Isolation of Araguspongine M, a New Stereoisomer of an Araguspongine/Xestospongin alkaloid, and Dopamine from the Marine Sponge Neopetrosia exigua Collected in Palau
Abstract
:Introduction
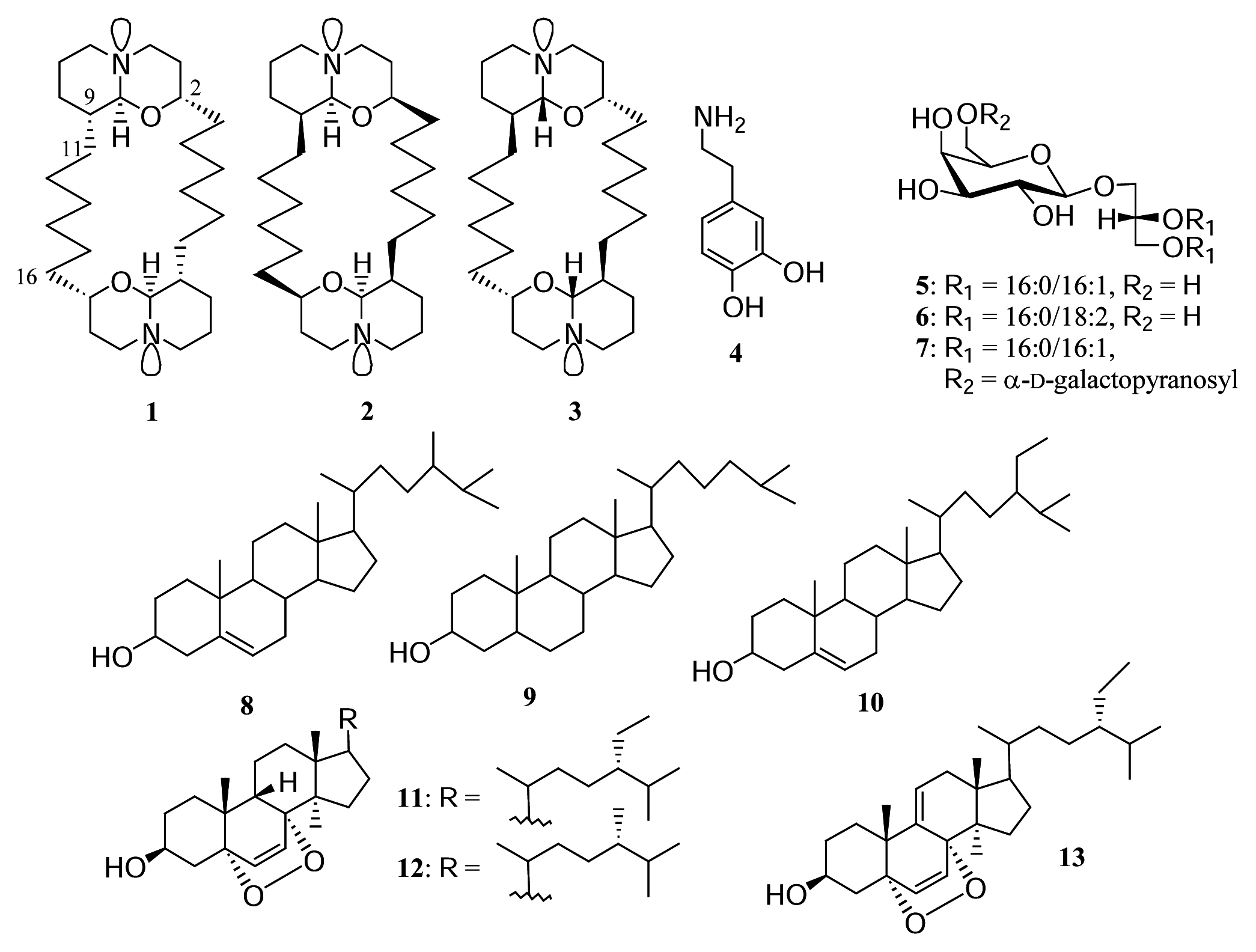
Results and discussion
Conclusions
Experimental data
Gerenal
Marine Sponge
Extraction and Isolation
TLC analysis of dopamine in marine sponges
Cytotoxicity assay
Antimicrobial activity
| Atom No. | 13C | 1H (J in Hz) |
|---|---|---|
| 2 | 76.4 | 3.68 br t (10.8) |
| 3 | 29.5 | 1.74 m, 2.26 m |
| 4 | 52.7 | 3.01 m, 3.54 br d (10.4) |
| 6 | 53.3 | 2.73 m, 3.35 br d (11.2) |
| 7 | 22.2 | 1.72 m, 2.33 m |
| 8 | 24.8 | 1.30 m, 1.43 m |
| 9 | 39.1 | 2.25 m |
| 10 | 94.9 | 4.09 d (10.0) |
| 11 | 30.5 | 1.36 m, 1.64 m |
| 12 | 28.7 | 1.14 m, 1.32 m |
| 13 | 31.1 | 1.16 m, 1.32 m |
| 14 | 27.3 | 1.78 m, 1.84 m |
| 15 | 24.3 | 1.14 m, 1.27 m |
| 16 | 34.5 | 1.48 m, 1.70 m |
Acknowledegments
- Sample Availability: Samples are available from the authors.
References and Notes
- Wilkinson, C. R. Significance of microbial symbionts in sponge evolution and ecology. Symbiosis 1987, 4, 135–146. [Google Scholar]
- Vacelet, J.; Donadey, C. Electron microscope study of the association between some sponges and bacteria. J. Exp. Mar. Ecol 1977, 30, 301–314. [Google Scholar]
- Willenz, P.; Hartman, W. D. Micromorphology and ultrastructure of Caribbean sclerosponges. I. Ceratoporella nicholsoni and Stromatospongia norae (Ceratoporellidae: Porifera). Mar. Biol 1989, 103, 387–402. [Google Scholar]
- Haygood, M. G.; Schmidt, E. W.; Davidson, S. K.; Faulkner, D. J. Microbial symbionts of marine invertebrates: Opportunities for microbial biotechnology. J. Mol. Microbiol. Biotechnol 1999, 1, 33–43. [Google Scholar]
- Faulkner, D. J.; Harper, M. K.; Haygood, M. G.; Salomon, C. E.; Schmidt, E. W. Symbiotic bacteria in sponges: Sources of bioactive substances. In Drugs from the Sea; Fusetani, N., Ed.; Kaeger: Basel, 2002; pp. 107–119. [Google Scholar]
- Thin slices and homogenized samples were prepared from N. exigua and observed under a microscope in a laboratory at the Palau International Coral Reef Center in March, 2004.
- Kobayashi, M.; Kawazoe, K.; Kitagawa, I. Araguspongines B, C, D, E, F, G, H, and J, new vasodilative bis-1-oxaquinolizidine alkaloids from an Okinawan marine sponge, Xestopongia sp. Chem. Pharm. Bull 1989, 37, 1676–1678. [Google Scholar]
- Kobayashi, M.; Miyamota, Y.; Aoki, S.; Murakami, N.; Kitagawa, I.; Ishida, T. Isomerization of dimeric 2, 9-disubstituted 1-oxaquinolizidine alkaloids and structural revision of araguspongines B and E, isolated from a marine sponge of Xestospongia sp. Heterocycles 1998, 47, 195–203. [Google Scholar]
- Morimoto, T.; Murakami, N.; Nagatsu, A.; Sakakibara, J. Studies on glycolipids VII. Isolation of two new sulfoquinovosyl diacylglycerols from green alga Chlorella vulgaris. Chem. Pharm. Bull 1993, 41, 1545–1548. [Google Scholar]
- Shirahashi, H.; Murakami, N.; Watanabe, M.; Nagatsu, A.; Sakakibara, J.; Tokuda, H.; Nishino, H.; Iwashima, A. Isolation and identification of anti-tumor-promoting principles from the fresh-water cyanobacterium Phormidium tenue. Chem. Pharm. Bull 1993, 41, 1664–1666. [Google Scholar]
- Oshima, Y.; Yamada, S. H.; Matsunaga, K.; Moriya, T.; Ohizumi, Y. A monogalactosyl diacylglycerol from a cultured marine dinoflagellate, Scrippsiella trochoidea. J. Nat. Prod 1994, 57, 534–536. [Google Scholar]
- Kobayashi, M.; Hayashi, K.; Kawazoe, K.; Kitagawa, I. Marine natural products. XXIX. Heterosigma-glycolipids I, II, III, and IV, four diacylglyceroglycolipids possessing ω3-polyunsaturated fatty acid residues, from the raphidophycean dinoflagellate Heterosigma akashiwo. Chem. Pharm. Bull 1992, 40, 1404–1410. [Google Scholar]
- Sassaki, G. L.; Gorin, P. A. J.; Iacomini, M. Characterization of lyso-galactolipids, C-2 and C-3 O-acyltrigalactosylglycerol isomers, obtained from the lichenized fungus Dictyonema glabratum. FEMS Microbiol. Lett 2001, 194, 155–158. [Google Scholar]
- Huang, P.; Karagianis, G.; Waterman, P.G. Chemical constituents from Typhonium flagelliforme. Zhong Yao Cai 2004, 27, 173–175. [Google Scholar]
- Xu, X.; Guan, Z.; Zeng, L.; Su, J. Study on the steroid constituents of soft coral Lobophytum microspiculatum. Se Pu 1999, 17, 225–228. [Google Scholar]
- Blumenberg, M.; Thiel, V.; Pape, T.; Michaelis, W. The steroids of hexactinellid sponges. Naturwissenschaften 2002, 89, 415–419. [Google Scholar]
- Gauvin, A.; Smadja, J.; Aknin, M.; Faure, R.; Gaydou, E. M. Isolation of bioactive 5α, 8α-epidioxy sterols from the marine sponge Luffariella cf. variabilis. Can. J. Chem 2000, 78, 986–992. [Google Scholar]
- Gunatilaka, A. A. L.; Gopichand, Y.; Francis, J. S.; Djerassi, C. Isolation and structure elucidation of nine new 5α,8α-epidoxy sterols from four marine organisms. J. Org. Chem 1981, 46, 3860–3866. [Google Scholar]
- Orabi, K. Y.; El Sayed, K. A.; Hamann, M. T.; Dunbar, D. C.; Al-Said, M. S.; Higa, T.; Kelly, M. Araguspongines K and L, new bioactive bis-oxaquinozilidine N-oxide alkaloids from red sea specimens of Xestospongia exigua. J. Nat. Prod 2002, 65, 1782–1785. [Google Scholar]
- Moon, S. S.; Macmillan, J. B.; Olmstead, M. M.; Ta, T. A.; Pessah, I. N.; Molinski, T. F. (+)-7S-hydroxyxestosponin A from the marine sponge Xestospongia sp. and absolute configuration of (+)-xestospongin D. J. Nat. Prod 2002, 65, 249–254. [Google Scholar]
- Nakagawa, M.; Endo, M.; Tanaka, N.; Lee, G.-P. Structures of xestospongin A, B, C and D, novel vasodilative compounds from marine sponge, Xestospongia exigua. Tetrahedron Lett 1984, 25, 3227–3230. [Google Scholar]
- Reddy, M. V. R.; Faulker, D. J. 3β, 3′β,-dimethylxestospongin C, a new bis-1–oxa quinolizidine alkaloid from the Palauan sponge Xestospongia sp. Nat. Prod. Lett 1997, 11, 53–59. [Google Scholar]
- Venkateswarlu, Y.; Reddy, M. V. R.; Rao, J. V. Bis-1-oxaquinolizidine from the sponge Haliclona exigua. J. Nat. Prod 1994, 57, 1283–1285. [Google Scholar]
- Bohlmannn, F. Zur konfigurationsbestimmung von chinolizin-derivaten. Angew. Chem 1957, 69, 641–644. [Google Scholar]
- Hoye, T. R.; North, J. T.; Yao, L. J. Conformational considerations in 1-oxaquinolizidines related to the Xestospongin/araguspongine family: Reassignment of stereostructures for araguspongines B and E. J. Org. Chem 1994, 59, 6904–6910, Structure correction of araguspongine B, J. Org. Chem. 1995, 60, 4958. [Google Scholar]
- Rao, J.; Desaiah, D.; Vig, P.; Venkateswarlu, Y. Marine biomolecules inhibit rat brain nitric oxide synthase activity. Toxicology 1998, 129, 103–112. [Google Scholar]
- Pettit, G.; Orr, B.; Herald, D.; Doubek, D.; Tackett, L.; Schmidt, J.; Boyd, M.; Pettit, R.; Hooper, J. Isolation and X-ray crystal structure of racemic xestospongin D from the Singapore marine sponge Niphates sp. Bioorg. Med. Chem. Lett 1996, 6, 1313–1318. [Google Scholar]
- Vassas, A.; Bourdy, G.; Paillard, J.; Lavayre, J.; Pais, M.; Quirion, J.; Debitus, C. Naturally occurring somatosatatin and vasoactive intestinal peptide inhibitors: Isolation of alkaloids from two marine sponges. Planta Med 1996, 62, 28–30. [Google Scholar]
- Pimentel, S. M. V.; Bojo, Z. P.; Roberto, A. V. D.; Lazaro, J. E. H.; Mangalindan, G. C.; Florentino, L. M.; Lim-Navarro, P.; Tasdemir, D.; Ireland, C. M.; Concepcion, G. P. Platelet aggregation inhibitors from Philippine marine invertebrate samples screened in a new microplate assay. Mar. Biotechnol 2003, 5, 395–400. [Google Scholar]
- Laycock, M. V.; Rogan, M. A. L-3, 4-Dihydroxyphenylalanine-3-sulfate from the brown alga, Ascophyllum nodosum. J. Nat. Prod 1984, 47, 1033–1036. [Google Scholar]
- Nelson, A. V. Chemical Defenses of Ulvaria obscura: Effect on Food Preference of Strongylocentrotus droebachiensis. M.S. Thesis, Western Washington University, Bellingham, WA, 2003; p. 49. [Google Scholar]
- Nelson, T. A.; Lee, D. J.; Smith, B. C. Are green tides harmful algal blooms? Toxic properties of water-soluble extracts from two bloom-forming macroalgae, Ulva fenestrata and Ulvaria obscura (Ulvophyceae). J. Phycol 2003, 39, 874–879. [Google Scholar]
- Davidson, B. S.; Molinski, T. F.; Barrows, L.; Ireland, C. M. Varacin: a novel benzopentathiepin from Lissoclinum vareau that is cytotoxic toward a human colon tumor. J. Am. Chem. Soc 1991, 113, 4709–4710. [Google Scholar]
- Litaudon, M.; Trigalo, F.; Martin, M. T.; Frappier, F.; Guyot, M. Lissoclinotoxins: Antibiotic polysulfur derivatives from the tunicate Lissoclinum perforatum. Revised structure of lissoclinotoxin A. Tetrahedron 1994, 50, 5323–5334. [Google Scholar]
- Compagnone, R. S.; Faulkner, D. J.; Carté, B. K.; Chan, G.; Freyer, A.; Hemling, M. E.; Hofmann, G. A.; Mattern, M. R. Pentathiepins and trithianes from two Lissoclinum species and a Eudistoma sp.: inhibitors of protein kinase C. Tetrahedron 1994, 50, 12785–12792. [Google Scholar]
- Searle, P. A.; Molinski, T. F. Five new alkaloids from the tropical ascidian, Lissoclinum sp. lissoclinotoxin A is chiral. J. Org. Chem 1994, 59, 6600–6605. [Google Scholar]
- Kikuchi, H.; Tsukitani, Y.; Manda, T.; Fujii, T.; Nakanishi, H.; Kobayashi, M.; Kitagawa, I. Marine natural products X. Pharmacologically active glycolipids from the Okinawan marine sponge Phyllospongia foliascens. Chem. Pharm. Bull 1982, 30, 3544–3547. [Google Scholar]
- Meguro, S.; Namikoshi, M.; Kobayashi, H. A new screening method for antimitotic substances and isolation of glycolipids as stimulators of tubulin polymerization form Okinawan sponge Pseudoceratian sp. J. Antibiot 2002, 55, 256–262. [Google Scholar]
- The voucher specimen of the same sponge is also kept in the Marine Biotechnology Institute, Japan as 02PA-006, which was identified by Professor P. Bergquist as Neopetrosia exigua Kirkpatrick (Order Petrosida, Family Petrosiidae).
- Namikoshi, M.; Negishi, R.; Nagai, H.; Dmitrenok, A.; Kobayashi, H. Three new chlorine containing antibiotics from a marine-derived fungus Aspergillus ostianus collected in Pohnpei. J. Antibiot 2003, 56, 755–761. [Google Scholar]
© 2004 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Liu, H.; Mishima, Y.; Fujiwara, T.; Nagai, H.; Kitazawa, A.; Mine, Y.; Kobayashi, H.; Yao, X.; Yamada, J.; Oda, T.; et al. Isolation of Araguspongine M, a New Stereoisomer of an Araguspongine/Xestospongin alkaloid, and Dopamine from the Marine Sponge Neopetrosia exigua Collected in Palau. Mar. Drugs 2004, 2, 154-163. https://doi.org/10.3390/md204154
Liu H, Mishima Y, Fujiwara T, Nagai H, Kitazawa A, Mine Y, Kobayashi H, Yao X, Yamada J, Oda T, et al. Isolation of Araguspongine M, a New Stereoisomer of an Araguspongine/Xestospongin alkaloid, and Dopamine from the Marine Sponge Neopetrosia exigua Collected in Palau. Marine Drugs. 2004; 2(4):154-163. https://doi.org/10.3390/md204154
Chicago/Turabian StyleLiu, Hongwei, Yuri Mishima, Takeshi Fujiwara, Hiroshi Nagai, Akira Kitazawa, Yuji Mine, Hisayoshi Kobayashi, Xinsheng Yao, Junko Yamada, Taiko Oda, and et al. 2004. "Isolation of Araguspongine M, a New Stereoisomer of an Araguspongine/Xestospongin alkaloid, and Dopamine from the Marine Sponge Neopetrosia exigua Collected in Palau" Marine Drugs 2, no. 4: 154-163. https://doi.org/10.3390/md204154




