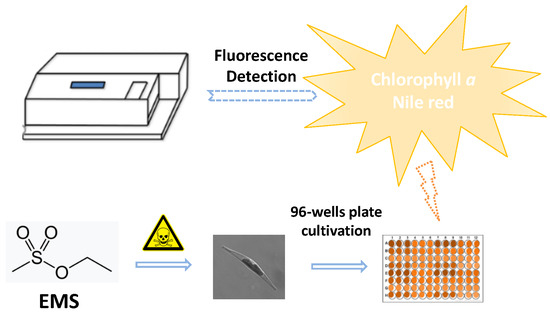
Chemical Mutagenesis and Fluorescence-Based High-Throughput Screening for Enhanced Accumulation of Carotenoids in a Model Marine Diatom Phaeodactylum tricornutum
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Doses of DPA on P. tricornutum Growth
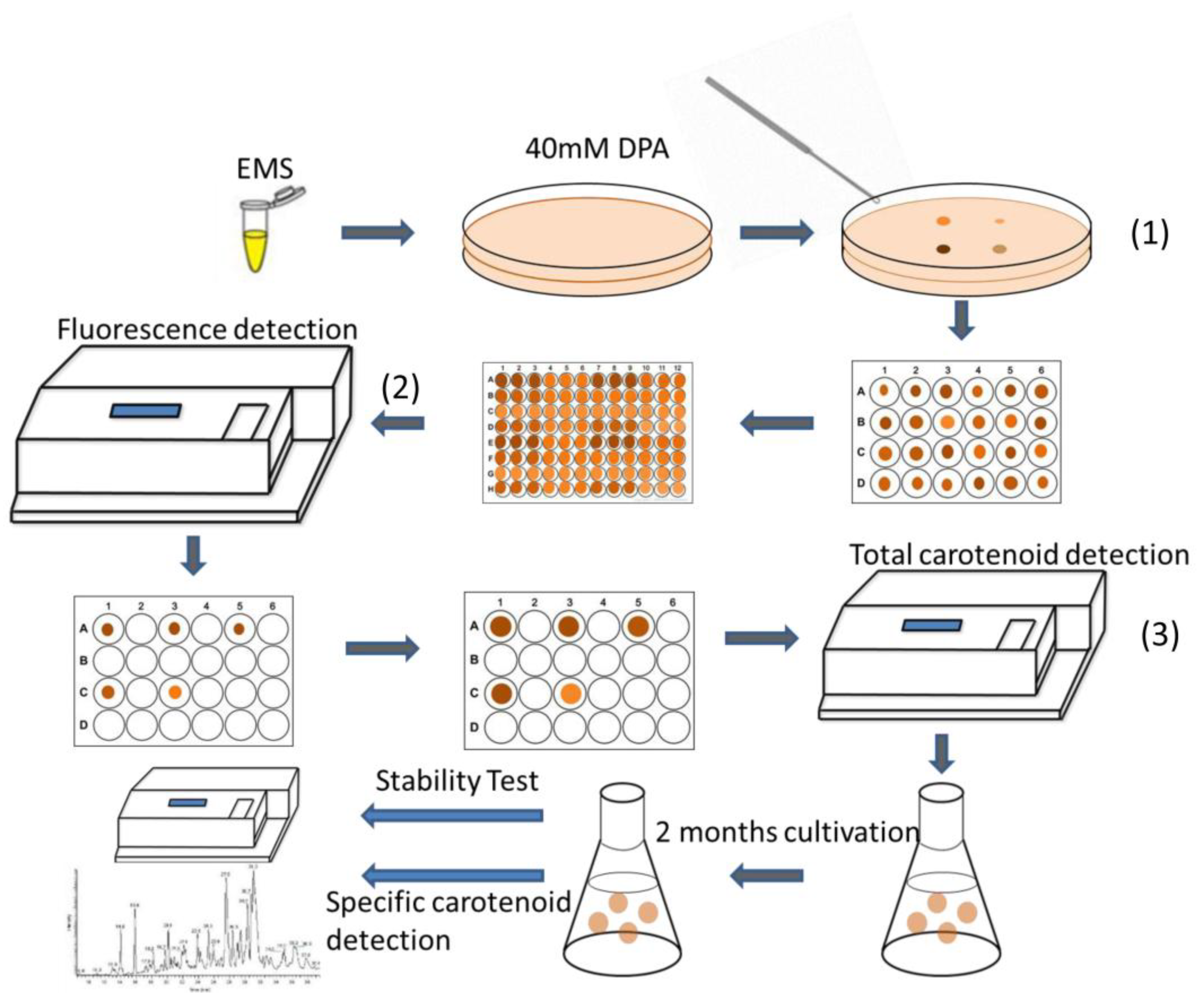
2.2. Effects of EMS and NTG on Creating Positive Mutants
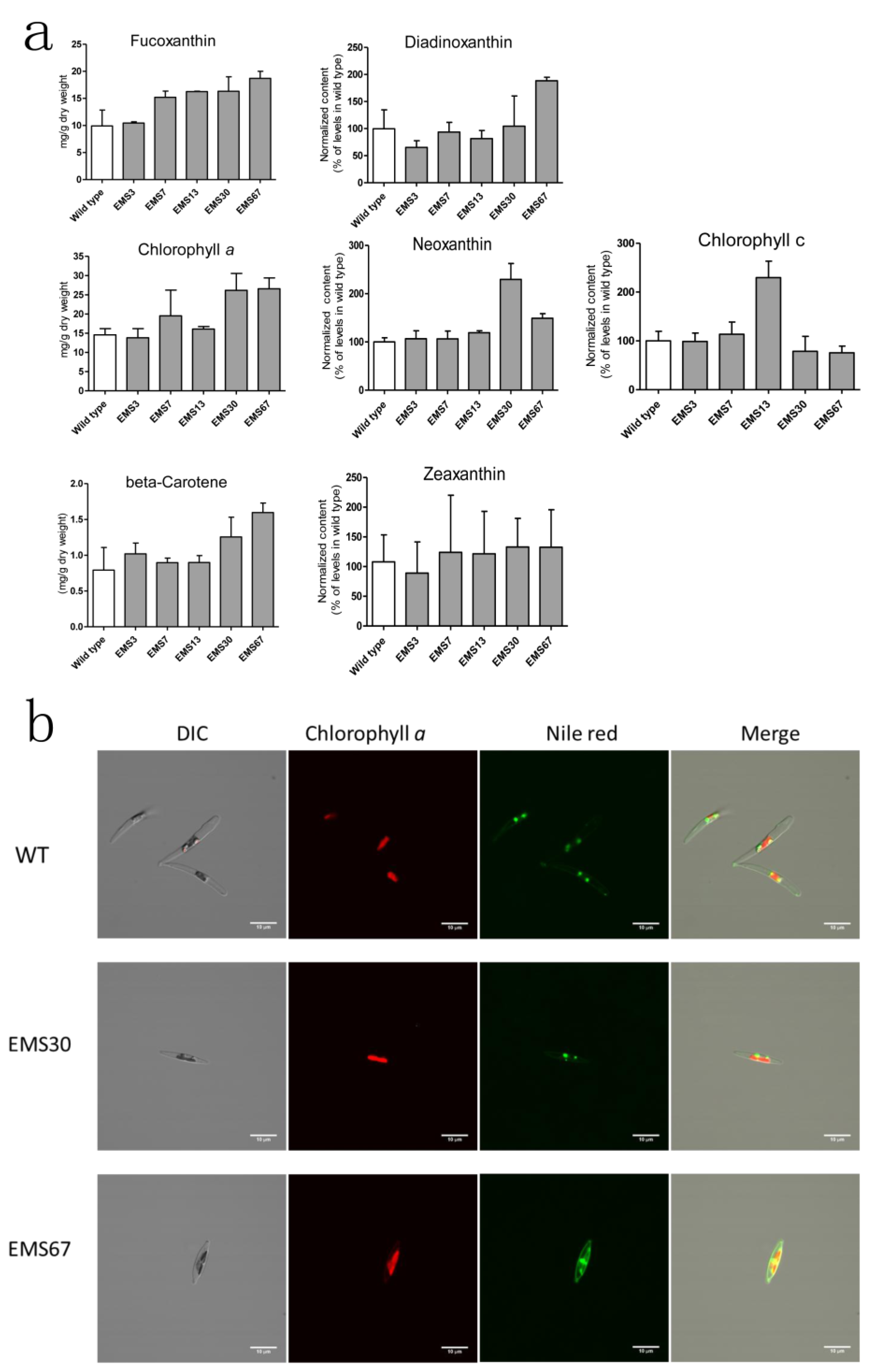
2.3. Correlations of Both Chlorophyll a and Lipids with Carotenoid Metabolism
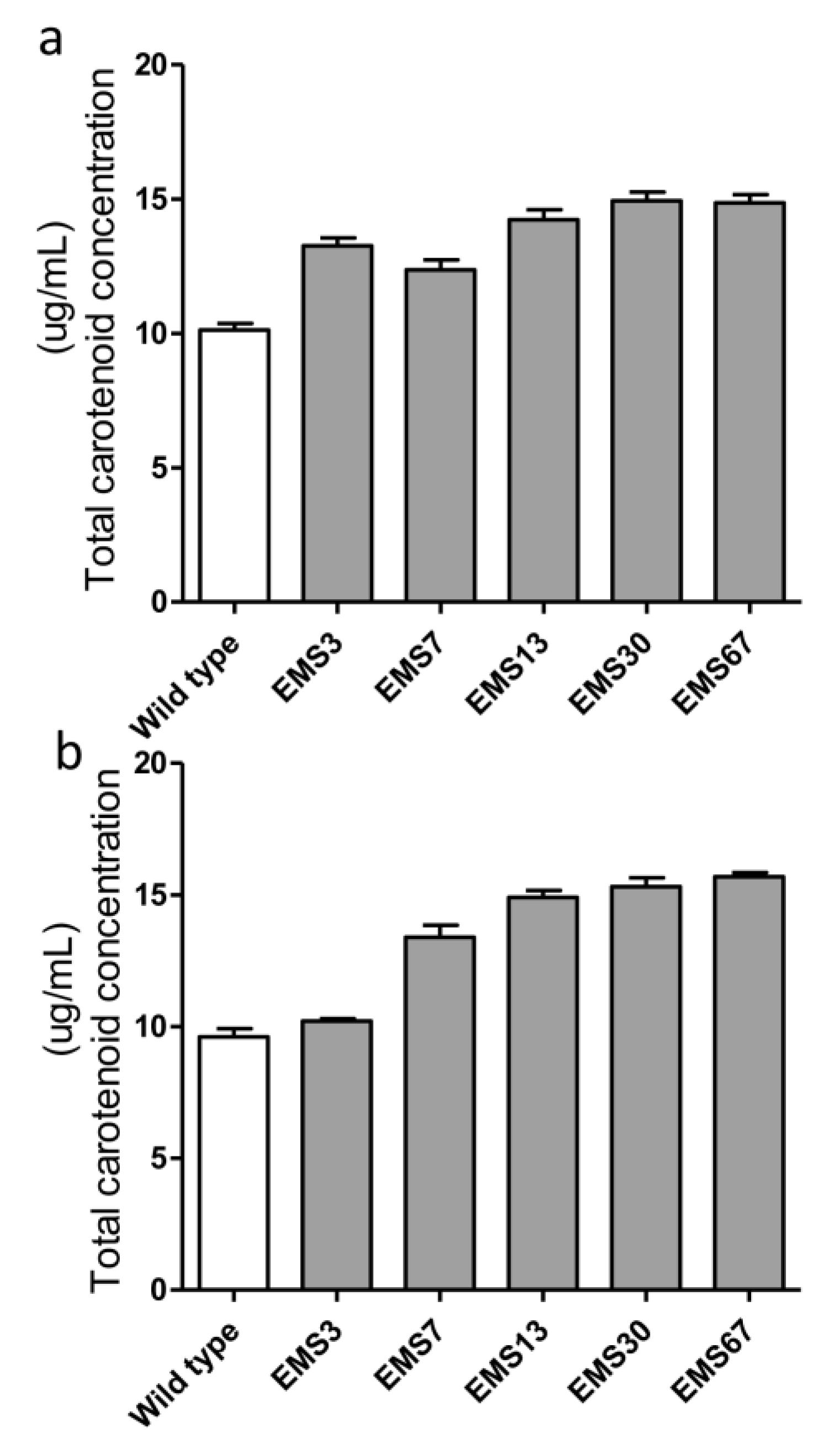
2.4. Detection and Analysis of Major Pigments and Lipids in the Diatom Dtrains
2.5. Assessment of Selected Mutant Stability for Carotenoid Accumulation
3. Discussion
4. Material and Methods
4.1. Cells and Chemicals
4.2. Diatom Culture and Growth
4.3. EMS and NTG Mutagenesis
4.4. Herbicide Test
4.5. Chlorophyll a Fluorescence and Nile Red Staining Measurement
4.6. Confocal Imaging
4.7. Spectrophotometer for Pigment Detection
4.8. LC-MS Determination and Analysis of Major Pigments
4.9. Metabolic Modeling Analysis
4.10. Data Processing and Analysis
4.11. High-Throughput Screening Method
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Magnusdottir, M.; Zhang, Y.; Brynjolfsson, S.; Fu, W. Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar. Drugs 2015, 13, 6138–6151. [Google Scholar] [CrossRef] [PubMed]
- De, M.A.; Agnès, M.; Juan, S.; Kehou, P.; Chris, B. Genetic and phenotypic characterization of phaeodactylum tricornutum (bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847. [Google Scholar] [CrossRef] [PubMed]
- Veith, T.; Büchel, C. The monomeric photosystem i-complex of the diatom phaeodactylum tricornutum binds specific fucoxanthin chlorophyll proteins (fcps) as light-harvesting complexes. Biochim. Biophys. Acta (BBA)-Bioenerget. 2007, 1767, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wichuk, K.; Brynjólfsson, S. Developing diatoms for value-added products: Challenges and opportunities. New Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: The Netherlands, Amsterdam, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Fu, W.; Chaiboonchoe, A.; Khraiwesh, B.; Sultana, M.; Jaiswal, A.; Jijakli, K.; Nelson, D.R.; Al-Hrout, A.A.; Baig, B.; Amin, A.; et al. Intracellular spectral recompositioning of light enhances algal photosynthetic efficiency. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S. Genetic and Biotechnological Development of the Pennate Marine Diatom Phaeodactylum Tricornutum for High-Value Bioproducts and Carbon Bio-Mitigation. Ph.D. Thesis, National University of Ireland, Galway, Ireland, 2014. [Google Scholar]
- Kamath, B.S.; Vidhyavathi, R.; Sarada, R.; Ravishankar, G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in haematococcus pluvialis mutants. Bioresour. Technol. 2008, 99, 8667–8673. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Yang, G.P.; Han, J.C.; Thomsen, L.; Pan, K.H. Breeding 3 elite strains of nannochloropsis oceanica by nitrosoguanidine mutagenesis and robust screening. Algal. Res. 2016, 19, 104–108. [Google Scholar] [CrossRef]
- Tripathi, U.; Venkateshwaran, G.; Sarada, R.; Ravishankar, G. Studies on haematococcus pluvialis for improved production of astaxanthin by mutagenesis. World J. Microbiol. Biotechnol. 2001, 17, 143–148. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Lu, W.; Xing, J.; Hui, B.; Han, Y. Screening and characterization of astaxanthin-hyperproducing mutants of haematococcus pluvialis. Biotechnol. Lett. 2003, 25, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.I.; Inostroza, I.; Pizarro, M.; Pérez, J. From genetic improvement to commercial-scale mass culture of a chilean strain of the green microalga haematococcus pluvialis with enhanced productivity of the red ketocarotenoid astaxanthin. AoB Plants 2013, 5, plt026. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Guan, B.; Kong, Q.; Sun, H.; Geng, Z.; Duan, L. Enhancement of astaxanthin production from haematococcus pluvialis mutants by three-stage mutagenesis breeding. J. Biotechnol. 2016, 236, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Tillich, U.M.; Lehmann, S.; Schulze, K.; Duhring, U.; Frohme, M. The optimal mutagen dosage to induce point-mutations in synechocystis sp pcc6803 and its application to promote temperature tolerance. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef]
- De Riso, V.; Raniello, R.; Maumus, F.; Rogato, A.; Bowler, C.; Falciatore, A. Gene silencing in the marine diatom phaeodactylum tricornutum. Nucleic Acids Res. 2009, 37, e96. [Google Scholar] [CrossRef] [PubMed]
- Daboussi, F.; Leduc, S.; Marechal, A.; Dubois, G.; Guyot, V.; Perez-Michaut, C.; Amato, A.; Falciatore, A.; Juillerat, A.; Beurdeley, M.; et al. Genome engineering empowers the diatom phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014, 5, 3831. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, H.; de la Jara, A.; Freijanes, K.; Carmona, L.; Ramos, A.A.; de Sousa Duarte, V.; Serafim Varela, J.C. Characterization of dunaliella salina strains by flow cytometry: A new approach to select carotenoid hyperproducing strains. Electron. J. Biotechnol. 2008, 11, 5–6. [Google Scholar] [CrossRef]
- Levering, J.; Broddrick, J.; Dupont, C.L.; Peers, G.; Beeri, K.; Mayers, J.; Gallina, A.A.; Allen, A.E.; Palsson, B.O.; Zengler, K. Genome-scale model reveals metabolic basis of biomass partitioning in a model diatom. PLoS ONE 2016, 11, e0155038. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, B.; Huang, A.; Huan, L.; He, L.; Lin, A.; Niu, J.; Wang, G. Detection of intracellular neutral lipid content in the marine microalgae prorocentrum micans and phaeodactylum tricornutum using nile red and bodipy 505/515. J. Appl. Phycol. 2014, 26, 1659–1668. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Beyer, P.; Lintig, J.V.; Hugueney, P.; Kleinig, H. Induced β-carotene synthesis driven by triacylglycerol deposition in the unicellular alga dunaliella bardawil. Plant Physiol. 1998, 116, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, H.; Martel, A.; Jiménez del Río, M.; García Reina, G. Oleic acid is the main fatty acid related with carotenogenesis in dunaliella salina. J. Appl. Phycol. 1999, 11, 15–19. [Google Scholar] [CrossRef]
- Grünewald, K.; Hirschberg, J.; Hagen, C. Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga haematococcus pluvialis. Biol. Chem. 2001, 276, 6023–6029. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Veith, T.; Brauns, J.; Weisheit, W.; Mittag, M.; Buchel, C. Identification of a specific fucoxanthin-chlorophyll protein in the light harvesting complex of photosystem i in the diatom cyclotella meneghiniana. Biochim. Biophys. Acta (BBA)-Bioenerget. 2009, 1787, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Zigmantas, D.; Hiller, R.G.; Sharples, F.P.; Frank, H.A.; Sundström, V.; Polívka, T. Effect of a conjugated carbonyl group on the photophysical properties of carotenoids. Phys. Chem. Chem. Phys. 2004, 6, 3009–3016. [Google Scholar] [CrossRef]
- Ikehata, H.; Ono, T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.B.; Talebi, A.B.; Shahrokhifar, B. Ethyl methane sulphonate (EMS) induced mutagenesis in malaysian rice (cv. Mr219) for lethal dose determination. Am. J. Plant Sci. 2012, 3, 1661. [Google Scholar] [CrossRef]
- Bode, S.; Quentmeier, C.C.; Liao, P.N.; Hafi, N.; Barros, T.; Wilk, L.; Bittner, F.; Walla, P.J. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. USA 2009, 106, 12311–12316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khozin-Goldberg, I.; Cohen, Z. Unraveling algal lipid metabolism: Recent advances in gene identification. Biochimie 2011, 93, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Mirón, A.; Cerón Garcı́a, M.C.; Garcı́a Camacho, F.; Molina Grima, E.; Chisti, Y. Growth and biochemical characterization of microalgal biomass produced in bubble column and airlift photobioreactors: Studies in fed-batch culture. Enzyme Microb. Technol. 2002, 31, 1015–1023. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Hall, D.O.; Guerrero, E.C.; Rao, K.K.; Grima, E.M. Outdoor production of phaeodactylum tricornutum biomass in a helical reactor. J. Biotechnol. 2003, 103, 137–152. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.Ø.; Paglia, G. UPLC-UV-MSE analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Vavitsas, K.; Rue, E.Ø.; Stefánsdóttir, L.K.; Gnanasekaran, T.; Blennow, A.; Crocoll, C.; Gudmundsson, S.; Jensen, P.E. Responses of synechocystis sp. Pcc 6803 to heterologous biosynthetic pathways. Microb. Cell Fact. 2017, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Su, Y.; Xu, M.; Bergmann, A.; Ingthorsson, S.; Rolfsson, O.; Salehi-Ashtiani, K.; Brynjolfsson, S.; Fu, W. Chemical Mutagenesis and Fluorescence-Based High-Throughput Screening for Enhanced Accumulation of Carotenoids in a Model Marine Diatom Phaeodactylum tricornutum. Mar. Drugs 2018, 16, 272. https://doi.org/10.3390/md16080272
Yi Z, Su Y, Xu M, Bergmann A, Ingthorsson S, Rolfsson O, Salehi-Ashtiani K, Brynjolfsson S, Fu W. Chemical Mutagenesis and Fluorescence-Based High-Throughput Screening for Enhanced Accumulation of Carotenoids in a Model Marine Diatom Phaeodactylum tricornutum. Marine Drugs. 2018; 16(8):272. https://doi.org/10.3390/md16080272
Chicago/Turabian StyleYi, Zhiqian, Yixi Su, Maonian Xu, Andreas Bergmann, Saevar Ingthorsson, Ottar Rolfsson, Kourosh Salehi-Ashtiani, Sigurdur Brynjolfsson, and Weiqi Fu. 2018. "Chemical Mutagenesis and Fluorescence-Based High-Throughput Screening for Enhanced Accumulation of Carotenoids in a Model Marine Diatom Phaeodactylum tricornutum" Marine Drugs 16, no. 8: 272. https://doi.org/10.3390/md16080272