Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms
Abstract
:1. Introduction
2. Algae
2.1. Macroalgae
2.2. Microalgae
3. Other Marine Sources
3.1. Fungi
3.2. Lichens
3.3. Bacteria
3.4. Cyanobacteria
3.5. Marine Animals
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Agar, N; Halliday, G; Barnetson, R; Ananthaswamy, H; Wheeler, M; Jones, A. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc Natl Acad Sci USA 2004, 101, 4954–4959. [Google Scholar]
- Ryu, B; Qian, ZJ; Kim, MM; Nam, KW; Kim, SK. Anti-photoaging activity and inhibition of matrixmetalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat Phys Chem 2009, 78, 98–105. [Google Scholar]
- Kondo, S. The roles of cytokines in photoaging. J Dermatol Sci 2000, 23, 30–36. [Google Scholar]
- Rittie, L; Fisher, GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev 2002, 1, 705–720. [Google Scholar]
- Kehrer, JP. Free radicals as mediators of tissue injury and disease. CRC Crit Rev Toxicol 1993, 23, 21–48. [Google Scholar]
- Aruoma, OI. Nutrition and health aspects of free radicals and antioxidant. Food Chem Toxicol 1994, 62, 671–683. [Google Scholar]
- Fiers, W; Bevaert, R; Declercq, W; Vandenabeele, P. More than one way to die: Apoptosis and necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar]
- Record, IR; Dreosti, IE; Konstantinopoulos, M; Buckley, RA. The influence of topical and systemic vitamin E on ultraviolet light-induced skin damage in hairless mice. Nutr Cancer 1991, 16, 219–226. [Google Scholar]
- Kawaguchi, Y; Tanaka, H; Okada, T; Konishi, H; Takahashi, M; Ito, M; Asai, J. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch Dermatol Res 1996, 288, 39–44. [Google Scholar]
- Bode, W; Fernandez-Catalan, C; Tschesche, H; Grams, F; Nagase, H; Maskos, K. Structural properties of matrix metalloproteinases. Cell Mol Life Sci 1999, 55, 639–652. [Google Scholar]
- Kang, S; Chung, JH; Lee, JH; Fisher, GJ; Wan, YS; Duell, EA; Voorhees, JJ. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol 2003, 120, 835–841. [Google Scholar]
- Inomata, S; Matsunaga, Y; Amano, S; Takada, K; Kobayashi, K; Tsunenaga, M; Nishiyama, T; Kohno, Y; Fukuda, M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol 2003, 120, 128–134. [Google Scholar]
- Kim, MM; Kim, SK. Chitooligosaccharides inhibit activation and expression of matrix metalloproteinase-2 in human dermal fibroblasts. FEBS Lett 2006, 580, 2661–2666. [Google Scholar]
- Kim, MM; van Ta, Q; Mendis, E; Rajapakse, N; Jung, WK; Byun, HG; Jeon, YJ; Kim, SK. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci 2006, 79, 1436–1443. [Google Scholar]
- Van Ta, Q; Kim, MM; Kim, SK. Inhibitory effect of chitooligosaccharides on matrix metalloproteinase-9 in human fibrosarcoma cells (HT1080). Mar. Biotechnol 2006, 8, 593–599. [Google Scholar]
- Rajapakse, N; Kim, MM; Mendis, E; Huang, RH; Kim, SK. Carboxylated chitooligosaccharides (CCOS) inhibit MMP-9 expression in human fibrosarcoma cells via down-regulation of AP-1. Biochim Biophys Acta 2006, 1760, 1780–1788. [Google Scholar]
- Rajapakse, N; Mendis, E; Kim, MM; Kim, SK. Sulfated glucosamine inhibits MMP-2 and MMP-9 expressions in human fibrosarcoma cells. Bioorg Med Chem 2007, 15, 4891–4896. [Google Scholar]
- Kong, CS; Kim, YA; Kim, MM; Park, JS; Kim, JA; Kim, SK; Lee, BJ; Nam, TJ; Seo, YW. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol In Vitro 2008, 22, 1742–1748. [Google Scholar]
- Zhang, C; Kim, SK. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: The current situation and future prospects. Mar Drugs 2009, 7, 71–84. [Google Scholar]
- Sinha, RP; Klisch, M; Gröniger, A; Häder, DP. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B Biol 1998, 47, 83–94. [Google Scholar]
- Sinha, RP; Singh, SP; Häder, DP. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B Biol 2007, 89, 29–35. [Google Scholar]
- Groniger, A; Sinha, RP; Klisch, M; Häder, DP. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—a database. J Photochem Photobiol B Biol 2000, 58, 115–122. [Google Scholar]
- Alapetite, C; Wachter, T; Sage, E; Moustacchi, E. Use of the alkaline comet assay to detect DNA repair deficiencies in human fibrobolasts exposed to UVC, UVB, UVA and gamma-rays. Int J Radiat Biol 1996, 69, 359–369. [Google Scholar]
- Marrot, L; Belaidi, JP; Chaubo, C; Meunier, JR; Perez, P; Agapakis-causse, C. An in vitro strategy to evaluate the phototoxicity of solar UV at the molecular and cellular level: Application to photoprotection assessment. Eur J Dermatol 1998, 8, 403–412. [Google Scholar]
- de Leeuw, SM; Smit, NPM; van Veldhoven, MV; Pennings, EdM; Pavel, S; Simons, WIMJ; Schothorst, AA. Melanin content of cultured human melanocytes and UV-induced cytotoxicity. J Photochem Photobiol B Biol 2001, 61, 106–113. [Google Scholar]
- Dawson, TL. Light-harvesting and light-protecting pigments in simple life forms. Coloration Technol 2007, 123, 129–142. [Google Scholar]
- Edwards, HGM; Moeller, R; Villar, SEJ; Horneck, G; Stackebrandt, E. Raman spectroscopic study of the photoprotection of extremophilic microbes against ultraviolet radiation. Int J Astrobiol 2006, 5, 313–318. [Google Scholar]
- Kumar, R; Patel, DD; Bansal, DD; Mishra, S; Mohammed, S; Arora, R; Sharma, A; Sharma, RK; Tripathi, RP. Singh, OV, Harvey, SP, Eds.; Extremophiles: Sustainable resource of natural compounds-extremolytes. In Sustainable Biotechnology, Sources of Renewable Energy; Springer: Dordrecht, Netherlands, 2009; pp. 279–294. [Google Scholar]
- Cahyana, AH; Shuto, Y; Kinoshita, Y. Pyropheophytin A as an antioxidative substance from the marine alga, Arame (Eicenia bicyclis). Biosci Biotechnol Agrochem 1992, 56, 1533–1535. [Google Scholar]
- Yan, X; Chuda, Y; Suzuki, M; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci Biotechnol Biochem 1999, 63, 605–607. [Google Scholar]
- Kang, HS; Chung, HY; Jung, HA; Son, BW; Choi, JS. A new phlorotannins from the brown alga Ecklonia stolonifera. Chem Pharm Bull 2003, 51, 1012–1014. [Google Scholar]
- Torres, MA; Barros, MP; Campos, SC; Pinto, E; Rajamani, S; Sayre, RT; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol Environ Saf 2008, 71, 1–15. [Google Scholar]
- Sabry, OMN; Andrews, S; McPhail, KL; Geoger, DE; Yokochi, A; LePag, KT; Murray, TF; Gerwick, WH. Neurotoxic meoditerpenoids from the tropical marine brown alga Stypopodium flabelliorme. J Nat Prod 2005, 68, 1022–1030. [Google Scholar]
- Heo, SJ; Park, EJ; Lee, KW; Jeon, YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Biores Technol 2005, 96, 1613–1623. [Google Scholar]
- Heo, SJ; Jeon, YJ. Radical scavenging capacity and cytoprotective effect of enzymatic digests of Ishige okamurae. J App Phycol 2008, 20, 1087–1095. [Google Scholar]
- Heo, SJ; Ko, SC; Cha, SH; Kang, DH; Park, HS; Choi, YU; Kim, D; Jung, WK; Jeon, YJ. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol In Vitro 2009, 23, 1123–1130. [Google Scholar]
- Hur, S; Lee, H; Kim, Y; Lee, BH; Shin, J; Kim, TY. Sargaquinoic acid and sargachromenol, extracts of Sargassum sagamianum, induce apoptosis in HaCaT cells and mice skin: Its potentiation of UVB-induced apoptosis. Eur J Pharmacol 2008, 582, 1–11. [Google Scholar]
- Tsang, CK; Ina, A; Goto, T; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar]
- de la Coba, F; Aguilera, J; de Gálvez, MV; Álvarez, M; Gallego, E; Figueroa, FL; Herrera, E. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J Dermatol Sci 2009, 55, 161–169. [Google Scholar]
- Lyons, NM; O’Brien, NM. Modulatory effects of an algal extract containing astaxanthin on A-irradiated cells in culture. J Dermatol Sci 2002, 30, 73–84. [Google Scholar]
- Heo, SJ; Jeon, YJ. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J Photochem Photobiol B Biol 2009, 95, 101–107. [Google Scholar]
- Fisher, GJ; Datta, SC; Talwar, HS; Wang, ZQ; Varani, J; Kang, S; Voorhees, JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar]
- Brenneisen, P; Oh, J; Wlaschek, M; Wenk, J; Briviba, K; Hommel, C; Herrmann, G; Sies, H; Scharffetter-Kochanek, K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem Photobiol 1996, 64, 877–85. [Google Scholar]
- Jeffrey, SW; MacTavish, HS; Dunlap, WC; Vesk, M; Groenewoud, K. Occurrence of UVA- and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar Ecol Prog Ser 1999, 189, 35–51. [Google Scholar]
- Bradbury, J. Nature’s Nanotechnologists: Unveiling the secrets of diatoms. Public Lib Sci Biol 2004, 2, 1512–1515. [Google Scholar]
- Hasle, G; Syvertsen, EE. Tomas, CR, Ed.; Marine diatoms. In Identifying Marine Diatoms and Dinoflagellates; Academic Press: San Diego, CA, USA, 1996; pp. 5–385. [Google Scholar]
- Zudaire, L; Roy, S. Photoprotection and long-term acclimation to UV radiation in the marine diatom Thalassiosira weissflogii. J Photochem Photobiol B Biol 2001, 62, 26–34. [Google Scholar]
- Helbling, EW; Chalker, BE; Dunlap, WC; Holm-Hansen, O; Villafañe, VE. Photoacclimation of Antarctic marine diatoms to solar ultraviolet radiation. J Exp Mar Biol Ecol 1996, 204, 85–101. [Google Scholar]
- El-Sayed, SZ; Stephens, FC; Bidigare, RR; Ondrusek, ME. Kerry, KR, Hempel, G, Eds.; Effect of ultraviolet radiation on Antarctic marine phytoplankton. In 5th SCAR Symposium on the Ecological Changes and the Conservation of Antarctic Ecosystems; Springer-Verlag: Berlin, Germany, 1990; pp. 379–385. [Google Scholar]
- Karentz, D. Ultraviolet tolerance mechanisms in Antarctic marine organisms, in Ultraviolet radiation in Antarctica: Measurements and biological effects. Antarctic Res Ser 1994, 62, 93–110. [Google Scholar]
- Hannach, G; Sigleo, AC. Photoinduction of UV-absorbing compounds in six species of marine phytoplankton. Mar Ecol Prog Ser 1998, 174, 207–222. [Google Scholar]
- Torres, A; Hochberg, M; Pergament, I; Smoum, R; Niddam, V; Dembitsky, VM; Temina, M; Dor, I; Lev, O; Srebnik, M; Enk, CD. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur J Biochem 2004, 271, 780–784. [Google Scholar]
- Kogej, T; Gostincar, C; Volkmann, M; Gorbushina, AA; Gunde-Cimerman, N. Mycosporines in extremophilic fungi—novel complementary osmolytes. Environ Chem 2006, 3, 105–110. [Google Scholar]
- Muller, K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 2001, 56, 9–16. [Google Scholar]
- Huneck, S. New results on the chemistry of lichen substances. Fortschritte der Chemie Organischer Naturstoffe 2001, 81, 1–276. [Google Scholar]
- Margolin, KA. Biochemotherapy for melanoma: rational therapeutics in the search for weapons of melanoma destruction. Cancer 2004, 101, 435–438. [Google Scholar]
- Sondak, VK; Sabel, MS; Mule, JJ. Allogeneic and autologous melanoma vaccines: Where have we been and where are we going. Clin Canc Res 2006, 12, 2337s–234s. [Google Scholar]
- Russo, PAJ; Halliday, GM. Inhibition of nitric oxide and reactive oxygen species production improves the ability of a sunscreen to protect from sunburn, immunosuppression and photocarcinogenesis. Brit J Dermatol 2006, 155, 408–415. [Google Scholar]
- Russo, A; Piovano, M; Lombardo, L; Garbarino, J; Cardile, V. Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci 2008, 83, 468–474. [Google Scholar]
- Rancan, F; Rosan, S; Boehm, K; Fernandez, E; Hidalgo, ME; Quilhot, W; Rubio, C; Boehm, F; Piazena, H; Oltmanns, U. Protection against UVB irradiation by natural filters extracted from lichens. J Photochem Photobiol B Biol 2002, 68, 133–139. [Google Scholar]
- Geng, J; Yu, SB; Wan, X; Wang, XJ; Shen, P; Zhou, P; Chen, XD. Protective action of bacterial melanin against DNA damage in full UV spectrums by a sensitive plasmid-based noncellular system. J Biochem Biophys Meth 2008, 70, 1151–1155. [Google Scholar]
- Wan, X; Liu, HM; Liao, Y. Isolation of a novel strain of Aeromonas media producing high levels of DOPA-melanin and assessment of the photoprotective role of the melanin in bioinsecticide applications. J Appl Microbiol 2007, 103, 2533–2541. [Google Scholar]
- Holmes, JD; Smith, PR; Richard, EG; Richardson, DJ; Russell, DA; Sodeau, JR. Bacterial photoprotection through extracellular cadmium sulfide crystallites. Photochem Photobiol 2008, 62, 1022–1026. [Google Scholar]
- Stewart, WDP. Some aspects of structure and function in N2-fixing cyanobacteria. Annu Rev Microbiol 1980, 34, 497–536. [Google Scholar]
- de Marsac, NT; Houmard, J. Adaptation of cyanobacteria to environmental stimuli: New steps towards molecular mechanisms. FEMS Microbiol Rev 1993, 104, 119–190. [Google Scholar]
- Haider, DP. Phototensory behavior in prokaryotes. Microbiol Rev 1987, 51, 1–21. [Google Scholar]
- Bedout, BM; Garcia-Pichel, F. UV-B induced vertical migrations of cyanobacteria in a microbial mat. Appl Environ Microbiol 1995, 61, 4215–4222. [Google Scholar]
- Burton, GW; Ingold, KU. β-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar]
- Vincent, WF; Quesada, A. Weiler, CS, Penhale, PA, Eds.; Ultraviolet radiation effects on cyanobacteria: Implication for Antarctic microbial ecosystems. In Ultraviolet radiation in antarctica: Measurements and biological effects; Antarctic Research Series, American Geophysical Union: Washington, DC, USA, 1994; Volume 62, pp. 111–124. [Google Scholar]
- Kim, ST; Sancar, A. Photorepair of nonadjacent pyrimidine dimmers by DNA photolyase. Photochem Photobiol 1995, 61, 171–174. [Google Scholar]
- Christopher, DA; Mullet, JE. Separate photosensory pathways coregulate blue light/ultraviolet-A-activated psbD-psbC transcription and light induced D2 and CP43 degradation in barley (Hordeum vulgare) chloroplasts. Plant Physiol 1994, 104, 1119–1129. [Google Scholar]
- de Marsac, NT. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol 1977, 130, 82–91. [Google Scholar]
- Garcia-Pichel, F; Wingard, CE; Castenholz, RW. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl Environ Microbiol 1993, 59, 170–176. [Google Scholar]
- Brenowitz, S; Castenholz, RW. Long-term effects of UV and visible irradiance on natural populations of a scytonemincontaining cyanobacterium (Calothrix sp). FEMS Microbiol Ecol 1997, 24, 343–352. [Google Scholar]
- Chen, HY; Chu, X; Yan, CL; Chen, XH; Sun, M; Wang, YJ; Wang, CB; Yu, WG. Polypeptide from Chlamys farreri attenuates murine thymocytes damage induced by ultraviolet B. Acta Pharmacol Sin 2007, 28, 1665–1670. [Google Scholar]
- Li, JL; Liu, N; Chen, XH; Sun, M; Wang, CB. Inhibition of UVA-induced apoptotic signaling pathway by polypeptide from Chlamys farreri in human HaCaT keratinocytes. Radiat Environ Biophys 2007, 46, 263–268. [Google Scholar]
- Xing, YX; Li, P; Miao, YX; Du, W; Wang, CB. Involvement of ROS/ASMase/JNK signalling pathway in inhibiting UVA-induced apoptosis of HaCaT cells by polypeptide from Chlamys farreri. Free Radic Res 2008, 42, 12–19. [Google Scholar]
- Bandaranayake, WM; des Rocher, A. Role of secondary metabolites and pigments in the epidermal tissues, ripe ovaries, viscera, gut contents and diet of the sea cucumber (Holothuria atra). Mar Biol 1999, 133, 163–169. [Google Scholar]
- Oyamada, C; Kaneniwa, M; Ebitani, K; Murata, M; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar Biotechnol 2008, 10, 141–150. [Google Scholar]
- Carefoot, TH; Harris, M; Taylor, BE. Mycosporine-like amino acids: Possible UV protection in eggs of the sea hare (Aplysia dactylomela). Mar Biol 1998, 130, 389–396. [Google Scholar]
| No. | Compound | Structure |
|---|---|---|
| 1. | Eckol | 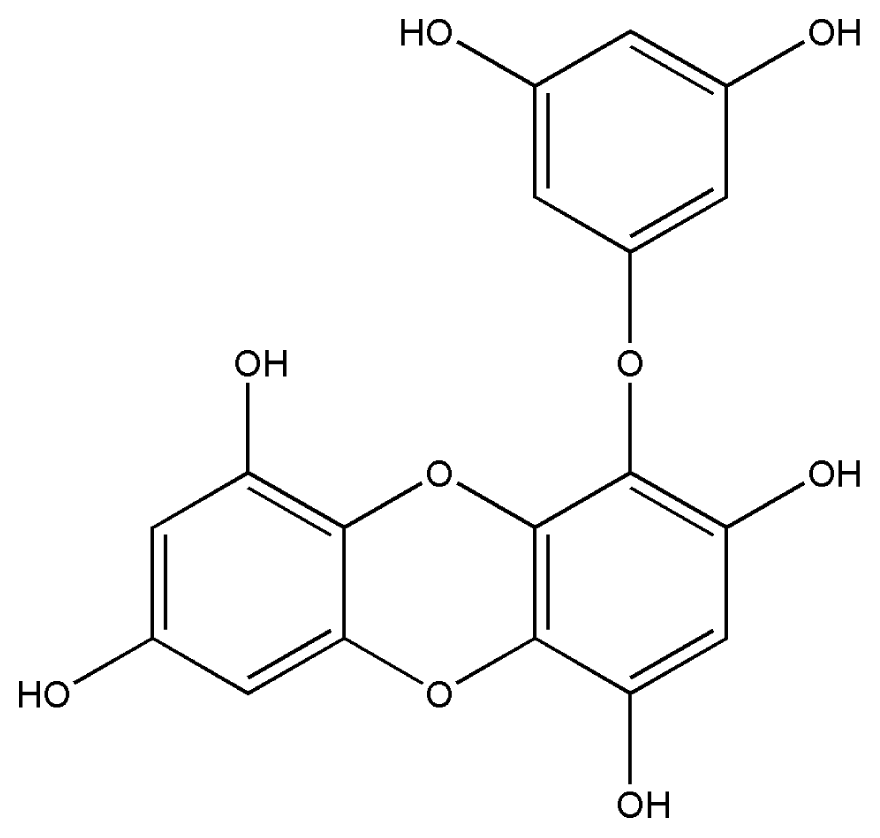 |
| 2. | Mycosporine methylamine-serine | 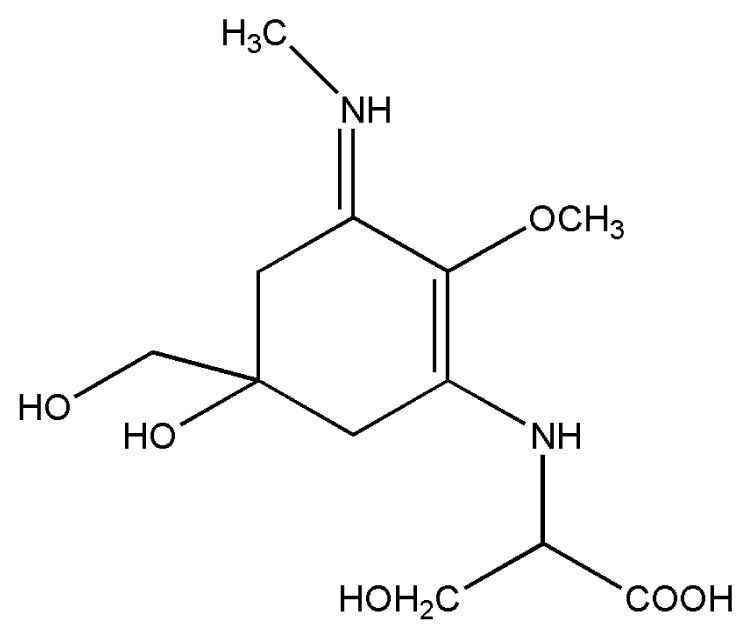 |
| 3. | Mycosporine-glycine |  |
| 4. | Palythene | 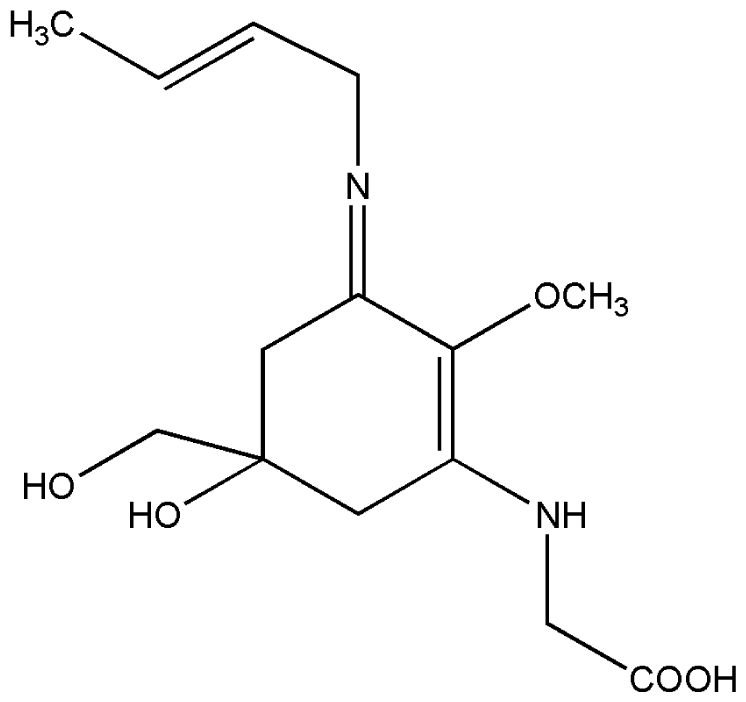 |
| 5. | Shinorine |  |
| 6. | Porphyra-334 | 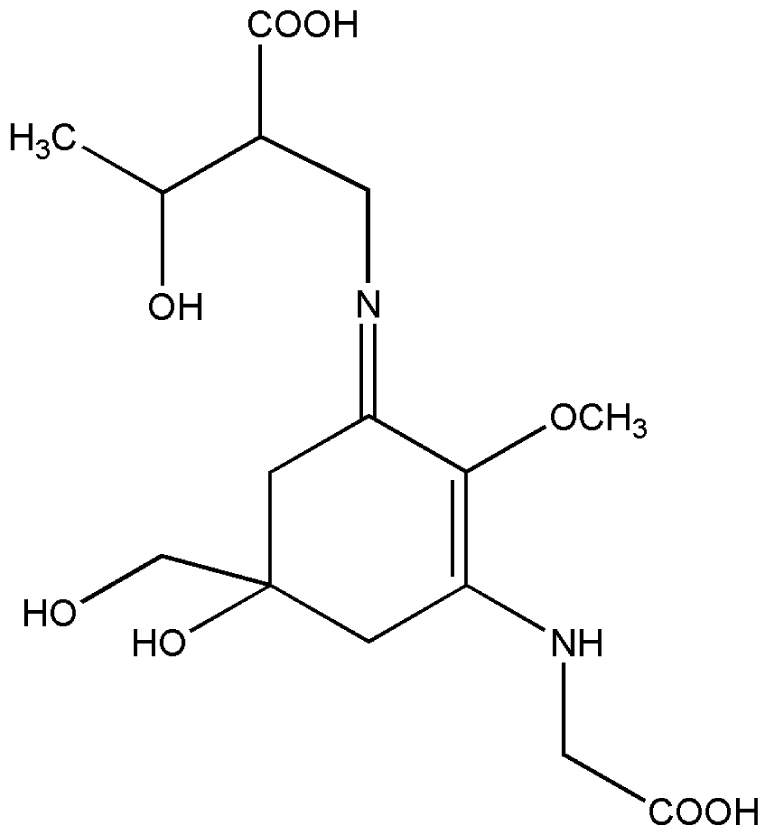 |
| 7. | Scytonemin | 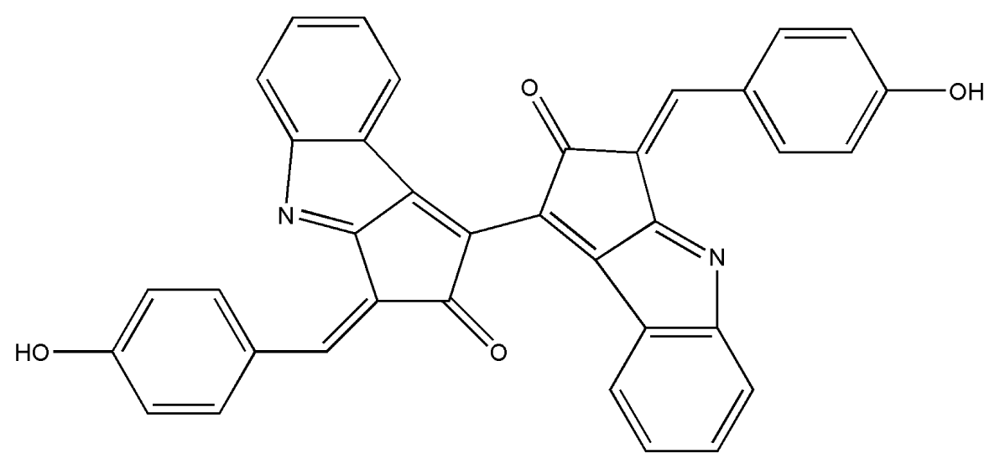 |
| 8. | Sargaquinoic acid | 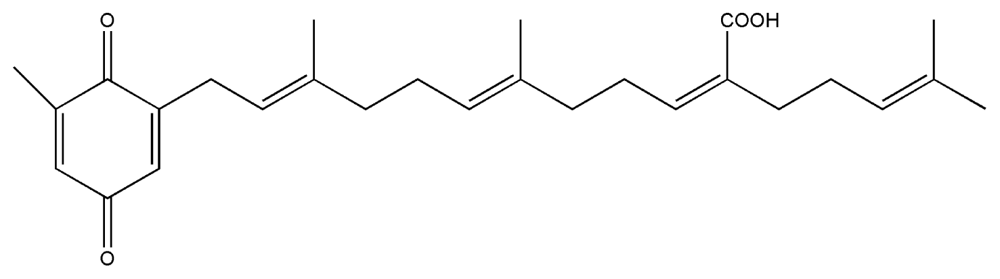 |
| 9. | Sargachromenol | 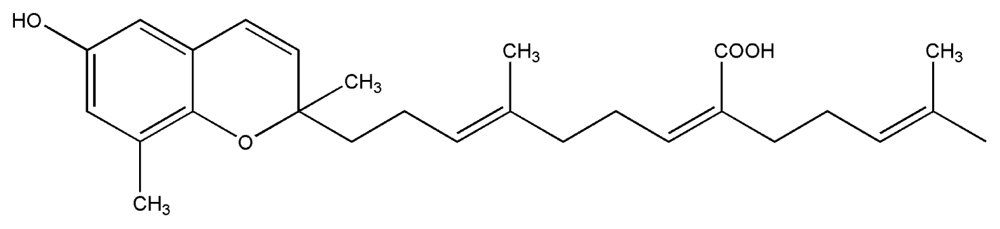 |
| 10. | Fucoxanthin | 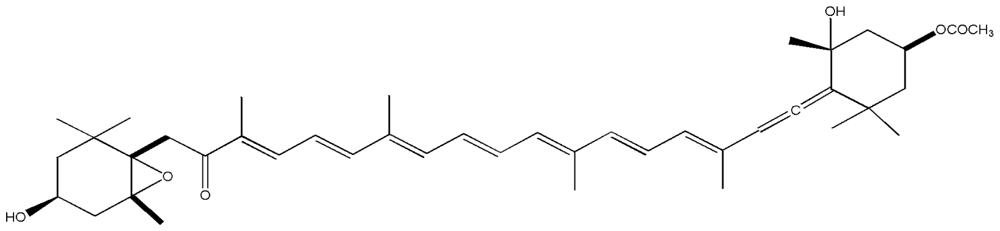 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pallela, R.; Na-Young, Y.; Kim, S.-K. Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms. Mar. Drugs 2010, 8, 1189-1202. https://doi.org/10.3390/md8041189
Pallela R, Na-Young Y, Kim S-K. Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms. Marine Drugs. 2010; 8(4):1189-1202. https://doi.org/10.3390/md8041189
Chicago/Turabian StylePallela, Ramjee, Yoon Na-Young, and Se-Kwon Kim. 2010. "Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms" Marine Drugs 8, no. 4: 1189-1202. https://doi.org/10.3390/md8041189
APA StylePallela, R., Na-Young, Y., & Kim, S.-K. (2010). Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms. Marine Drugs, 8(4), 1189-1202. https://doi.org/10.3390/md8041189





