Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo
Abstract
:1. Introduction
2. Results
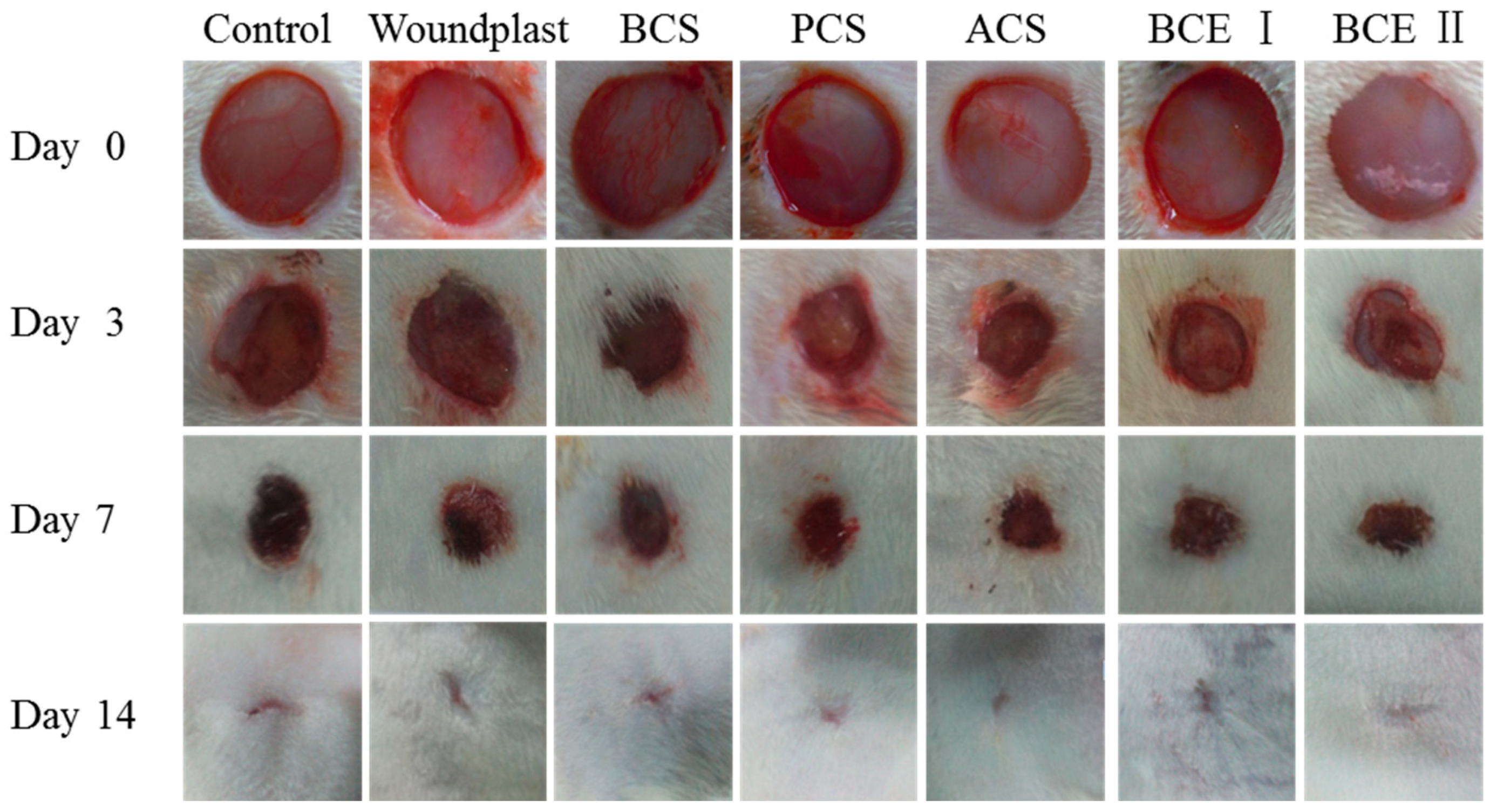
2.1. Macroscopic Observation of the Wounds
2.2. The Quantification of Aggregate Protein at the Wound Site
2.3. Hydroxyproline (Hyp) Content at the Wound Site
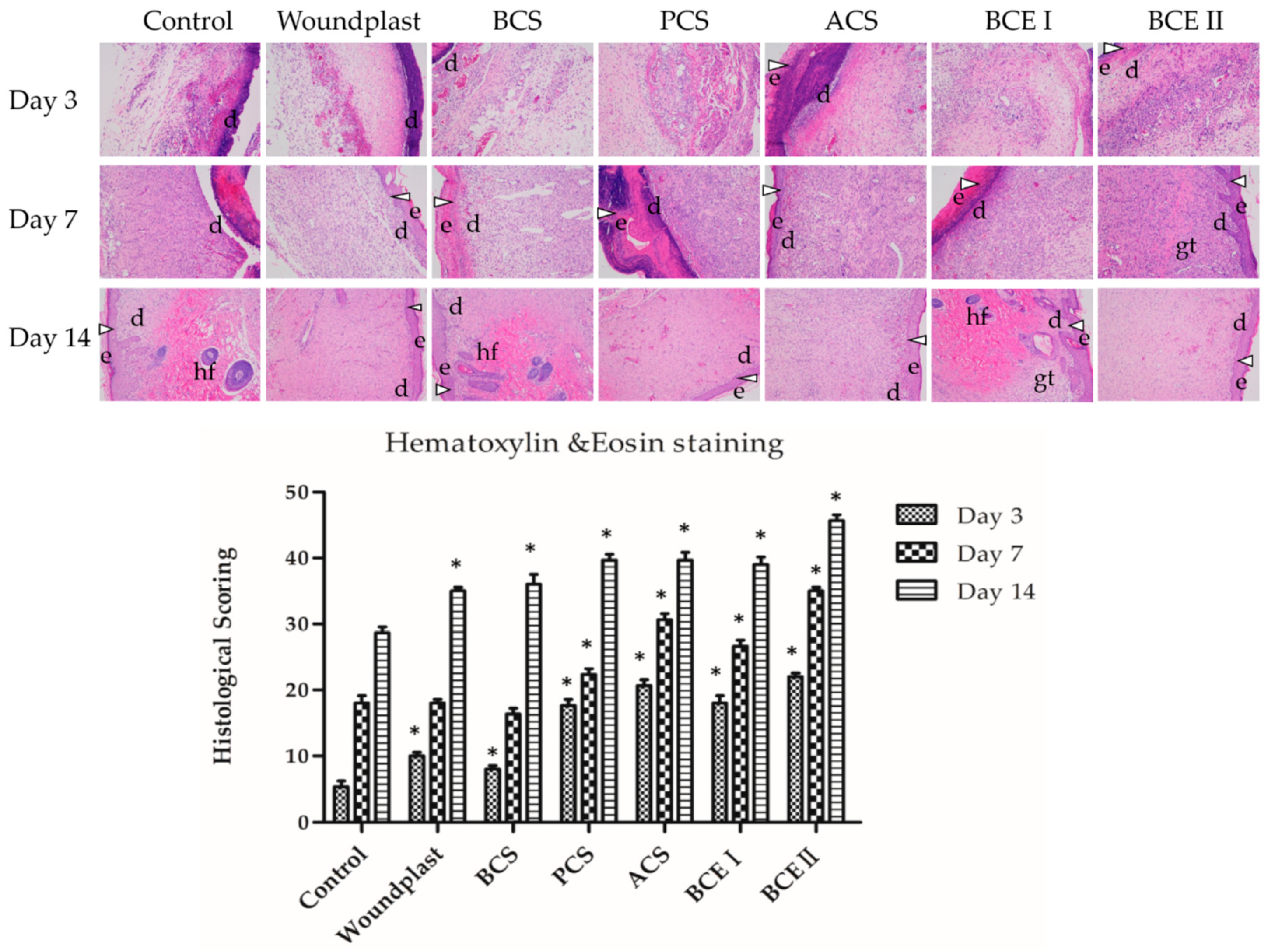
2.4. Histopathological Examination
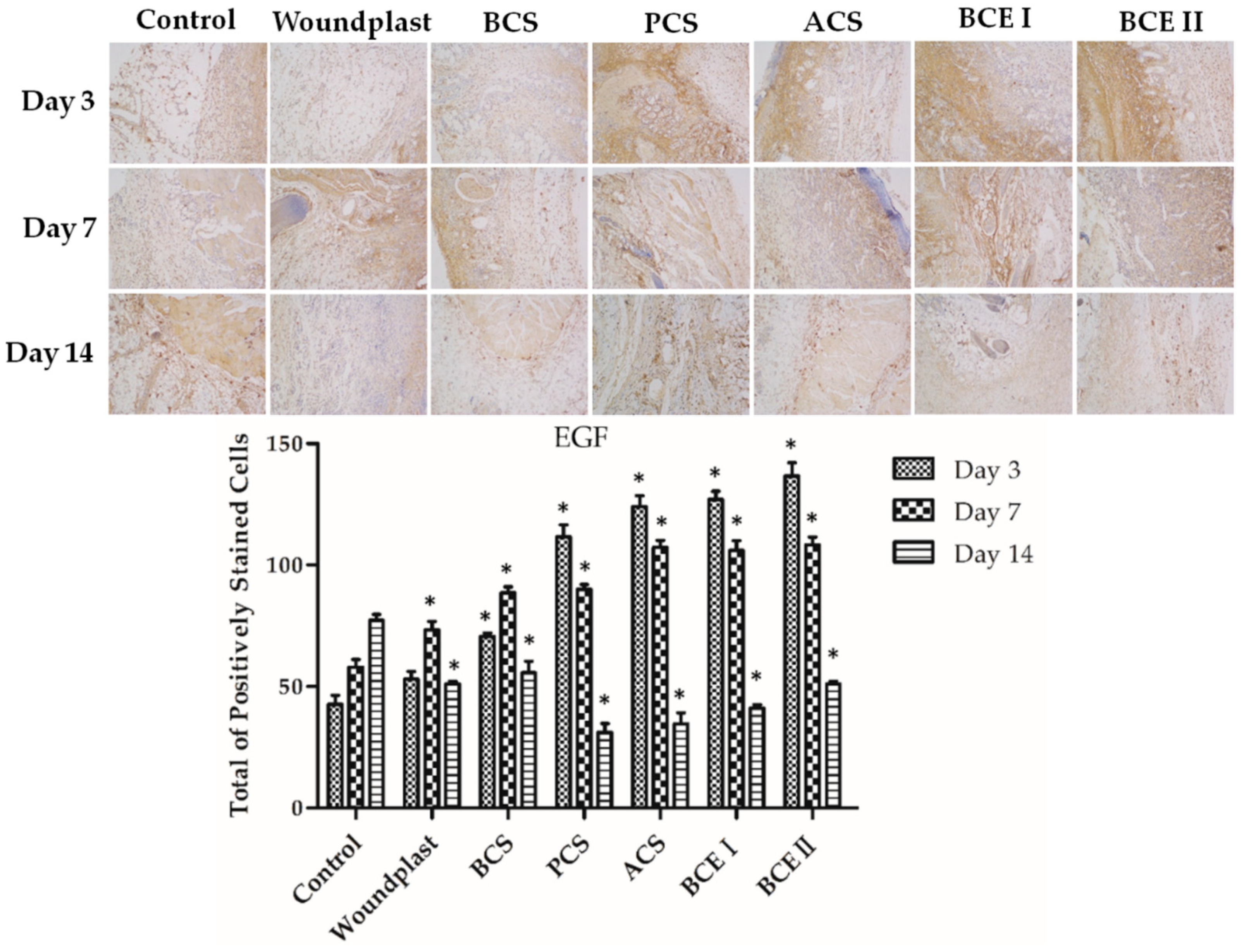
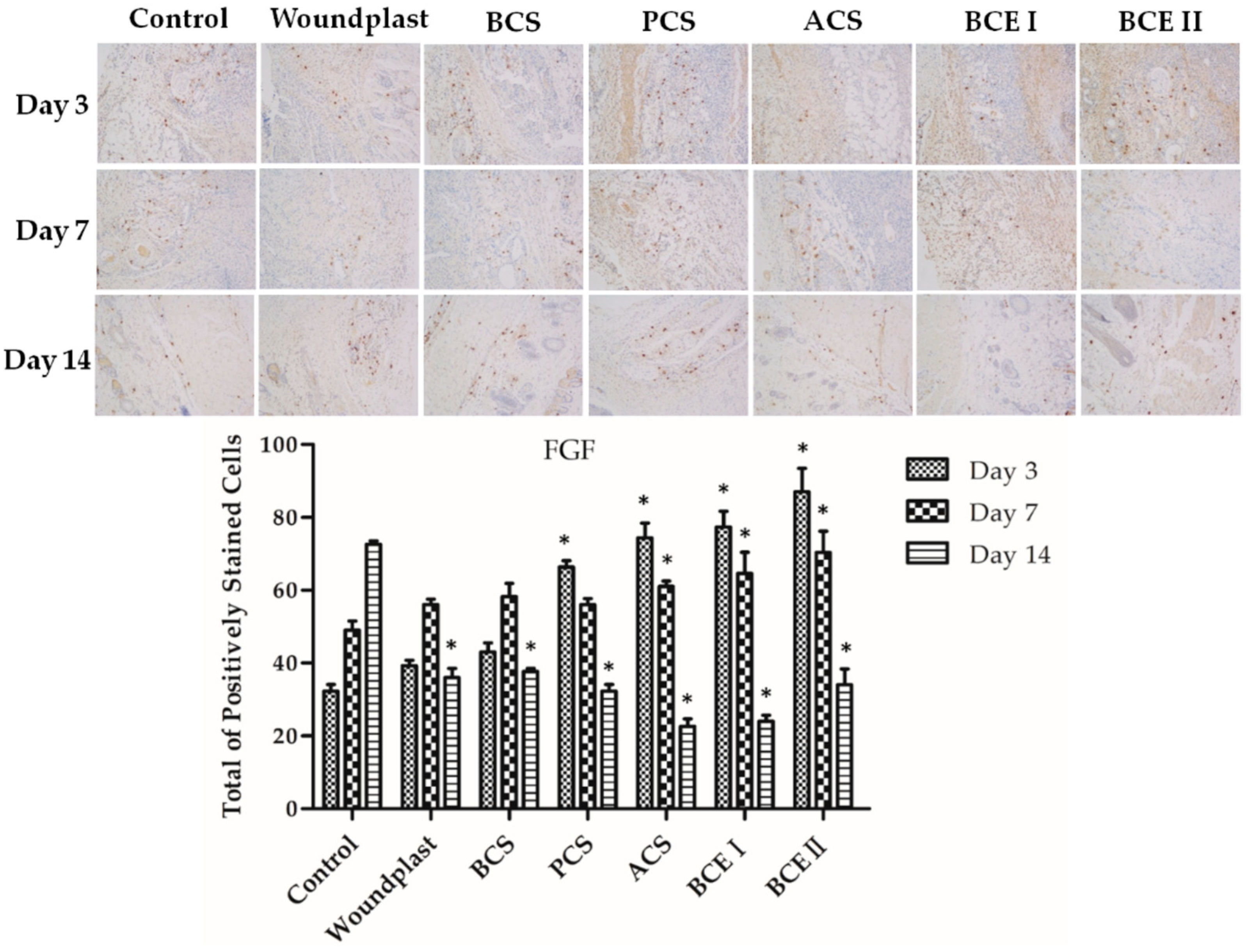
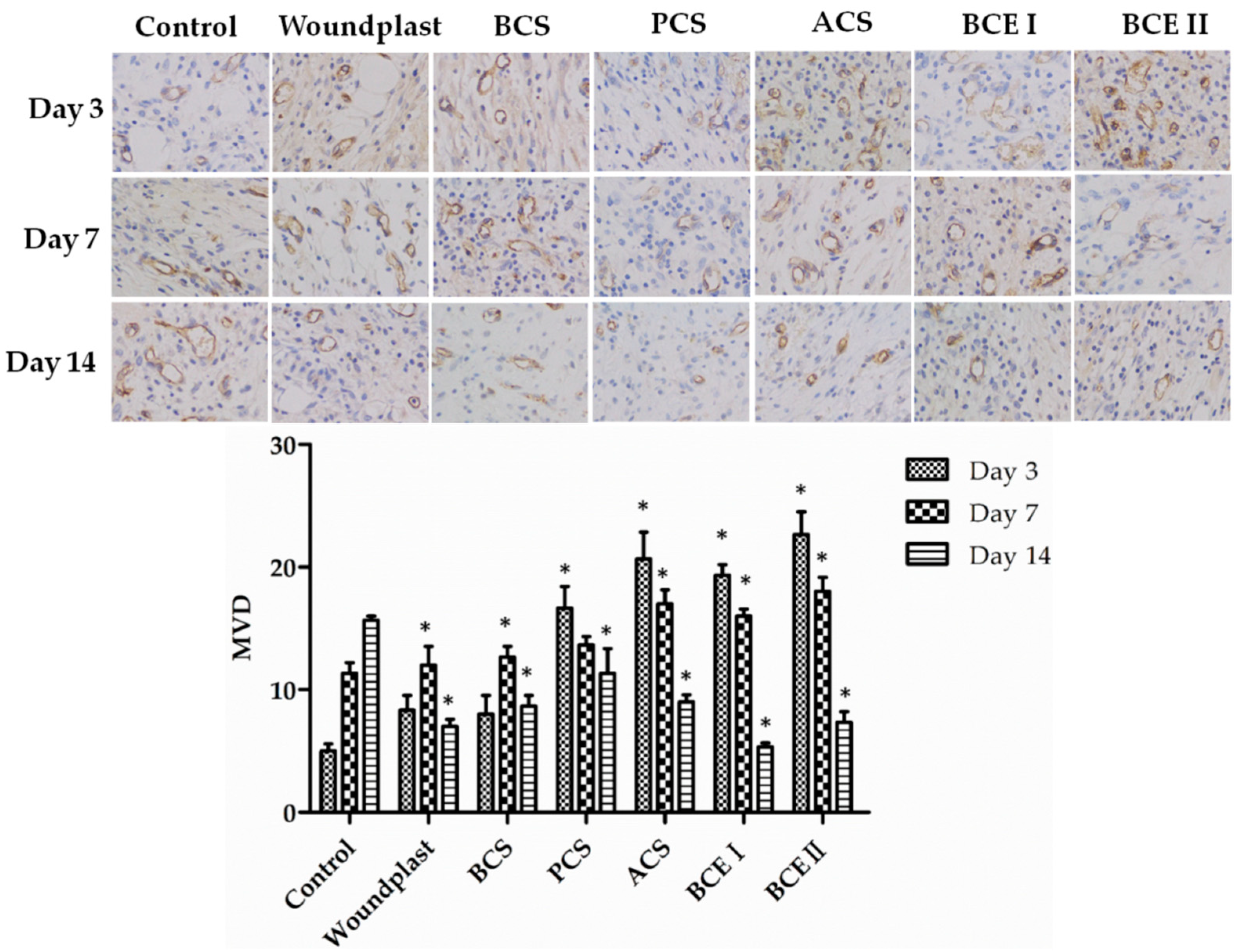
2.5. Collagen Promotes the Expression of EGF, FGF, and CD31 in the Wounds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Skin Wound Healing in SD Rats
4.3. Determination of Total Protein and Hydroxyproline Content
4.4. Histopathological Examination
4.5. Immuno-Histochemical Examinations
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L.M. Co-electrospun nanofibrous membranes of collagen and zein for wound healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057. [Google Scholar] [CrossRef]
- De Almeida, E.B.; Cardoso, J.C.; de Lima, A.K.; de Oliveira, N.L.; de Pontes, N.T.; Lima, S.O.; Souza, I.C.L.; de Albuquerque, R.L.C. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J. Ethnopharmacol. 2013, 147, 419–425. [Google Scholar] [CrossRef]
- Banerjee, P.; Mehta, A.; Shanthi, C. Investigation into the cyto-protective and wound healing properties of cryptic peptides from bovine achilles tendon collagen. Chem.-Biol. Interact. 2014, 211, 1–10. [Google Scholar] [CrossRef]
- Castillo-Briceno, P.; Bihan, D.; Nilges, M.; Hamaia, S.; Meseguer, J.; Garcia-Ayala, A.; Farndale, R.W.; Mulero, V. A role for specific collagen motifs during wound healing and inflammatory response of fibroblasts in the teleost fish gilthead seabream. Mol. Immunol. 2011, 48, 826–834. [Google Scholar] [CrossRef]
- Holmer, C.; Praechter, C.; Mecklenburg, L.; Heimesaat, M.; Rieger, H.; Pohlen, U. Anastomotic stability and wound healing of colorectal anastomoses sealed and sutured with a collagen fleece in a rat peritonitis model. Asian J. Surg. 2014, 37, 35–45. [Google Scholar] [CrossRef]
- Jeevithan, E.; Shakila, R.J.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Physico-functional and mechanical properties of chitosan and calcium salts incorporated fish gelatin scaffolds. Int. J. Biol. Macromol. 2013, 60, 262–267. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, S.H.; Chen, W.J.; Hung, K.C.; Lin, Y.H.; Liu, S.J.; Hsieh, M.J.; Pang, J.H.S.; Juang, J.H. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J. Colloid Interface Sci. 2015, 439, 88–97. [Google Scholar] [CrossRef]
- Jeevithan, E.; Jingyi, Z.; Bao, B.; Shujun, W.; JeyaShakila, R.; Wu, W.H. Biocompatibility assessment of type-II collagen and its polypeptide for tissue engineering: Effect of collagen’s molecular weight and glycoprotein content on tumor necrosis factor (fas/apo-1) receptor activation in human acute t-lymphocyte leukemia cell line. RSC Adv. 2016, 6, 14236–14246. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Jeevithan, E.; Zhang, J.Y.; Wang, N.P.; He, L.; Bao, B.; Wu, W.H. Physico-chemical, antioxidant and intestinal absorption properties of whale shark type-ii collagen based on its solubility with acid and pepsin. Process Biochem. 2015, 50, 463–472. [Google Scholar] [CrossRef]
- Muthukumar, T.; Anbarasu, K.; Prakash, D.; Sastry, T.P. Effect of growth factors and pro-inflammatory cytokines by the collagen biocomposite dressing material containing macrotyloma uniflorum plant extract-in vivo wound healing. Colloids Surf. B Biointerfaces 2014, 121, 178–188. [Google Scholar] [CrossRef]
- Elango, J.; Lee, J.W.; Wang, S.; Henrotin, Y.; de Val, J.; Regenstein, J.M.; Lim, S.Y.; Bao, B.; Wu, W. Evaluation of differentiated bone cells proliferation by blue shark skin collagen via biochemical for bone tissue engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef]
- Ramasamy, P.; Shanmugam, A. Characterization and wound healing property of collagen-chitosan film from sepia kobiensis (hoyle, 1885). Int. J. Biol. Macromol. 2015, 74, 93–102. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Salama, A.H. Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: Preparation, evaluation and in-vivo wound healing assessment. Eur. J. Pharm. Sci. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Kim, K.O.; Lee, Y.; Hwang, J.W.; Kim, H.; Kim, S.M.; Chang, S.W.; Lee, H.S.; Choi, Y.S. Wound healing properties of a 3-D scaffold comprising soluble silkworm gland hydrolysate and human collagen. Colloids Surf. B 2014, 116, 318–326. [Google Scholar] [CrossRef]
- Dang, Q.F.; Liu, H.; Yan, J.Q.; Liu, C.S.; Liu, Y.; Li, J.; Li, J.J. Characterization of collagen from haddock skin and wound healing properties of its hydrolysates. Biomed. Mater. 2015, 10, 015022. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chao, Y.-K.; Chang, S.-H.; Chen, W.-J.; Hung, K.-C.; Liu, S.-J.; Juang, J.-H.; Chen, Y.-T.; Wang, F.-S. Nanofibrous rhPDGF-eluting PLGA–collagen hybrid scaffolds enhance healing of diabetic wounds. RSC Adv. 2016, 6, 6276–6284. [Google Scholar] [CrossRef]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maity, P.P.; Moulik, D.; Dhara, S. Accelerating full thickness wound healing using collagen sponge of mrigal fish (cirrhinus cirrhosus) scale origin. Int. J. Biol. Macromol. 2016, 93, 1507–1518. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Suesca, E.; Casadiegos, S.; Leal, E.C.; Fontanilla, M.R.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta 2014, 1842, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wang, N.P.; Xue, Y.; Ding, T.T.; Liu, X.; Mo, X.M.; Sun, J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf. B 2016, 143, 415–422. [Google Scholar] [CrossRef]
- Ye, B.H.; Luo, X.S.; Li, Z.W.; Zhuang, C.P.; Li, L.H.; Lu, L.; Ding, S.; Tian, J.H.; Zhou, C.R. Rapid biomimetic mineralization of collagen fibrils and combining with human umbilical cord mesenchymal stem cells for bone defects healing. Mater. Sci. Eng. C 2016, 68, 43–51. [Google Scholar] [CrossRef]
- Zhang, J.; Jeevithan, E.; Bao, B.; Wang, S.; Gao, K.; Zhang, C.; Wu, W. Structural characterization, in-vivo acute systemic toxicity assessment and in-vitro intestinal absorption properties of tilapia (Oreochromis niloticus) skin acid and pepsin solublilized type I collagen. Process Biochem. 2016, 51, 2017–2025. [Google Scholar] [CrossRef]
- Jeevithan, E.; Bao, B.; Zhang, J.Y.; Hong, S.T.; Wu, W.H. Purification, characterization and antioxidant properties of low molecular weight collagenous polypeptide (37 kDa) prepared from whale shark cartilage (Rhincodon typus). J. Food Sci. Technol. 2015, 52, 6312–6322. [Google Scholar] [CrossRef]
- Elango, J.; Zhang, J.Y.; Bao, B.; Palaniyandi, K.; Wang, S.J.; Wu, W.H.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef]
- Jeevithan, E.; Wu, W.H.; Wang, N.P.; Lan, H.; Bao, B. Isolation, purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus albimarginatus) skeletal and head bone. Process Biochem. 2014, 49, 1767–1777. [Google Scholar] [CrossRef]
- Jeevithan, E.; Bao, B.; Bu, Y.S.; Zhou, Y.; Zhao, Q.B.; Wu, W.H. Type II collagen and gelatin from silvertip shark (Carcharhinus albimarginatus) cartilage: Isolation, purification, physicochemical and antioxidant properties. Mar. Drugs 2014, 12, 3852–3873. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Huang, R.; Li, W.Z.; Lv, X.X.; Lei, Z.J.; Bian, Y.Q.; Deng, H.B.; Wang, H.J.; Li, J.Q.; Li, X.Y. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2018. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkoli, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Moreno-Arotzena, O.; Meier, J.G.; del Amo, C.; Garcia-Aznar, J.M. Characterization of fibrin and collagen gels for engineering wound healing models. Materials 2015, 8, 1636–1651. [Google Scholar] [CrossRef]
- Ramanathan, G.; Thyagarajan, S.; Sivagnanam, U.T. Accelerated wound healing and its promoting effects of biomimetic collagen matrices with siderophore loaded gelatin microspheres in tissue engineering. Mater. Sci. Eng. C 2018, 93, 455–464. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Ahearne, M.; Wilson, S.L.; Liu, K.K.; Rauz, S.; El Haj, A.J.; Yang, Y. Influence of cell and collagen concentration on the cell-matrix mechanical relationship in a corneal stroma wound healing model. Exp. Eye Res. 2010, 91, 584–591. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Rudenko, T.G.; Istranov, L.P.; Guller, A.E.; Borodulin, R.R.; Vanin, A.F. Dinitrosyl iron complexes with glutathione incorporated into a collagen matrix as a base for the design of drugs accelerating skin wound healing. Eur. J. Pharm. Sci. 2015, 78, 8–18. [Google Scholar] [CrossRef]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef]
- Kwan, K.H.L.; Liu, X.L.; To, M.K.T.; Yeung, K.W.K.; Ho, C.M.; Wong, K.K.Y. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Nanomedicine 2011, 7, 497–504. [Google Scholar] [CrossRef]
- Liu, N.; Ding, D.; Hao, W.; Yang, F.; Wu, X.; Wang, M.; Xu, X.; Ju, Z.; Liu, J.P.; Song, Z.; et al. Htert promotes tumor angiogenesis by activating VEGF via interactions with the Sp1 transcription factor. Nucleic Acids Res. 2016, 44, 8693–8703. [Google Scholar] [CrossRef]
| Groups | Total Protein (mg/mL) | ||
|---|---|---|---|
| 3 Days | 7 Days | 14 Days | |
| Control | 7.93 ± 0.6 a | 8.03 ± 1.1 a | 9.15 ± 0.7 a |
| Woundplast | 8.12 ± 0.6 b | 9.15 ± 0.4 b | 10.3 ± 0.7 cd |
| BCS | 8.61 ± 0.4 bc | 9.52 ± 1.4 bc | 9.94 ± 1.1 b |
| PCS | 8.48 ± 0.8 bc | 9.58 ± 0.8 bc | 11.7 ± 1.3 bc |
| ACS | 8.65 ± 0.4 c | 9.66 ± 1.3 b | 13.3 ± 0.8 c |
| BCE I | 8.79 ± 0.9 c | 10.2 ± 0.8 c | 11.4 ± 0.4 bd |
| BCE II | 8.85 ± 0.5 c | 10.5 ± 0.7 c | 11.3 ± 0.5 bd |
| Groups | Hydroxyproline Content (mg/g Wet Skin) | ||
|---|---|---|---|
| 3 Days | 7 Days | 14 Days | |
| Control | 5.26 ± 0.33 a | 6.12 ± 0.29 a | 6.79 ± 0.39 a |
| Woundplast | 5.46 ± 0.41 ab | 6.38 ± 0.49 a | 7.29 ± 0.18 ab |
| BCS | 5.60 ± 0.24 ab | 6.29 ± 0.36 a | 7.02 ± 0.39 ab |
| PCS | 5.47 ± 0.36 ab | 6.55 ± 0.44 b | 7.32 ± 0.43 b |
| ACS | 5.87 ± 0.42 b | 6.65 ± 0.34 b | 7.41 ± 0.42 b |
| BCE I | 5.42 ± 0.25 ab | 6.42 ± 0.35 a | 6.77 ± 0.33 a |
| BCE II | 5.66 ± 0.12 b | 6.68 ± 0.54 b | 7.22 ± 0.27 ab |
| 0 | 1–3 | 4–6 | 7–9 | |
|---|---|---|---|---|
| Inflammatory cells | Abundant | Moderate | Scant | Rarely |
| Fibroblast content | None | Scant | Moderate | Abundant |
| Re-epithelialization | None | Partial | Thin | Complete |
| Collagen deposition | None | Scant | Moderate | Abundant |
| Revascularizations | None | Scant | Moderate | Abundant |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33. https://doi.org/10.3390/md17010033
Chen J, Gao K, Liu S, Wang S, Elango J, Bao B, Dong J, Liu N, Wu W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Marine Drugs. 2019; 17(1):33. https://doi.org/10.3390/md17010033
Chicago/Turabian StyleChen, Jingjing, Kaili Gao, Shu Liu, Shujun Wang, Jeevithan Elango, Bin Bao, Jun Dong, Ning Liu, and Wenhui Wu. 2019. "Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo" Marine Drugs 17, no. 1: 33. https://doi.org/10.3390/md17010033





