(−)-Homosalinosporamide A and Its Mode of Proteasome Inhibition: An X-ray Crystallographic Study
Abstract
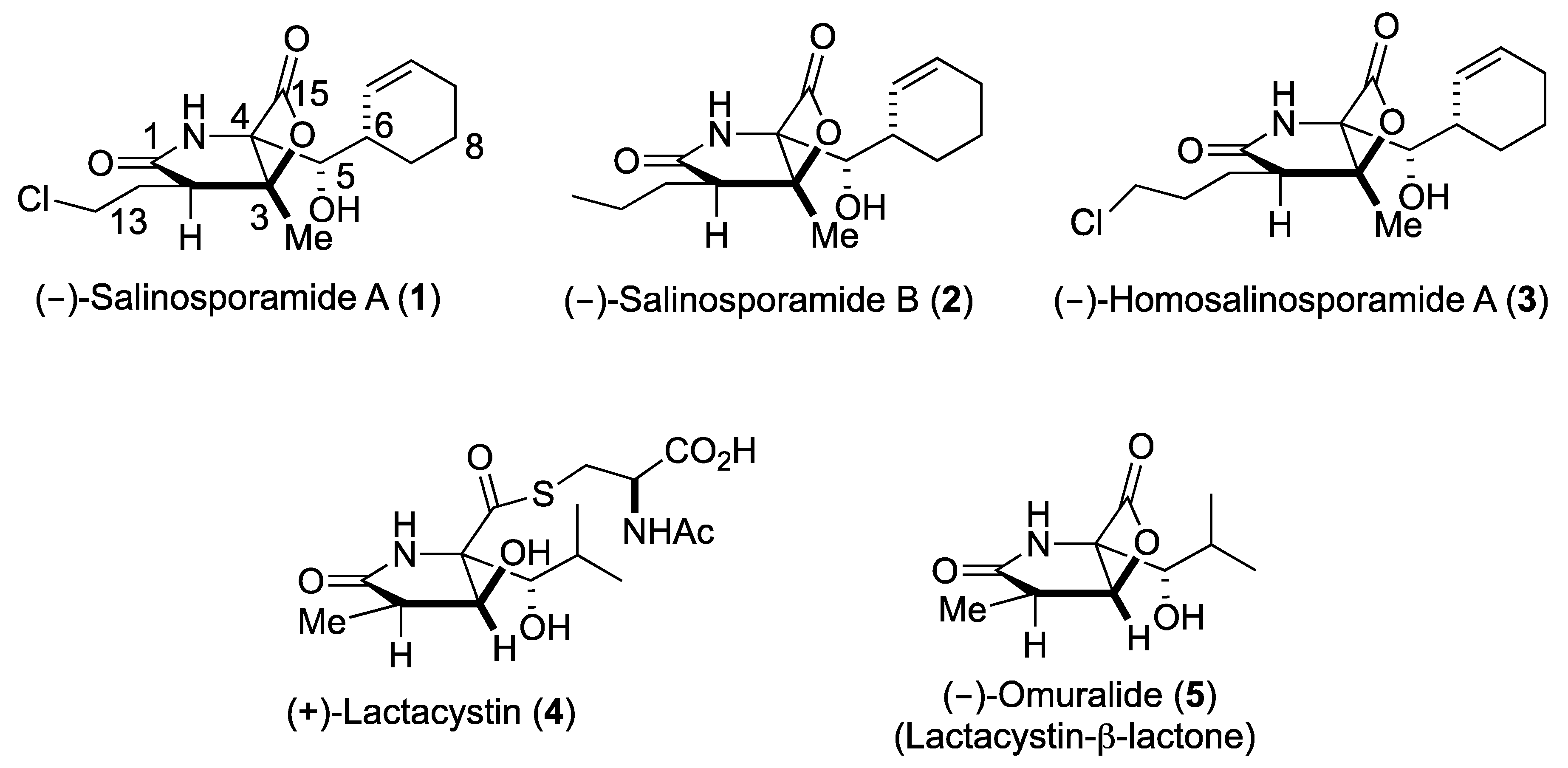
:1. Introduction
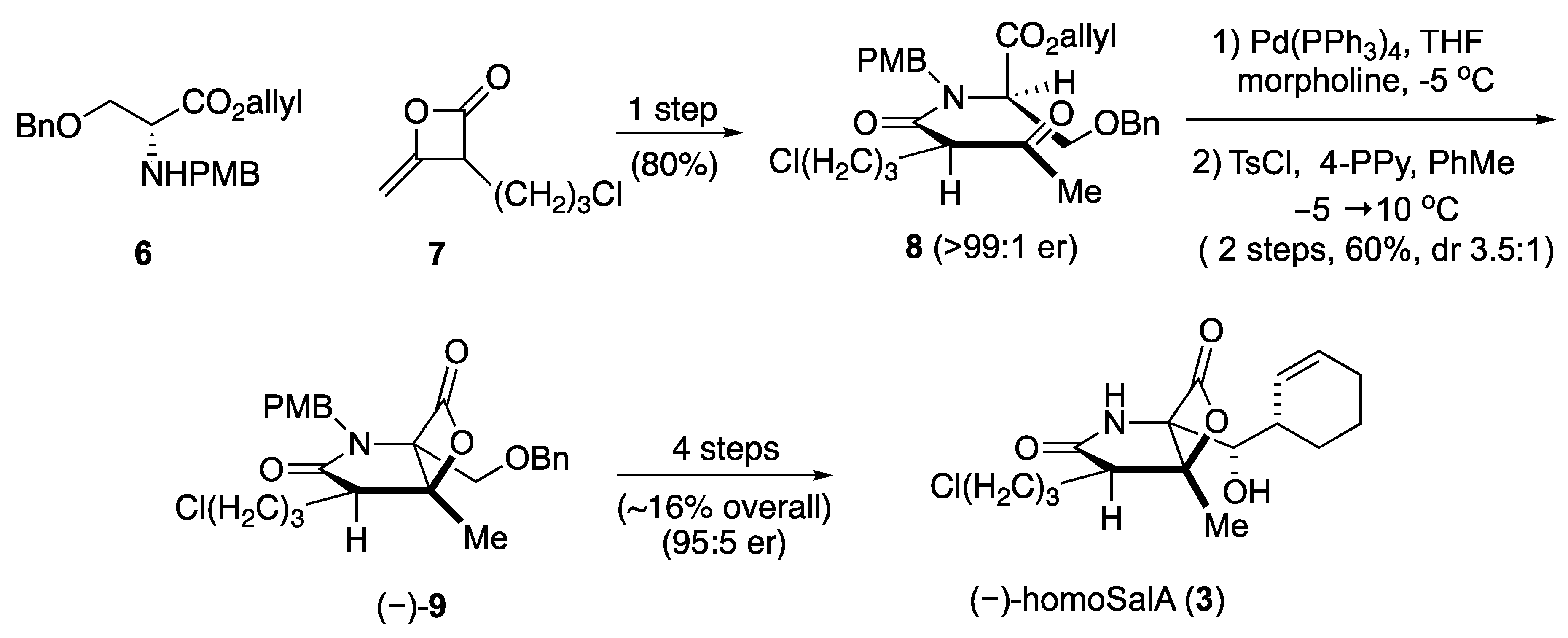
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Voorhees, P.M.; Dees, E.C.; O’Neil, B.; Orlowski, R.Z. The proteasome as a target for cancer therapy. Clin. Cancer Res. 2003, 9, 6316–6325. [Google Scholar] [PubMed]
- Huber, E.M.; Heinemeyer, W.; Groll, M. Bortezomib-resistant mutant proteasomes: Structural and biochemical evaluation with carfilzomib and ONX 0914. Structure 2015, 23, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Scalzulli, E.; Grammatico, S.; Vozella, F.; Petrucci, M.T. Proteasome inhibitors for the treatment of multiple myeloma. Expert Opin. Pharmacother. 2018, 19, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Arastu-Kapur, S.; Anderl, J.L.; Kraus, M.; Parlati, F.; Shenk, K.D.; Lee, S.J.; Muchamuel, T.; Bennett, M.K.; Driessen, C.; Ball, A.J.; et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clin. Cancer Res. 2011, 17, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Gräwert, M.A.; Groll, M. Exploiting nature’s rich source of proteasome inhibitors as starting points in drug development. Chem. Commun. 2012, 48, 1364–1378. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.M.; Groll, M. Inhibitors for the immuno- and constitutive proteasome: Current and future trends in drug development. Angew. Chem. Int. Ed. Engl. 2012, 51, 8708–8720. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Matsuzaki, K.; Fujimoto, T.; Kosuge, K.; Furuya, T.; Fujita, S.; Nakagawa, A.J. Structure of Lactacystin, a new microbial metabolite which induces differentiation of neuroblastoma cells. J. Antibiot. 1991, 44, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Fenteany, G.; Standaert, R.F.; Lane, W.S.; Choi, S.; Corey, E.J.; Schreiber, S.L. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 1995, 268, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Ditzel, L.; Löwe, J.; Stock, D.; Bochtler, M.; Bartunik, H.D.; Huber, R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 1997, 386, 463. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Huber, R.; Potts, B.C.M. Crystal structures of salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of β-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. Soc. 2006, 128, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Catley, L.; Li, G.; Podar, K.; Hideshima, T.; Velankar, M.; Mitsiades, C.; Mitsiades, N.; Yasui, H.; Letai, A.; et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 2005, 8, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenical, W.; Jensen, P.R.; Palladino, M.A.; Lam, K.S.; Lloyd, G.K.; Potts, B.C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulder, T.A.M.; Moore, B.S. Salinosporamide natural products: Potent 20S proteasome inhibitors as promising cancer chemotherapeutics. Angew. Chem. Int. Ed. Engl. 2010, 49, 9346–9367. [Google Scholar] [CrossRef] [PubMed]
- Macherla, V.R.; Mitchell, S.S.; Manam, R.R.; Reed, K.A.; Chao, T.-H.; Nicholson, B.; Deyanat-Yazdi, G.; Mai, B.; Jensen, P.R.; Fenical, W.F.; et al. Structure-activity relationship studies of salinosporamide A (NPI-0052), a novel marine derived proteasome inhibitor. J. Med. Chem. 2005, 48, 3684–3687. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.C.; Corey, E.J. Proteasome inhibition by a totally synthetic β-lactam related to salinosporamide A and omuralide. J. Am. Chem. Soc. 2005, 127, 15386–15387. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.R.; Saravanan, P.; Corey, E.J. A simple stereocontrolled synthesis of salinosporamide A. J. Am. Chem. Soc. 2004, 126, 6230–6231. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.R.; Fournier, J.-F.; Reddy, B.V.S.; Corey, E.J. New synthetic route for the enantioselective total synthesis of salinosporamide A and biologically active analogues. Org. Lett. 2005, 7, 2699–2701. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Danishefsky, S.J. Total synthesis of salinosporamide A. J. Am. Chem. Soc. 2005, 127, 8298–8299. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Midori, M.; Kawano, K.; Ishihara, J.; Hatakeyama, S. Entry to heterocycles based on indium-catalyzed conia-ene reactions: Asymmetric synthesis of (−)-salinosporamide A. Angew. Chem. Int. Ed. 2008, 47, 6244–6246. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Sugiyama, K.; Arima, S.; Harigaya, Y.; Nagamitsu, T.; Omura, S. Total synthesis of salinosporamide A. Org. Lett. 2008, 10, 4239–4242. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Yokoshima, S.; Fukuyama, T. Total synthesis of salinosporamide A. Org. Lett. 2011, 13, 3028–3031. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.L.; Moore, B.S. Biosynthetic convergence of salinosporamides A and B in the marine actinomycete salinispora tropica. Org. Lett. 2007, 9, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Eustaquio, A.S.; Moore, B.S. Mutasynthesis of fluorosalinosporamide, a potent and reversible inhibitor of the proteasome. Angew. Chem. Int. Ed. 2008, 47, 3936–3938. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; Gulder, T.A.M.; Kale, A.J.; Andrew, J.; Hughes, C.C.; Moore, B.S. Function-oriented biosynthesis of β-lactone proteasome inhibitors in salinispora tropica. J. Med. Chem. 2009, 52, 6163–6167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hazzard, C.; Eustaquio, A.S.; Reynolds, K.A.; Moore, B.S. Biosynthesis of salinosporamides from α,β-unsaturated fatty acids: Implications for extending polyketide synthase diversity. J. Am. Chem. Soc. 2009, 131, 10376–10377. [Google Scholar] [CrossRef] [PubMed]
- Eusthquio, A.S.; McGlinchey, R.P.; Liu, Y.; Hazzard, C.; Beer, L.L.; Florova, G.; Alhamadsheh, M.M.; Lechner, A.; Kale, A.J.; Kobayashi, Y.; et al. Biosynthesis of the salinosporamide A polyketide synthase substrate chloroethylmalonyl-coenzyme A from S-adenosyl-l-methionine. Proc. Natl. Acad. Sci. USA 2009, 106, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Potts, B.C. Proteasome structure, function, and lessons learned from beta-lactone inhibitors. Curr. Top. Med. Chem. 2011, 11, 2850–2878. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Ma, G.; Romo, D. A1,3-strain enabled retention of chirality during bis-cyclization of β-ketoamides: Total synthesis of (−)-salinosporamide A and (−)-homosalinosporamide A. Chem. Commun. 2010, 46, 4803–4805. [Google Scholar] [CrossRef] [PubMed]
- Cortez, G.S.; Tennyson, R.; Romo, D. Intramolecular, nucleophile-catalyzed aldol-lactonization (NCAL) reactions: Catalytic, asymmetric synthesis of bicyclic β-lactones. J. Am. Chem. Soc. 2001, 123, 7945–7946. [Google Scholar] [CrossRef] [PubMed]
- Cortez, G.S.; Oh, S.-H.; Romo, D. Bicyclic β-lactones via intramolecular NCAL reactions with cinchona alkaloids: Effect of the C9-substituent on enantioselectivity and catalyst conformation. Synthesis 2001, 11, 1731–1736. [Google Scholar] [CrossRef]
- Oh, S.-H.; Cortez, G.S.; Romo, D. Asymmetric synthesis of bicyclic β-lactones via the intramolecular, nucleophile-catalyzed aldol lactonization: Improved efficiency and expanded scope. J. Org. Chem. 2005, 70, 2835–2838. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Jacobsen, E.N. α, β-Unsaturated β-Silyl Imide Substrates for Catalytic, Enantioselective Conjugate Additions: A Total Synthesis of (+)-Lactacystin and the Discovery of a New Proteasome Inhibitor. J. Am. Chem. Soc. 2006, 128, 6810–6812. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Balskus, E.P.; Jacobsen, E.N. Structural analysis of spiro β-Lactone proteasome inhibitors. J. Am. Chem. Soc. 2008, 130, 14981–14983. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Ma, G.; Gladysheva, T.; Fremgen, T.; Romo, D. Bioinspired total synthesis and human proteasome inhibitory activity of (−)-salinosporamide A, (−)-homosalinosporamide A, and derivatives obtained via organonucleophile promoted bis-cyclizations. J. Org. Chem. 2011, 76, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Huber, R. Purification, crystallization and X-ray analysis of the yeast 20S proteasomes. Methods Enzymol. 2005, 398, 329–336. [Google Scholar] [PubMed]
- Groll, M.; Gallastegui, N. Analysing properties of proteasome inhibitors using kinetic and X-ray crystallographic studies. Methods Mol. Biol. 2012, 832, 373–390. [Google Scholar]
- Huber, E.M.; Heinemeyer, W.; Li, X.; Arendt, C.S.; Hochstrasser, M.; Groll, M. A unified mechanism for proteolysis and autocatalytic activation in the 20S proteasome. Nat. Commun. 2016, 7, 10900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.L.; Cui, H.; Beck, P.; Dubiella, C.; Voss, C.; Krüger, A.; Schmidt, B.; Groll, M. Systematic comparison of peptidic proteasome inhibitors highlights the α-ketoamide electrophile as an auspicious reversible lead motif. Angew. Chem. Int. Ed. Engl. 2014, 53, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Manam, R.R.; McArthur, K.A.; Chao, T.H.; Weiss, J.; Ali, J.A.; Palombella, V.J.; Groll, M.; Lloyd, G.K.; Palladino, M.A.; Neuteboom, S.T.; et al. Leaving groups prolong the duration of 20S proteasome inhibition and enhance the potency of salinosporamides. J. Med. Chem. 2008, 51, 6711–6724. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; McArthur, K.A.; Macherla, V.R.; Manam, R.R.; Potts, B.C. Snapshots of the Fluorosalinosporamide/20S Complex Offer Mechanistic Insights for Fine Tuning Proteasome Inhibition. J. Med. Chem. 2009, 52, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 1993, 26, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Brünger, A.T.; Adams, P.D.; Clore, G.M.; DeLano, W.L.; Gros, P.; Grosse-Kunstleve, R.W.; Jiang, J.-S.; Kuszewski, J.; Nilges, M.; Pannu, N.S.; et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998, 1, 905–921. [Google Scholar]
- Brünger, A.T. X-PLOR Version 3.1: A System for X-ray Crystallography and NMR; Yale University Press: New Haven, CT, USA, 1992. [Google Scholar]
- Turk, D. MAIN software for density averaging, model building, structure refinement and validation. Acta Crystallogr. D Biol. Crystallogr. 2013, D69, 1342–1357. [Google Scholar] [CrossRef] [PubMed]
- Brünger, A.T.; Free, R. value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 1992, 355, 472–475. [Google Scholar] [CrossRef] [PubMed]

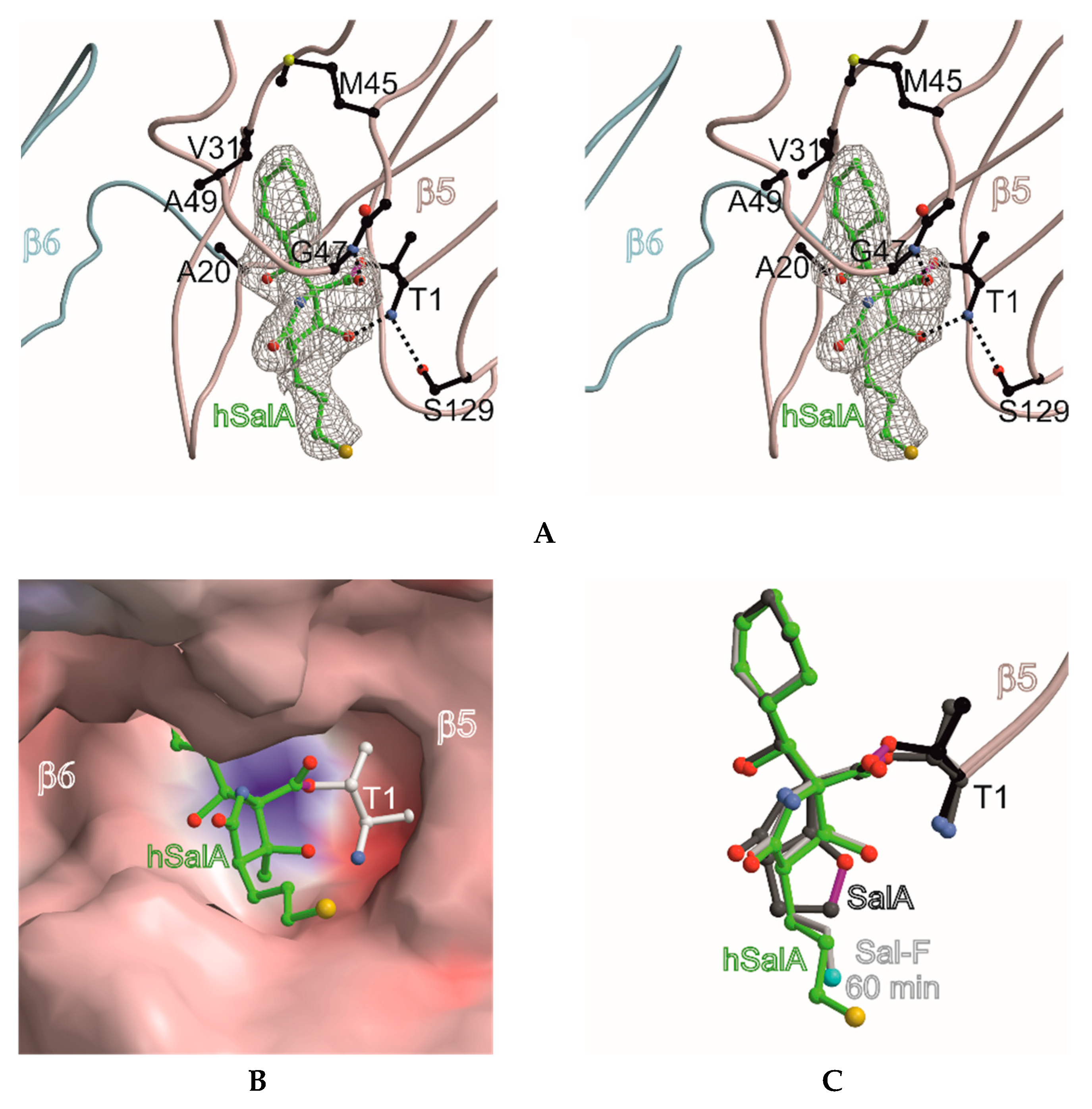
| Compound | ChT-L Activity 20S Proteasome (IC50, nM) | C-L Activity 20S Proteasome (IC50, nM) | T-L Activity 20S Proteasome (IC50, nM) |
|---|---|---|---|
| (–)-SalA (1) | 0.8 ± 0.08 | 111 ± 22 | 39 ± 7 |
| (–)-homoSalA (3) | 0.7 ± 0.04 | 144 ± 12 | 118 ± 28 |
| yCP:HomoSalA | |
|---|---|
| Data Collection | |
| Beamline | X06SA, SLS |
| Wavelength (Å) | 1.0 |
| Space group | P21 |
| Cell dimensions | |
| a, b, c (Å) | 135.0, 299.8, 144.4 |
| α, β, γ (°) | 90.0, 112.6, 90.0 |
| Resolution (Å) a | 50–2.9 (30.0–2.9) |
| No. observations | 682,573 |
| No. unique reflections b | 208,316 |
| Rmerge (%) c | 8.5 (42.4) |
| I/σ (I) | 13.1 (2.8) |
| Completeness (%) | 92.1 (91.8) |
| Redundancy | 3.2 (3.0) |
| Refinement | |
| Resolution (Å) | 15–2.9 |
| No. reflections working set | 196,716 |
| No. reflections test set | 10,353 |
| Rwork/Rfree (%) d | 19.7/23.3 |
| No. atoms | |
| Protein | 50,209 |
| Ligand | 132 |
| Water/ions/solvents | 701 |
| R.m.s. deviations e | |
| Bond lengths (Å) | 0.007 |
| Bond angles (°) | 1.1 |
| Average B-factor (Å2) | 46.6 |
| Ramachandran Plot (%) f | 97.8/2.0/0.2 |
| PDB accession code | 6GOP |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groll, M.; Nguyen, H.; Vellalath, S.; Romo, D. (−)-Homosalinosporamide A and Its Mode of Proteasome Inhibition: An X-ray Crystallographic Study. Mar. Drugs 2018, 16, 240. https://doi.org/10.3390/md16070240
Groll M, Nguyen H, Vellalath S, Romo D. (−)-Homosalinosporamide A and Its Mode of Proteasome Inhibition: An X-ray Crystallographic Study. Marine Drugs. 2018; 16(7):240. https://doi.org/10.3390/md16070240
Chicago/Turabian StyleGroll, Michael, Henry Nguyen, Sreekumar Vellalath, and Daniel Romo. 2018. "(−)-Homosalinosporamide A and Its Mode of Proteasome Inhibition: An X-ray Crystallographic Study" Marine Drugs 16, no. 7: 240. https://doi.org/10.3390/md16070240







