Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods
Abstract
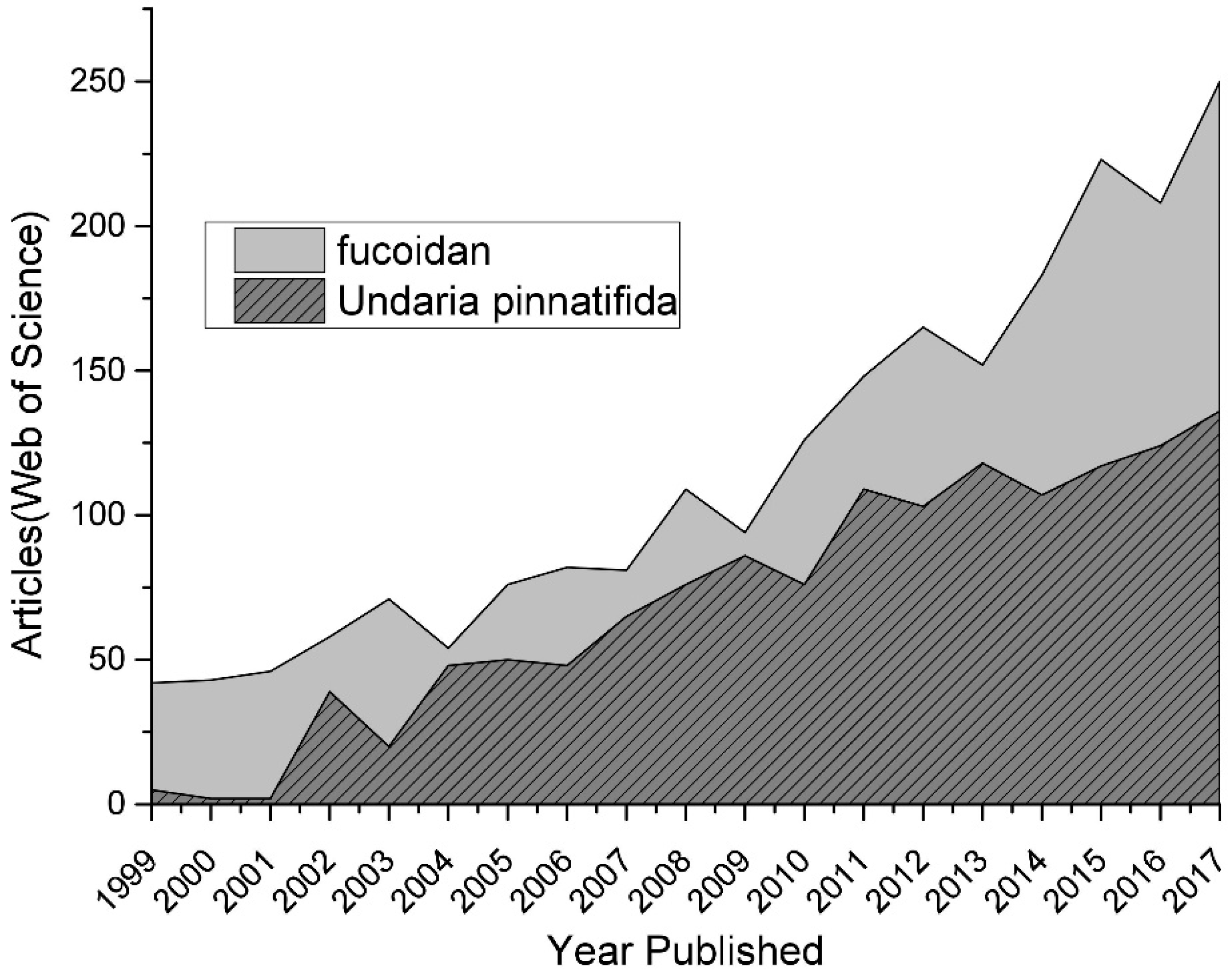
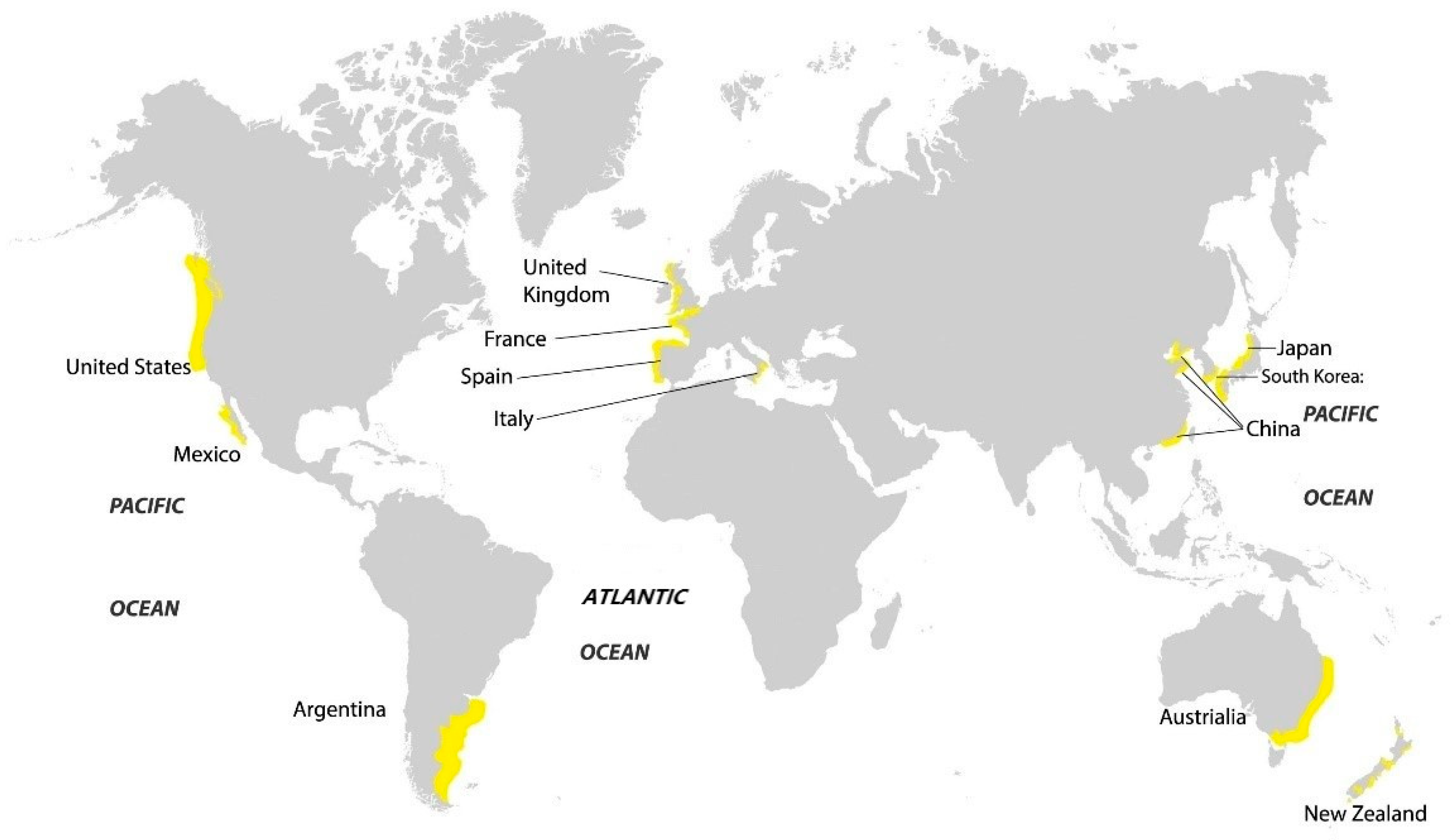
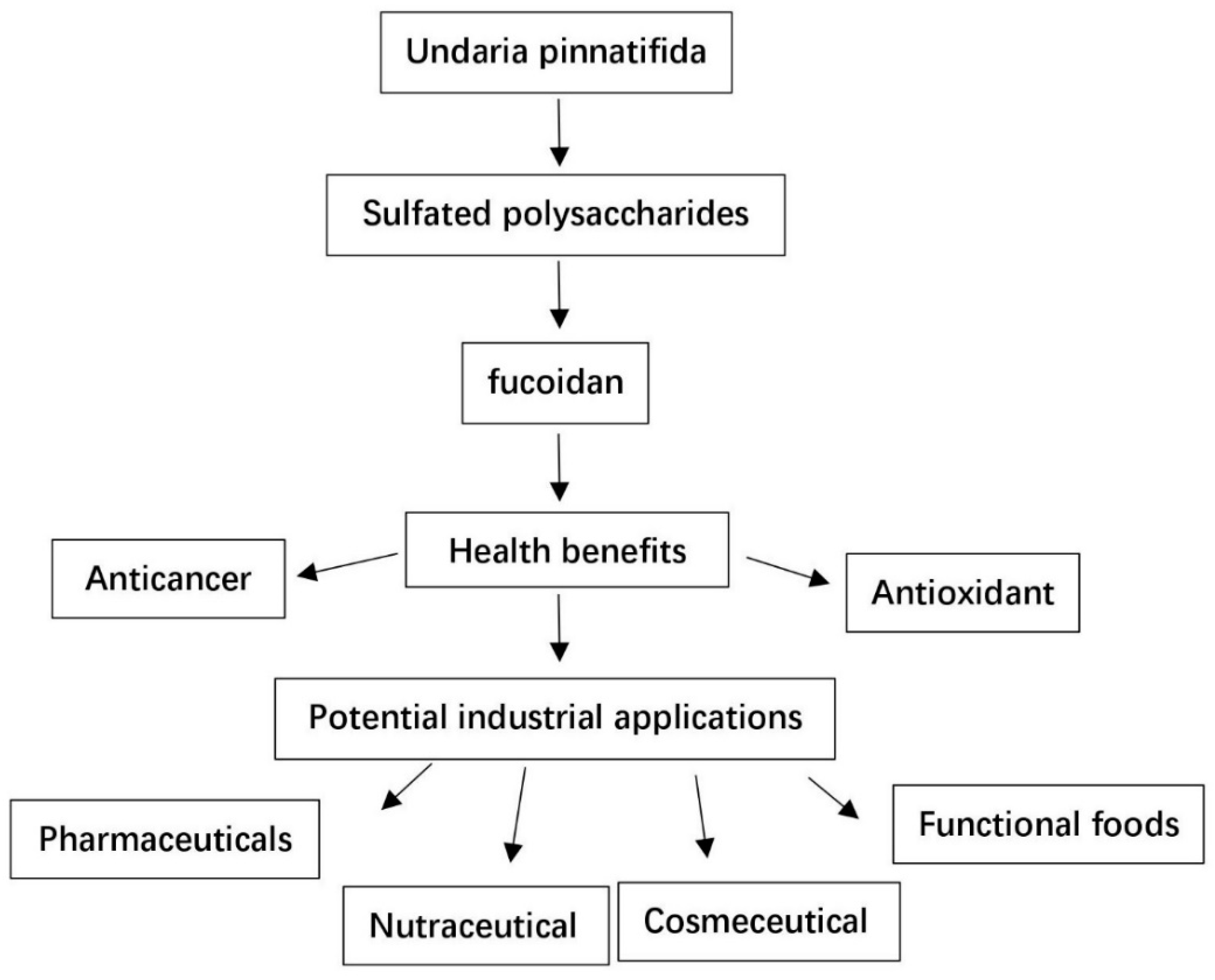
:1. Introduction
2. The Extraction of Fucoidan from U. pinnatifida
3. The Structure and Function of Fucoidan from U. pinnatifida
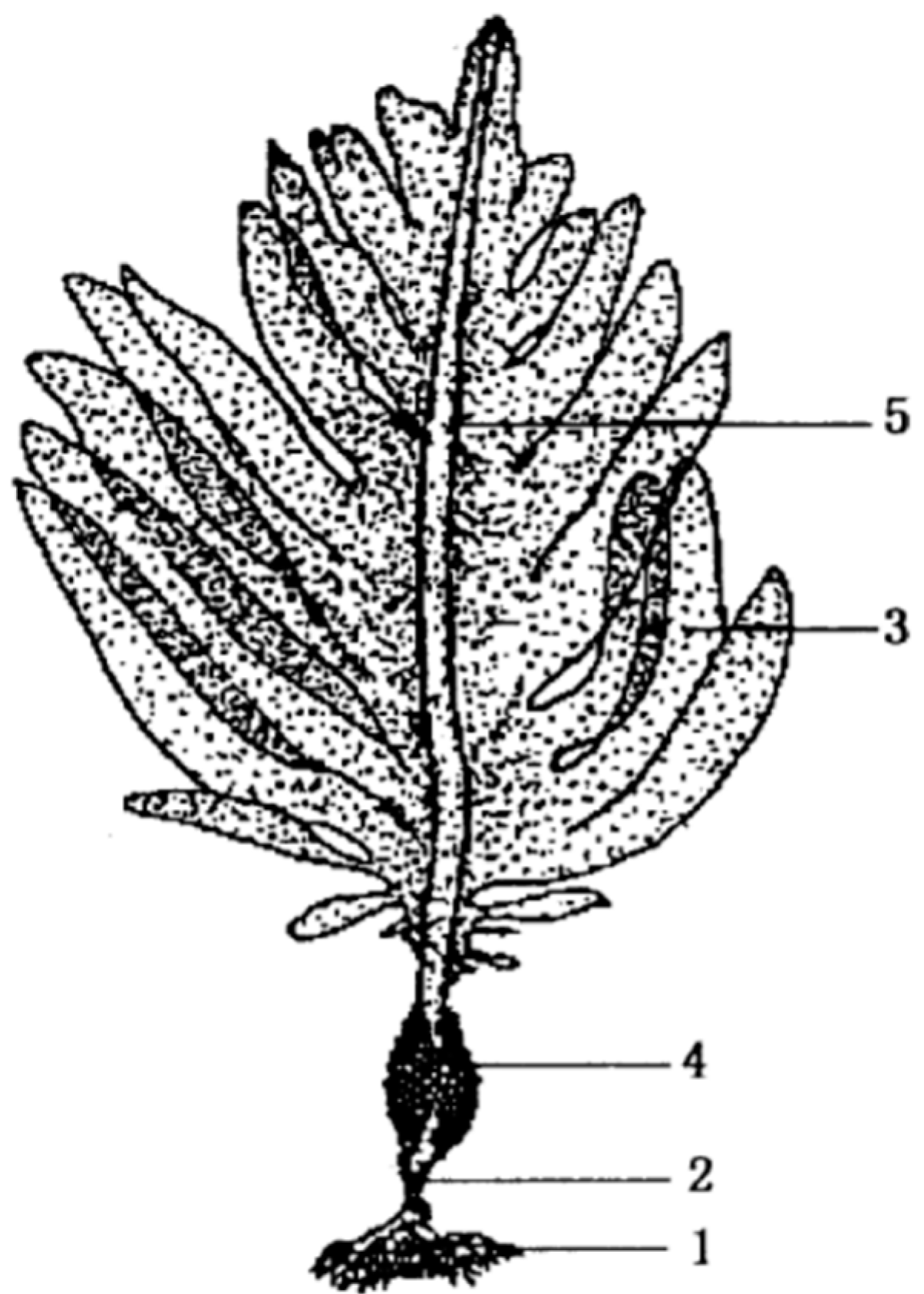
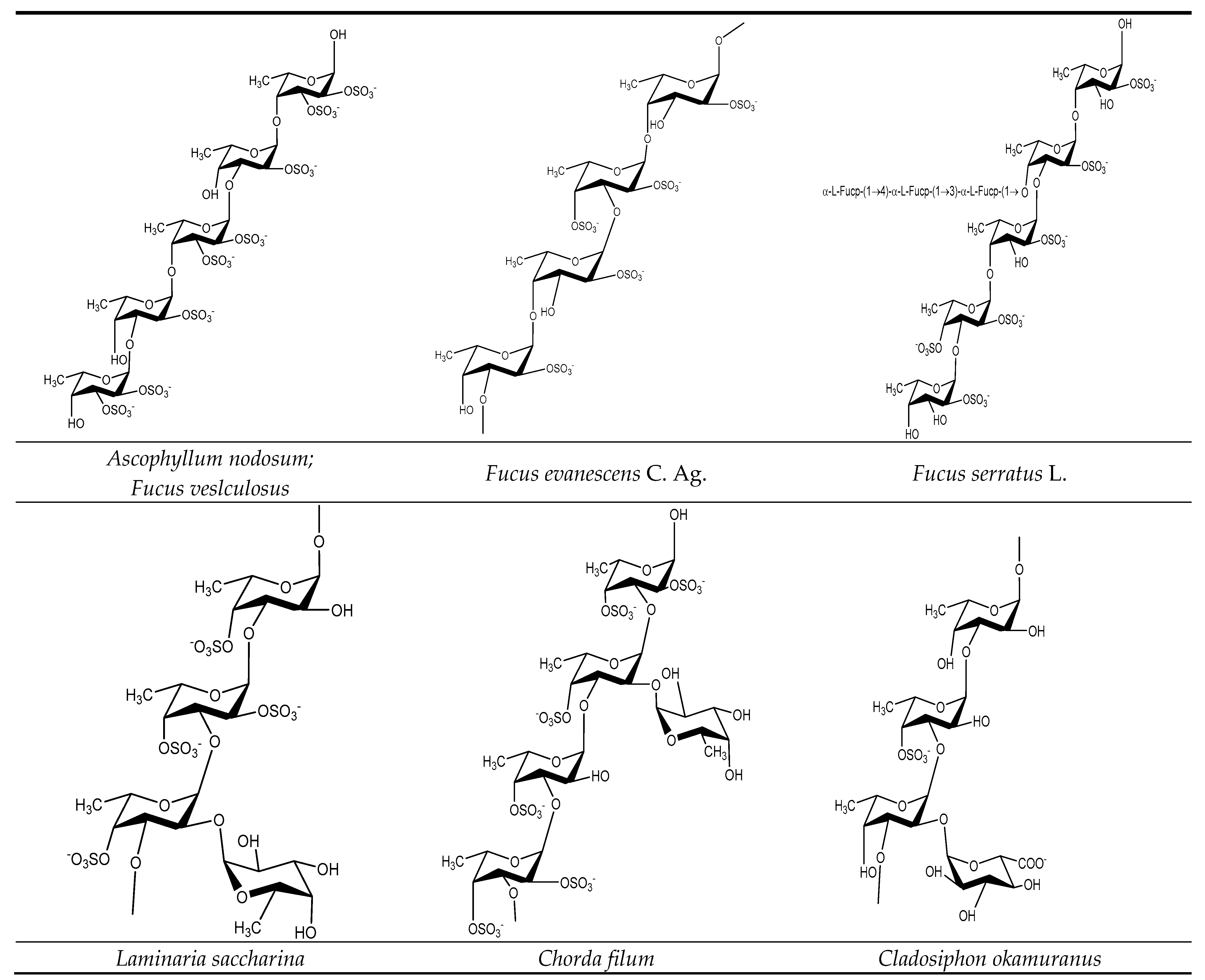
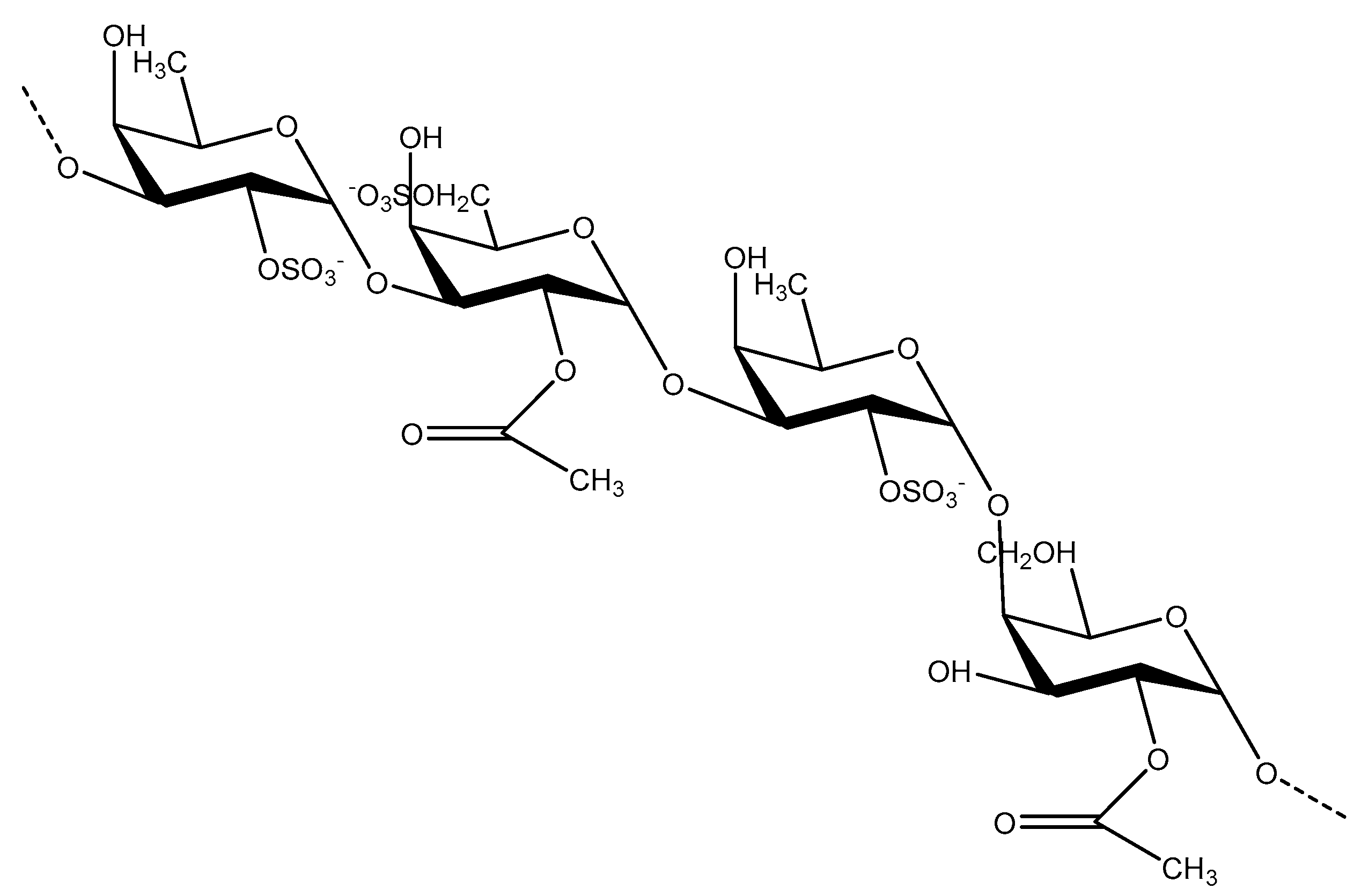
3.1. Fucoidan Basic Structure
3.2. Structure Characterization and Structure–Activity Relationship
3.2.1. Anticancer Properties of Fucoidan from U. pinnatifida
3.2.2. Antioxidant Activities of Fucoidan from U. pinnatifida
3.2.3. Anticoagulant Activity of Fucoidan from U. pinnatifida
3.2.4. Antibacterial Activity
4. Fucoidan as Functional Food and Therapeutic Agent
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Kim, S.K. Fucoidan, A Sulfated Polysaccharides from Brown Algae as Therapeutic Target for Cancer; Springer International Publishing: Cham, Switzerland, 2015; pp. 145–164. ISBN 978-3-319-07144-2. [Google Scholar]
- Kylin, H. Biochemistry of sea algae. HZ Physiol. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Campbell, M.L.; Mcennulty, F.; Moore, K.M.; Murfet, N.B.; Robertson, B.; Schaffelke, B. Efficacy of physical removal of a marine pest: The introduced kelp Undaria pinnatifida in a Tasmanian Marine Reserve. Biol. Invasions 2005, 7, 251–263. [Google Scholar] [CrossRef]
- Fletcher, R.L.; Farrell, P. Introduced brown algae in the North East Atlantic, with particular respect to Undaria pinnatifida (Harvey) suringar. Helgoländer Meeresuntersuchungen 1998, 52, 259–275. [Google Scholar] [CrossRef]
- Wallentinus, I. Alien Species Alert: Undaria pinnatifida:(Wakame or Japanese Kelp); The Council: Göteborg, Sweden, 2007; ISBN 8774820559. [Google Scholar]
- Helen, F.J. Method and composition for the treatment of a viral infection. Arch. Oral. Biol. 2006, 41, 299–305. [Google Scholar]
- Yang, C.; Chung, D.; Shina, I.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.D. Review of Research on Undaria pinnatifida in New Zealand and Its Potential Impacts on the Eastern Coast of the South Island; Department of Conservation: Wellington, New Zealand, 2004; ISBN 0-478-22090-1. [Google Scholar]
- Nishino, T.; Nagumo, T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res. 1991, 214, 193. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, T.; Lang, S.; Ulber, R.; Kai, M. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Rani, V. Influence of Species, Geographic Location, Seasonal Variation and Extraction Method on the Fucoidan Yield of the Brown Seaweeds of Gulf of Mannar, India. Indian J. Pharm. Sci. 2017, 79, 65–71. [Google Scholar] [CrossRef]
- Jia Yucui, Y.B.J.Y. Research on extracting technique of fucoidin from Undaria pinnatifuta. China Mod. Med. 2009, 16, 69. [Google Scholar]
- Kim, W.J.; Kim, S.M.; Kim, H.G.; Oh, H.R.; Lee, K.B.; Lee, Y.K.; Park, Y.I. Purification and anticoagulant activity of a fucoidan from Korean Undaria pinnatifida sporophyll. J. Microbiol. Biotechnol. 2007, 21, 1043–1048. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Song, K.M.; Su, J.H.; Lee, J.E.; Kim, S.H.; Yong, H.K.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High yield ultrasonication extraction method for Undaria pinnatifida sporophyll and its anti-inflammatory properties associated with AP-1 pathway suppression. LWT Food Sci. Technol. 2015, 64, 1315–1322. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.; Fang, H. Study on Extraction of sulfated polysaccharides from Chinese Cabbage by complex enzymatic hydrolysis. Food Sci. 1999, 20, 26–29. [Google Scholar]
- Waffenschmidt, S.; Jaenicke, L. Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal. Biochem. 1987, 165, 337–340. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly changes in the content and monosaccharide composition of fucoidan from Undaria pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Hemmingson, J.A.; Falshaw, R.; Furneaux, R.H.; Thompson, K. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta). J. Appl. Phycol. 2006, 18, 185–193. [Google Scholar] [CrossRef]
- Sakai, T.; Ishizuka, K.; Shimanaka, K.; Ikai, K.; Kato, I. Structures of Oligosaccharides Derived from Cladosiphon okamuranus Fucoidan by Digestion with Marine Bacterial Enzymes. Mar. Biotechnol. 2003, 5, 536–544. [Google Scholar] [PubMed]
- Koo, J.G.; Jo, K.S.; Do, J.R.; Woo, S.J. Isolation and purification of fucoidans from Laminaria religiosa and Undaria pinnatifida in Korea. Korean J. Fish. Aquat. Sci. 1995, 28, 227–236. [Google Scholar]
- Park, E.J.; Choi, J.I. Melanogenesis inhibitory effect of low molecular weight fucoidan from Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 2213–2217. [Google Scholar] [CrossRef]
- Qiu, X.; Amarasekara, A.; Doctor, V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr. Polym. 2006, 63, 224–228. [Google Scholar] [CrossRef]
- Synytsya, A.; WooJung, K.; SungMin, K.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; YongIl, P. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Sekkal, M.; Legrand, P. A spectroscopic investigation of the carrageenans and agar in the 1500–100 cm−1 spectral range. Spectrochim. Acta A Mol. Spectr. 1993, 49, 209–221. [Google Scholar] [CrossRef]
- Yuan, M.X.; Fei, W.Y.; Yanmei, Z.; Yanyan, K.; Iiang, Z. PoJysaccharjdes from Undaria pinnatifida. Chin. Tradit. Herbal Drug 2006, 37, 362–365. [Google Scholar]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honya, M.; Mori, H.; Anzai, M.; Araki, Y.; Nisizawa, K. Monthly changes in the content of fucans, their constituent sugars and sulphate in cultured Laminaria japonica. Hydrobiologia 1999, 398–399, 411–416. [Google Scholar] [CrossRef]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal. Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Thakur, V.; Lu, J.; Roscilli, G.; Aurisicchio, L.; Cappelletti, M.; Pavoni, E.; White, W.L.; Bedogni, B. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget 2017, 8, 17887–17899. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; White, W.; Lu, J. Extracts from New Zealand Undaria pinnatifida Containing Fucoxanthin as Potential Functional Biomaterials against Cancer in Vitro. J. Funct. Biomater. 2014, 5, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, J.; Li, W.; Yan, Y.; Liu, M.; Wang, G.; Li, Q.; Yang, B.; Yao, X.; Zheng, J. Expression and clinical significance of the nin one binding protein and p38 MAPK in prostate carcinoma. Int. J. Clin. Exp. Pathol. 2013, 6, 2300–2311. [Google Scholar] [PubMed]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan Derived from Undaria pinnatifida Induces Apoptosis in Human Hepatocellular Carcinoma SMMC-7721 Cells via the ROS-Mediated Mitochondrial Pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lahrsen, E.; Liewert, I.; Alban, S. Gradual degradation of fucoidan from Fucus vesiculosus and its effect on structure, antioxidant and antiproliferative activities. Carbohydr. Polym. 2018, 192, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between Sulfate Groups and Biological Activities of Fucans. Thromb. Res. 2000, 100, 453. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, C.; Cai, Y.; Wang, D.; Fang, Y.U. The study of antioxidant activities of fucoidan from Laminaria japonica. High Technol. Lett. 2005, 11, 91–94. [Google Scholar]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Seng, J.L.; Wan, M.W.A.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift fur Lebensmittel-Untersuchung und Forschung 1993, 196, 329. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.; Ushakova, N.; Zyuzina, K.; Bilan, M.; Elizarova, A.; Somonova, O.; Madzhuga, A.; Krylov, V.; Preobrazhenskaya, M.; Usov, A.; et al. Influence of Fucoidans on Hemostatic System. Mar. Drugs 2013, 11, 2444–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, T.; Nagumo, T.; Kiyohara, H.; Yamada, H. Structural characterization of a new anticoagulant fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res. 1991, 211, 77–90. [Google Scholar] [CrossRef]
- Zayed, A.; Hahn, T.; Rupp, S.; Krämer, R.; Ulber, R. Fucoidan as a natural anticoagulant, antiviral and anti-cancer drug. German Pharm-Tox Summit 2018, 391, S7–S8. [Google Scholar]
- Faggio, C.; Pagano, M.; Morabito, M.; Minicante, S.A.; Arfuso, F.; Genovese, G. In vitro assessment of the effect of Undaria pinnatifida extracts on erythrocytes membrane integrity and blood coagulation parameters of Equus caballus. Coast Life Med. J. 2014, 16, e249. [Google Scholar]
- Casella, S.; Giannetto, C.; Giudice, E.; Marafioti, S.; Fazio, F.; Assenza, A.; Piccione, G. ADP-induced platelet aggregation after addition of tramadol in vitro in fed and fasted horses plasma. Res. Vet. Sci. 2013, 94, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Lippi, G.; Koutts, J. Laboratory testing of anticoagulants: The present and the future. Pathology 2011, 43, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Pereira, M.S. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999, 9, 225. [Google Scholar] [CrossRef]
- Ibtissam, C.; Hassane, R.; Martinezlopez, J.; Seglar, J.F.D.; Vidal, J.A.G.; Hassan, B.; Mohamed, K. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. Afr. J. Biotechnol. 2009, 8, 1258–1262. [Google Scholar]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomin, V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta 2012, 1820, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Song, J.K. Fucoidan from Undaria pinnatifida regulates type II collagen and COX-2 expression via MAPK and PI3K pathways in rabbit articular chondrocytes. Biologia 2017, 72, 1362–1369. [Google Scholar] [CrossRef]
- Tsai, H.; Tai, C.; Huang, C.; Chang, F.; Wang, J. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; Mcguinness, G.; Olesen, I.; Fitton, J.H. The Effect of Undaria pinnatifida Fucoidan on the Pharmacokinetics of Letrozole and Tamoxifen in Patients with Breast Cancer. Integr. Cancer Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Research and Development of Two Kinds of Functional Food. Ph.D. Dissertation, Ocean University of China, Beijing, China, 2011. [Google Scholar]
- Kong, F.D.; Xu, B.; Zu, G.R.; Sun, H.; Liu, Z.F. Study on the processing technology of the fermented beverage of the Chinese Cabbage. China Brew. 2011, 1, 186–189. [Google Scholar]
- Dong, S.; Hou, W.; Dong, J.; Duan, Z.; Zhao, J.; Dong, X. A Kind of Spaghetti and Its Production Method. Chinese Patent No. CN 104509780A, 15 April 2015. [Google Scholar]
- Nagai, T.; Suzuki, N.; Nagashima, T. Angiotensin I-converting enzyme inhibitory activities of beverages made from sea algae and commercially available tea extracts. J. Food Agric. Environ. 2006, 4, 35–48. [Google Scholar]
- Olivero-David, R.; Schultz-Moreira, A.; Vã, Z.M.; Gonzã, L.L.; Bastida, S.; Benedã, J.; Sanchez-Reus, M.I.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Effects of Nori- and Wakame-enriched meats with or without supplementary cholesterol on arylesterase activity, lipaemia and lipoproteinaemia in growing Wistar rats. Br. J. Nutr. 2011, 106, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Lã Pez-Lã Pez, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larrea, M.T.; Sã, N.F.; Cofrades, S.; Nez-Colmenero, F.J. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat. Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Chun, K.R.; Kim, J.K.; Suk, K.; Jung, Y.M.; Lee, W.H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother. Res. 2010, 24, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul. Fibrinol. 2009, 20, 607. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hayashi, K.; Kanekiyo, K.; Ohta, Y.; Jungbum, L.; Hashimoto, M.; Nakano, T.; Torrence, P.F. Promising Antiviral Glyco-Molecules from an Edible Alga; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 166–182. ISBN 978-470-11879-5. [Google Scholar]
- Chen, J.H.; Lim, J.D.; Sohn, E.H.; Choi, Y.S.; Han, E.T. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.R.K.; Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
| Extraction Method | Rationale | Properties | Extraction Conditions | Yield | Reference |
|---|---|---|---|---|---|
| Hot water extraction | A method based on the solubility of fucoidan in hot water and the insolubility in ethanol and other organic solvents. | Low cost, simple operation, but time-consuming and using a large amount of solvent. | U. pinnatifida was washed by ethanol at 80 °C for 1 h, then was extracted in 60-time weight of water for 7 h at 100 °C. Supernatant was precipitated with ethanol. | 12.9% | Jia et al. [15] |
| Dilute acid extraction | Based on the solubility of fucoidan in dilute hydrochloric acid aqueous solution. It is difficult for fucoidan to dissolve at lower pH value but easy for part of its sodium salt. | The extraction rate is not high, and the structure of fucoidan is easy to be destroyed which affects the bioactivity. | 250 g dried sporophyll was added to 4 L of 0.1 N HCl for 24 h at ambient temperature. The extract was filtered, and the filtrate was neutralized with 1 N NaOH. Fucoidan was precipitated with 3:1 volume of 75% ethanol. | 3.9% | Kim et al. [16] |
| Microwave-assisted extraction | Microwave radiation penetration has high energy, which can shortening extraction time to improve efficiency and reduces the use of organic solvent. | Fucoidan is extracted more selectively and quicker with better yields, using less energy and solvent, reducing costs and waste, and less destructive to the structure | 1 g milled dry seaweed was suspended in 25 mL of distilled water and placed into the extraction vessel. The suspensions were irradiated under 120 psi pressure for 1 min. | 18.2% | Rodriguez-Jasso et al. [17] |
| Ultra-sonication extraction | Ultrasonication produces cavitation, directs dynamic shock waves on the surface of materials. It breaks the cell wall of organic materials and facilitate the extraction of fucoidan. | Higher extraction yields and lower damage to fucoidan structure | 10 g powdered materials were added to 0.1 N HCl (PH = 2) and treated by ultrasonication at room temperature, 80% amplitude for 6 h. The supernatant was neutralized (PH = 7) with 0.1 N NaOH. | 33.0 ± 0.4% | Song et al. [18] |
| Ultra-filtration membrane extraction | It is the use of enzymes to disrupt the structure of cell walls, promote the dissolution of fucoidan, and greatly shorten the extraction time. | Extract fucoidan effectively while maintaining its structure and biological activity | Algae powder:water at 1:20 ratio was pressed at 25 °C to become seaweed slurry. Slurry at PH = 6.0 was added 2.0% enzyme and reacted for 2 h at 40 °C, then heated to 80 °C rapidly and extracted for 1 h, centrifuged, dialyzed, precipitated by ethanol, and finally dried. | 7.76% | Wang et al. [19] |
| Region | Process | Mass Fraction | Ref. |
|---|---|---|---|
| China | Extracted and purified polysaccharide from Wakame | 43.20% polysaccharide, 12.70% sulfate, 9.78% glucuronic acid | [2] |
| South Korea | Purified fucoidan by HPLC | 52.34% neutral sugar, 26.2% uronic acid, 7.4% sulfate ester | [16] |
| New Zealand | U. pinnatifida was harvested from Port Underwood, New Zealand | Monosaccharide composition: fucose (39.24%), xylose (28.85%), galactose (26.48%), mannose (5.04%), glucose (0.95%). The minor components: sulfate (15.02%), uronic acid (1.24%), and protein (0.36%). | [30] |
| Function | Origin | Effective Molecule/Fraction | Mode of Action | Ref. |
|---|---|---|---|---|
| Anti-lung carcinoma | Japan | Low molecular weight fucoidan fraction (5–30 kDa) | Induce apoptotic damage to A-549 cell lines | [35] |
| Anti-colon adenocarcinoma | Japan | Low molecular weight fucoidan fraction (5–30 kDa) | Induce apoptotic damage to WiDr cell lines. | [35] |
| Anti-breast cancer | Japan | A partially acetylated galactofucan with a high degree of sulfation and its main chain is built up of (1→3)- and/or (1→4)-a-l-fucopyranose residues. | Induce apoptosis in cancer cell lines as well as having antimetastatic activity blocking the interactions between cancer cells and the basement membrane. | [2] |
| Anti-melanoma | Japan | Same as the above. | Same as the above. | [2] |
| Korea | Fucoidan with low molecular weights of 89, 35, 17, and 6 kDa (prepared by radiation-degradation of a 378 kDa fucoidan) | Fucoidan inhibits tyrosinase and increases radical scavenging activity. | [26] | |
| Antioxidant | Korea | Fucoidan with high sulfate content | Fucoidan directly scavenges the free radicals produced inside the body, effectively abrogate oxidative stress. | [47] |
| Anticoagulant | Italy | Sulfonated polysaccharides of fucoidan | The sulfonated polysaccharides interfere with both the extrinsic and intrinsic pathways of coagulation inhibiting clot formation, that has an action similar to that carried out by heparin. | [51] |
| Antibacterial | Korea | Sulphated polysaccharides of fucoidan | Inhibition of peptidoglycan formation, or the presence of special cell wall components of Gram-negative bacteria that act as a barrier for fucoidan. | [55] |
| Functional Food | Processes/Preparation | Related Parameters | Food Sensory Evaluation | Results | Ref. |
|---|---|---|---|---|---|
| Cookie | Raw materials were mixed with water to form a dough piece with 2 mm thick, finally baked | Wakame powder 20% | Crisp taste, delicious flavor | Have the effect of reducing weight and blood glucose. | [63] |
| Beverages | Raw juice was obtained after U. pinnatifida was fermented by 8% inoculated yeast at 30 °C for 24 h. | 50% raw wakame juice. | Rich aroma of wakame and fermentation, sweet and sour taste, no smell and other odor. | Lowering blood pressure, lipids, and cholesterols, impeding platelet aggregation and preventing arteriosclerosis. | [64] |
| Noodles | Wakame, wheat flour and wheat gluten flour were used. | No details | Yellow-green color, slight fragrance of wakame. | Suitable for patients with high blood pressure, glucose and lipids | [65] |
| CVDs-related parameters in foods using U. pinnatifida | |||||
| Tea | Wakame was dried with hot air, crushed to pieces, boiled with water, and filtrated. | ACE inhibition IC50: 26.4 ± 1.05 mg/mL. | Green color | Contains rich minerals and suppresses hypertensive activity of angiotensin I. | [66] |
| Restructured meat | Dried wakame was homogenized with raw meat. | U. pinnatifida at 5% | No details | Moderately ameliorated lipid profile in hypercholesterolemic rats. | [67] |
| Gel/emulsion meat systems | No details | U. pinnatifida at 5.6% | No details | Increase n-3 PUFA and antioxidant, Decrease n-6/n-3 PUFA ratio and sodium. | [68] |
| Therapeutic Items | Trial | Administration | Observation | Ref. |
|---|---|---|---|---|
| Arthritis | Collagen-induced arthritis in mice | 100 kDa and 1 kDa fractions orally administered daily 300 mg/kg for 49 days | 1 kDa fraction effectively inhibited whereas 100 kDa exacerbated disease | [69] |
| Blood Homeostasis | Healthy human subjects | 3 g of U. pinnatifida fucoidan were ingested daily for 12 days by healthy subjects | A significant prolongation of global clotting time was noted | [70] |
| Influenza | In vivo | Orally delivered less than 1 mcg/mL | A marked inhibitory effect on the recent H1N1 Influenza A virus | [71] |
| Mouse model | 5 mg per day orally delivered | Strongly inhibited Influenza A infection | [72] | |
| Malaria | Plasmodium berghei-infected mice | Orally delivered for 4 days | A 37% suppressive effect and a significant delay in the deaths from anemia | [73] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. https://doi.org/10.3390/md16090321
Zhao Y, Zheng Y, Wang J, Ma S, Yu Y, White WL, Yang S, Yang F, Lu J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Marine Drugs. 2018; 16(9):321. https://doi.org/10.3390/md16090321
Chicago/Turabian StyleZhao, Yu, Yizhou Zheng, Jie Wang, Shuyi Ma, Yiming Yu, William Lindsey White, Shiping Yang, Fan Yang, and Jun Lu. 2018. "Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods" Marine Drugs 16, no. 9: 321. https://doi.org/10.3390/md16090321
APA StyleZhao, Y., Zheng, Y., Wang, J., Ma, S., Yu, Y., White, W. L., Yang, S., Yang, F., & Lu, J. (2018). Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Marine Drugs, 16(9), 321. https://doi.org/10.3390/md16090321






