, CDCl3) δ 7.60–7.59 (m, 1H), 7.58–7.57 (m, 1H), 6.84 (d, J = 9.0 Hz, 1H), 5.73 (s, 1H), 4.05 (t, J = 6.6 Hz, 2H), 3.87 (s, 3H), 1.92–1.79 (m, 2H), 1.05 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.91, 149.92, 145.49, 123.30, 122.81, 115.68, 110.70, 70.66, 51.78, 22.55, 10.51. MS: m/z 210 (M+); HRMS calcd for C11H14O4: 210.0887 (M+), found: 210.0886.
methyl 4-(allyloxy)-3-hydroxybenzoate (4)
White solid. Mp 65–67 °C; 1H NMR (400 MHz, CDCl3) δ 7.61–7.59 (m, 1H), 7.58 (d, J = 2.1 Hz, 1H), 6.87 (d, J = 8.3 Hz, 1H), 6.06 (dt, J = 16.0, 5.5 Hz, 1H), 5.67 (s, 1H), 5.38 (dd, J = 26.5, 14.5 Hz, 2H), 4.67 (d, J = 5.5 Hz, 2H), 3.88 (s, 3H). MS: m/z 208 (M+).
methyl 3-hydroxy-4-(prop-2-yn-1-yloxy)benzoate (5)
White solid. Mp 107–109 °C; 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 1H), 7.59 (d, J = 2.1 Hz, 1H), 6.99 (d, J = 8.6 Hz, 1H), 5.78 (s, 1H), 4.81 (s, 2H), 3.87 (s, 3H), 2.58 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 166.79, 148.40, 145.62, 124.49, 122.49, 116.35, 111.73, 57.13, 51.82. MS: m/z 206 (M+); HRMS calcd for C11H10O4: 206.0574 (M+), found: 206.0572.
methyl 3,4-bis(benzyloxy)benzoate (7)
Pale yellow solid. Mp 55–57 °C; 1H NMR (400 MHz, CDCl3) δ 7.67–7.61 (m, 2H), 7.50–7.28 (m, 10H), 6.94 (d, J = 8.3 Hz, 1H), 5.20 (d, J = 10.5 Hz, 4H), 3.87 (s, 3H). MS: m/z 348 (M+).
methyl 3,4-dipropoxybenzoate (8)
White solid. Mp 39–41 °C; 1H NMR (400 MHz, CDCl3) δ 7.63 (dd, J = 8.4, 2.0 Hz, 1H), 7.54 (d, J = 2.0 Hz, 1H), 6.87 (d, J = 8.5 Hz, 1H), 4.01 (td, J = 6.6, 2.1 Hz, 4H), 3.88 (s, 3H), 1.92–1.78 (m, 4H), 1.05 (t, J = 7.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 167.11, 153.42, 148.67, 123.67, 122.63, 114.71, 112.34, 71.01, 70.68, 51.99, 22.72, 22.64, 10.58, 10.54. MS: m/z 252 (M+); HRMS calcd for C14H20O4: 252.1356 (M+), found: 252.1354.
3-hydroxy-4-((3-methylbut-2-en-1-yl)oxy)benzaldehyde (9)
Brown solid. Mp 65–67 °C; 1H NMR (300 MHz, CDCl3) δ 9.82 (s, 1H), 7.42 (s, 2H), 6.97 (s, 1H), 5.80 (s, 1H), 5.48 (s, 1H), 4.67 (s, 2H), 1.80 (d, J = 15.3 Hz, 6H). MS: m/z 206 (M+).
3-hydroxy-4-(isopentyloxy)benzaldehyde (10)
Brown oil. 1H NMR (300 MHz, CDCl3) δ 9.84 (s, 1H), 7.42 (d, J = 10.2 Hz, 2H), 6.96 (d, J = 8.0 Hz, 1H), 5.72 (s, 1H), 4.18 (t, J = 6.4 Hz, 2H), 1.80 (dt, J = 21.9, 6.7 Hz, 3H), 1.02 (s, 3H), 1.00 (s, 3H). MS: m/z 208 (M+).
3-hydroxy-4-propoxybenzaldehyde (11)
White-like solid. Mp 71–73 °C; 1H NMR (300 MHz, CDCl3) δ 9.83 (s, 1H), 7.43 (dd, J = 8.2, 5.0 Hz, 2H), 6.95 (d, J = 8.1 Hz, 1H), 5.75 (s, 1H), 4.12 (t, J = 6.6 Hz, 2H), 1.91 (dd, J = 14.0, 6.6 Hz, 2H), 1.09 (t, J = 7.4 Hz, 3H). MS: m/z 180 (M+).
3-hydroxy-4-(prop-2-yn-1-yloxy)benzaldehyde (12)
Pale white solid. Mp 82–84 °C; 1H NMR (300 MHz, CDCl3) δ 9.85 (s, 1H), 7.45 (dt, J = 8.6, 2.2 Hz, 2H), 7.10 (d, J = 8.2 Hz, 1H), 5.77 (s, 1H), 4.87 (d, J = 2.4 Hz, 2H), 2.63 (t, J = 2.2 Hz, 1H). MS: m/z 176 (M+).
4-(allyloxy)-3-hydroxybenzaldehyde (13)
Pale white solid. Mp 55–57 °C; 1H NMR (400 MHz, CDCl3) δ 9.84 (s, 1H), 7.45 (d, J = 2.0 Hz, 1H), 7.41 (dd, J = 8.3, 2.0 Hz, 1H), 6.97 (d, J = 8.3 Hz, 1H), 6.07 (ddd, J = 22.8, 10.9, 5.5 Hz, 1H), 5.80 (s, 1H), 5.41 (dddd, J = 20.6, 10.5, 2.6, 1.3 Hz, 2H), 4.71 (dt, J = 5.5, 1.4 Hz, 2H). MS: m/z 178 (M+).
3,4-bis((3-methylbut-2-en-1-yl)oxy)benzaldehyde (16)
Colorless oil. 1H NMR (300 MHz, CDCl3) δ 9.82 (s, 1H), 7.49–7.35 (m, 2H), 6.96 (d, J = 8.7 Hz, 1H), 5.56–5.46 (m, 2H), 4.67 (dd, J = 13.0, 6.6 Hz, 4H), 1.80 (s, 6H), 1.77 (s, 6H). MS: m/z 274 (M+).
3,4-bis(prop-2-yn-1-yloxy)benzaldehyde (17)
Pale yellow solid. Mp 100–102 °C; 1H NMR (300 MHz, CDCl3) δ 9.88 (s, 1H), 7.63–7.44 (m, 2H), 7.18 (d, J = 8.2 Hz, 1H), 4.85 (dd, J = 9.3, 2.4 Hz, 4H), 2.63–2.49 (m, 2H). MS: m/z 214 (M+).
3,4-bis(allyloxy)benzaldehyde (18)
Red oil. 1H NMR (300 MHz, CDCl3) δ 9.83 (s, 1H), 7.46–7.39 (m, 2H), 6.98 (d, J = 8.4 Hz, 1H), 6.17–6.00 (m, 2H), 5.46 (d, J = 18.7 Hz, 2H), 5.33 (dd, J = 11.2, 6.9 Hz, 2H), 4.69 (dd, J = 10.1, 5.2 Hz, 4H). MS: m/z 218 (M+);
3,4-bis(isopentyloxy)benzaldehyde (19)
Brown oil. 1H NMR (400 MHz, CDCl3) δ 9.83 (s, 1H), 7.33 (d, J = 53.8 Hz, 2H), 6.95 (d, J = 8.0 Hz, 1H), 4.08 (dd, J = 12.7, 6.5 Hz, 4H), 1.84 (dd, J = 12.9, 6.4 Hz, 2H), 1.73 (dd, J = 13.3, 6.6 Hz, 4H), 0.97 (d, J = 6.5 Hz, 12H). 13C NMR (101 MHz, CDCl3) δ 191.33, 154.59, 149.52, 130.01, 126.79, 111.84, 110.84, 67.64, 37.91, 37.78, 25.33, 22.75. MS: m/z 278 (M+); HRMS calcd for C17H26O3: 278.1876 (M+), found: 278.1873.
3,4-dipropoxybenzaldehyde (20)
Brown oil. 1H NMR (300 MHz, CDCl3) δ 9.82 (s, 1H), 7.45–7.38 (m, 2H), 6.96 (d, J = 7.9 Hz, 1H), 4.05 (dd, J = 14.0, 6.9 Hz, 4H), 1.98–1.81 (m, 4H), 1.07 (dd, J = 11.1, 6.8 Hz, 6H). MS: m/z 222 (M+).
3,4-bis(prop-2-yn-1-yloxy)benzoic acid (21)
White solid. Mp 157–159 °C; 1H NMR (400 MHz, CDCl3) δ 7.83–7.71 (m, 2H), 7.11 (d, J = 8.5 Hz, 1H), 4.83 (dd, J = 9.9, 2.4 Hz, 4H), 2.55 (q, J = 2.4 Hz, 2H). MS: m/z 230 (M+).
3,4-bis(isopentyloxy)benzoic acid (22)
White solid. Yield, %. Mp 137–139 °C; 1H NMR (300 MHz, CDCl3) δ 7.72 (d, J = 8.4 Hz, 1H), 7.59 (s, 1H), 6.90 (d, J = 8.5 Hz, 1H), 4.10 (q, J = 6.3 Hz, 4H), 1.88 (dt, J = 13.5, 6.7 Hz, 2H), 1.81–1.69 (m, 4H), 1.00 (d, J = 6.5 Hz, 12H). MS: m/z 294 (M+).
3,4-dipropoxybenzoic acid (23)
White solid. Mp 133–135 °C; 1H NMR (300 MHz, CDCl3) δ 7.71 (d, J = 9.9 Hz, 1H), 7.58 (s, 1H), 6.90 (d, J = 8.4 Hz, 1H), 4.04 (dd, J = 11.2, 6.6 Hz, 4H), 1.96–1.81 (m, 4H), 1.08 (t, J = 7.4 Hz, 6H). MS: m/z 238 (M+).
(E)-methyl 3-(3-hydroxy-4-(prop-2-yn-1-yloxy)phenyl)acrylate (24)
White solid. Mp 103–105 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 15.9 Hz, 1H), 7.15 (d, J = 2.1 Hz, 1H), 7.03 (dd, J = 8.5, 1.9 Hz, 1H), 6.97 (d, J = 8.4 Hz, 1H), 6.30 (d, J = 15.9 Hz, 1H), 5.67 (s, 1H), 4.79 (d, J = 2.4 Hz, 2H), 3.79 (s, 3H), 2.58 (t, J = 2.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 167.75, 146.53, 146.35, 144.56, 129.26, 123.99, 121.59, 116.61, 113.88, 112.59, 111.70, 57.03, 51.78. MS: m/z 232 (M+); HRMS calcd for C13H12O4: 232.0730 (M+), found: 232.0732.
(E)-methyl 3-(3,4-bis(prop-2-yn-1-yloxy)phenyl)acrylate (25)
White solid. Mp 96–98 °C; 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 15.9 Hz, 1H), 7.25–7.11 (m, 2H), 7.04 (d, J = 8.4 Hz, 1H), 6.31 (d, J = 15.9 Hz, 1H), 4.77 (dd, J = 2.4, 1.2 Hz, 4H), 3.79 (s, 3H), 2.57–2.48 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 167.59, 149.54, 147.63, 144.37, 128.62, 123.25, 116.47, 114.45, 113.93, 78.23, 78.11, 76.44, 76.40, 57.10, 56.85, 51.74. MS: m/z 270 (M+); HRMS calcd for C16H14O4: 270.0887 (M+), found: 270.0888.
3.2.2. General Procedure 2: Synthesis of Compounds 6, 14–15
A mixture of di-substituted 3,4-dihydroxybenzoic acid methyl ester or 3,4-dihydroxybenzaldehyde with 10% Pd/C in 10% K2CO3–MeOH was stirred at room temperature for an appropriate time. After the catalyst was filtered, the filtrate was concentrated in vacuo. The mixture was extracted with AcoEt. The organic extracts were washed with brine and dried. The solvent was evaporated under reduced pressure to give a residue, which was purified by column-chromatography on silica gel.
methyl 3-(allyloxy)-4-hydroxybenzoate (6)
White solid. Mp 46–48 °C; 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 10.3 Hz, 1H), 7.56 (s, 1H), 6.96 (d, J = 3.9 Hz, 1H), 6.15–6.04 (m, 1H), 6.03 (s, 1H), 5.40 (dd, J = 26.7, 13.7 Hz, 2H), 4.68 (d, J = 5.5 Hz, 2H), 3.89 (s, 3H). MS: m/z 208 (M+).
4-hydroxy-3-((3-methylbut-2-en-1-yl)oxy)benzaldehyde (14)
Brown oil. 1H NMR (400 MHz, CDCl3) δ 9.79 (s, 1H), 7.41 (s, 1H), 7.38 (s, 1H), 7.02 (d, J = 7.9 Hz, 1H), 6.53 (s, 1H), 5.46 (t, J = 6.3 Hz, 1H), 4.62 (d, J = 6.8 Hz, 2H), 1.78 (s, 3H), 1.74 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 191.02, 151.79, 146.44, 139.68, 129.52, 127.56, 118.24, 114.29, 109.75, 65.77, 25.38, 18.30. MS: m/z 206 (M+); HRMS calcd for C12H14O3: 206.09387 (M+), found: 206.0938.
3-(allyloxy)-4-hydroxybenzaldehyde (15)
Pale white solid. Mp 113–115 °C; 1H NMR (300 MHz, CDCl3) δ 9.80 (s, 1H), 7.42 (s, 2H), 7.06 (d, J = 14.5 Hz, 1H), 6.22 (s, 1H), 6.18–5.98 (m, 1H), 5.41 (dd, J = 21.1, 14.9 Hz, 2H), 4.70 (d, J = 9.2 Hz, 2H). MS: m/z 178 (M+).
(E)-methyl 3-(3,4-dihydroxyphenyl)acrylate (26)
Pale white solid. Mp 164–166 °C; 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 22.4 Hz, 1H), 7.08 (d, J = 2.0 Hz, 1H), 7.01 (dd, J = 8.2, 2.0 Hz, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.27 (d, J = 15.9 Hz, 1H), 5.58 (s, 2H), 3.79 (s, 3H). MS: m/z 194 (M+).
methyl 3-(isopentyloxy)-4-methoxybenzoate (27)
Pale yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.63 (dd, J = 8.4, 2.0 Hz, 1H), 7.53 (d, J = 2.0 Hz, 1H), 6.85 (d, J = 8.5 Hz, 1H), 4.06 (t, J = 6.9 Hz, 2H), 3.88 (s, 3H), 3.86 (s, 3H), 1.83 (dt, J = 13.0, 6.5 Hz, 1H), 1.74 (q, J = 6.9 Hz, 2H), 0.96 (d, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 167.02, 153.41, 148.21, 123.32, 122.69, 113.59, 110.49, 67.51, 56.02, 51.98, 37.96, 25.14, 22.67. MS: m/z 252 (M+); HRMS calcd for C14H20O4: 252.1356 (M+), found: 252.1354.
3-methylbut-2-en-1-yl 4-methoxy-3-((3-methylbut-2-en-1-yl)oxy)benzoate (28)
Colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 42.4 Hz, 2H), 6.83 (s, 1H), 5.45 (s, 2H), 4.68 (d, J = 61.2 Hz, 4H), 3.88 (s, 3H), 1.75 (s, 12H). 13C NMR (101 MHz, CDCl3) δ 166.61, 153.33, 147.82, 138.86, 138.22, 123.53, 122.87, 119.56, 118.96, 115.70, 113.68, 110.27, 65.97, 61.78, 55.73, 25.90, 18.27, 18.14. MS: m/z 304 (M+); HRMS calcd for C18H24O4: 304.1669 (M+), found: 304.1667.
3-methylbut-2-en-1-yl 3-hydroxy-4-methoxybenzoate (29)
White solid. Mp 154–156 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 6.0 Hz, 2H), 6.82 (d, J = 9.0 Hz, 1H), 5.96 (s, 1H), 5.43 (t, J = 7.8 Hz, 1H), 4.76 (d, J = 7.1 Hz, 2H), 3.88 (s, 3H), 1.74 (d, J = 8.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 166.37, 150.57, 145.25, 138.83, 123.68, 122.64, 118.75, 115.78, 109.93, 61.78, 55.91, 25.58, 18.08. MS: m/z 236 (M+); HRMS calcd for C13H16O4: 236.1043 (M+), found: 236.1042.
3.2.3. Synthesis of 3,4-bis(prop-2-yn-1-yloxy)benzamide (30)
The mixture of 3,4-dihydroxybenzoic acid methyl ester and propargyl bromide were dissolved in acetone and 5 equiv K2CO3 were added. Through general procedure 1, di-substituted compound was obtained. Then it was dissolved in MeOH, 5 equiv of the amine were added and the mixture was stirred at 40 °C for 24 h. The mixture were poured into water and extracted with AcoEt. Removing the water of the aqueous layer and the target compound was obtained as white solid. Mp 145–147 °C; 1H NMR (400 MHz, DMSO) δ 7.83 (s, 1H), 7.60–7.49 (m, 2H), 7.22 (s, 1H), 7.10 (d, J = 8.5 Hz, 1H), 4.85 (dd, J = 14.6, 2.4 Hz, 4H), 3.57 (dt, J = 7.6, 2.4 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 167.14, 149.27, 146.16, 127.43, 121.25, 113.57, 113.04, 79.03, 78.88, 78.56, 78.44, 56.09, 56.00. MS: m/z 230 (M+ + H); HRMS calcd for C13H11NO3: 229.0733 (M+), found: 229.0732.
methyl 3-hydroxy-4-methoxybenzoate (31)
White solid. Mp 65–67 °C; 1H NMR (400 MHz, CDCl3) δ 7.64–7.57 (m, 2H), 6.87 (d, J = 8.4 Hz, 1H), 5.62 (s, 1H), 3.95 (s, 3H), 3.88 (s, 3H). MS: m/z 182 (M+).
(E)-methyl 3-(4-hydroxy-3-((3-methylbut-2-en-1-yl)oxy)phenyl)acrylate (32)
Yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 15.8 Hz, 1H), 7.06 (d, J = 9.6 Hz, 2H), 6.91 (d, J = 8.0 Hz, 1H), 6.27 (d, J = 15.9 Hz, 1H), 5.96 (s, 1H), 5.48 (s, 1H), 4.61 (s, 2H), 3.79 (s, 3H), 1.79 (d, J = 19.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 167.90, 148.51, 146.09, 145.22, 139.49, 126.93, 123.01, 118.85, 115.21, 114.86, 110.84, 66.03, 51.71, 25.85, 18.39. MS: m/z 262 (M+); HRMS calcd for C15H18O4: 262.1200 (M+), found: 262.1199.