The Efficacy of MAG-DHA for Correcting AA/DHA Imbalance of Cystic Fibrosis Patients
Abstract
:1. Introduction
2. Results
2.1. Lipid Profile of Erythrocytes Following MAG-DHA Supplementation
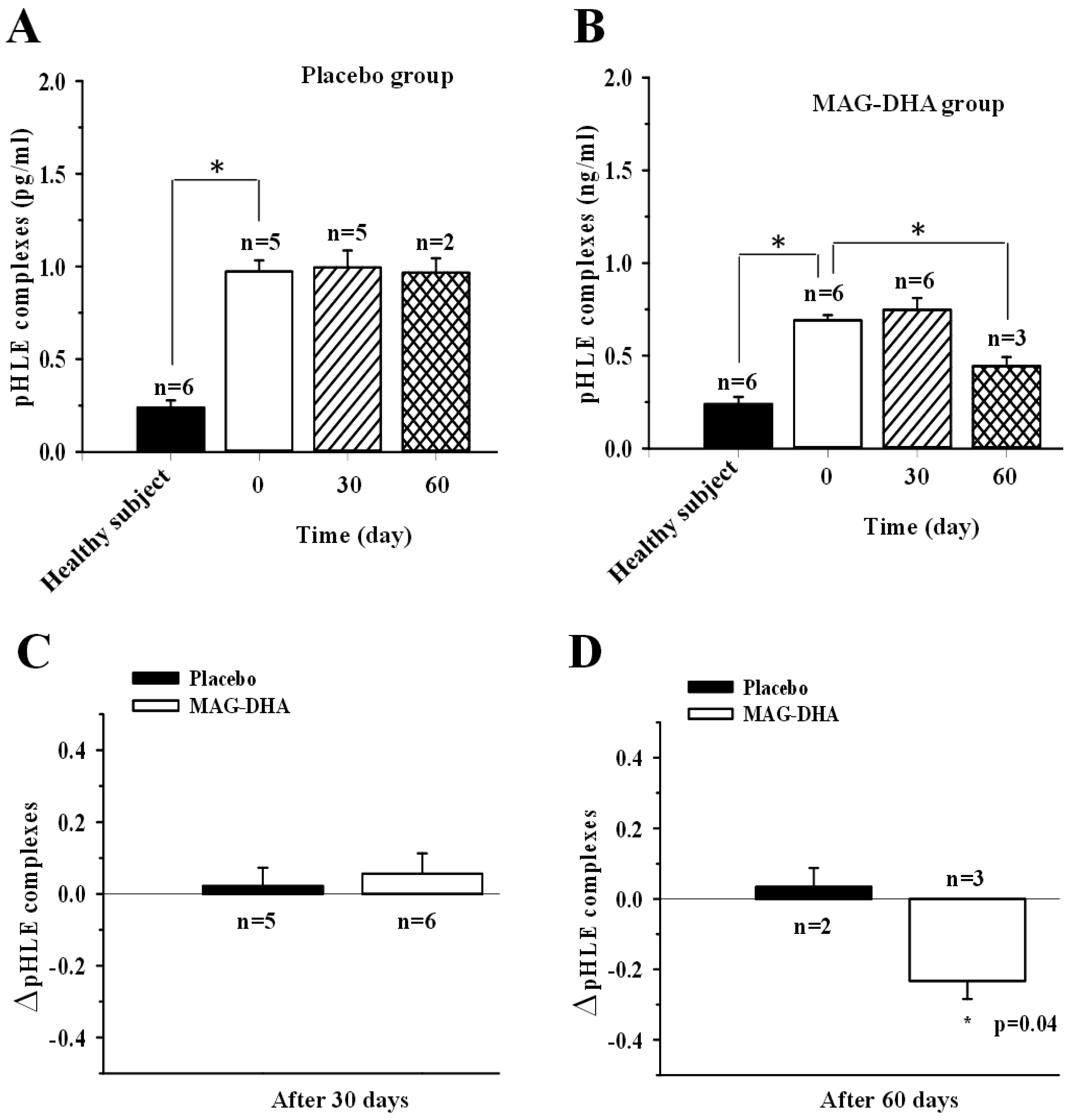
2.2. pHLE Complexes Levels in CF Patients after MAG-DHA or Placebo Supplementations
2.3. Effect of MAG-DHA Intake on Circulating IL-6 Levels in CF Patients
3. Discussion
MAG-DHA Increased DHA Bioavailability and Decreased Inflammatory Markers
4. Methods
4.1. MAG-DHA
4.2. Clinical Study
4.3. ELISA Assays
4.4. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morin, C.; Fortin, S.; Guibert, C.; Rousseau, É. 5 3 and 6 CYP450 Eicosanoid Derivatives: Key Lipid Mediators in the Regulation of Pulmonary Hypertension. 2011. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.464.2852 (accessed on 1 May 2018).
- Fortin, S. Polyunsaturated Fatty Acid Monoglycerides, Derivatives, and Uses Thereof. CA2672513, 2008; CA2677670, 2010; US8119690, 2011.
- Fortin, S. Compositions Comprising Polyunsaturated Fatty Acid Monoglycerides or Derivatives Thereof and Uses Thereof. US819690, 2012; US8222295, 2012.
- Morin, C.; Cantin, A.M.; Rousseau, É.; Sirois, M.; Sirois, C.; Rizcallah, E.; Fortin, S. Proresolving Action of Docosahexaenoic Acid Monoglyceride in Lung Inflammatory Models Related to Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Kerem, B.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Sinaasappel, M.; Stern, M.; Littlewood, J.; Wolfe, S.; Steinkamp, G.; Heijerman, H.G.; Robberecht, E.; Döring, G. Nutrition in patients with cystic fibrosis: A European Consensus. J. Cyst. Fibros. 2002, 1, 51–75. [Google Scholar] [CrossRef]
- Steinkamp, G.; Wiedemann, B. Relationship between nutritional status and lung function in cystic fibrosis: Cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax 2002, 57, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Freedman, S.D.; Blanco, P.G.; Zaman, M.M.; Shea, J.C.; Ollero, M.; Hopper, I.K.; Weed, D.A.; Gelrud, A.; Regan, M.M.; Laposata, M.; et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004, 350, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Rosenlund, M.L.; Kim, H.K.; Kritchevsky, D. Essential fatty acids in cystic fibrosis. Nature 1974, 251, 719. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Levy, E.; Ronco, N.; Smith, L.; Galéano, N.; Roy, C.C. Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. J. Lipid Res. 1989, 30, 1483–1490. [Google Scholar] [PubMed]
- Landon, C.; Kerner, J.A.; Castillo, R.; Adams, L.; Whalen, R.; Lewiston, N.J. Oral correction of essential fatty acid deficiency in cystic fibrosis. J. Parenter. Enteral Nutr. 1981, 5, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Strandvik, B.; Brönnegård, M.; Gilljam, H.; Carlstedt-Duke, J. Relation between defective regulation of arachidonic acid release and symptoms in cystic fibrosis. Scand. J. Gastroenterol. Suppl. 1988, 143, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Strandvik, B.; Svensson, E.; Seyberth, H.W. Prostanoid biosynthesis in patients with cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids 1996, 55, 419–425. [Google Scholar] [CrossRef]
- Rogiers, V.; Vercruysse, A.; Dab, I.; Crokaert, R.; Vis, H.L. Fatty acid pattern of platelet phospholipids in cystic fibrosis. Eur. J. Pediatr. 1984, 142, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Kosorok, M.R.; Rock, M.J.; Laxova, A.; Zeng, L.; Lai, H.C.; Hoffman, G.; Laessig, R.H.; Splaingard, M.L. Early d4iagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics 2001, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lévy, E.; Roy, C.; Lacaille, F.; Lambert, M.; Messier, M.; Gavino, V.; Lepage, G.; Thibault, L. Lipoprotein abnormalities associated with cholesteryl ester transfer activity in cystic fibrosis patients: The role of essential fatty acid deficiency. Am. J. Clin. Nutr. 1993, 57, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Hanssens, L.; Thiébaut, I.; Lefèvre, N.; Malfroot, A.; Knoop, C.; Duchateau, J.; Casimir, G. The clinical benefits of long-term supplementation with omega-3 fatty acids in cystic fibrosis patients—A pilot study. Prostaglandins Leukot. Essent. Fatty Acids 2016, 108, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Coste, T.C.; Armand, M.; Lebacq, J.; Lebecque, P.; Wallemacq, P.; Leal, T. An overview of monitoring and supplementation of omega 3 fatty acids in cystic fibrosis. Clin. Biochem. 2007, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- De Vizia, B.; Raia, V.; Spano, C.; Pavlidis, C.; Coruzzo, A.; Alessio, M. Effect of an 8-month treatment with omega-3 fatty acids (eicosapentaenoic and docosahexaenoic) in patients with cystic fibrosis. J. Parenter. Enteral Nutr. 2003, 27, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.P.; Manner, T.; Furst, P.; Askanazi, J. The use of an intravenous fish oil emulsion enriched with omega-3 fatty acids in patients with cystic fibrosis. Nutrition 1996, 12, 334–339. [Google Scholar] [CrossRef]
- Leggieri, E.; De Biase, R.V.; Savi, D.; Zullo, S.; Halili, I.; Quattrucci, S. Clinical effects of diet supplementation with DHA in pediatric patients suffering from cystic fibrosis. Minerva Pediatr. 2013, 65, 389–398. [Google Scholar] [PubMed]
- Olveira, G.; Olveira, C.; Acosta, E.; Espíldora, F.; Garrido-Sánchez, L.; García-Escobar, E.; Rojo-Martínez, G.; Gonzalo, M.; Soriguer, F. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch. Bronconeumol. 2010, 46, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Bilodeau, G.; Larivée, P.; Richter, M.V. Plasma biomarkers and cystic fibrosis lung disease. Clin. Investig. Med. 2012, 35, E173–E181. [Google Scholar] [CrossRef]
- Eckrich, J.; Zissler, U.M.; Serve, F.; Leutz, P.; Smaczny, C.; Schmitt-Grohé, S.; Fussbroich, D.; Schubert, R.; Zielen, S.; Eickmeier, O. Airway inflammation in mild cystic fibrosis. J. Cyst. Fibros. 2017, 16, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Strandvik, B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Al-Turkmani, M.R.; Andersson, C.; Alturkmani, R.; Katrangi, W.; Cluette-Brown, J.E.; Freedman, S.D.; Laposata, M. A mechanism accounting for the low cellular level of linoleic acid in cystic fibrosis and its reversal by DHA. J. Lipid Res. 2008, 49, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M.; Davidson, A.G. Cystic fibrosis and nutrition: Linking phospholipids and essential fatty acids with thiol metabolism. Annu. Rev. Nutr. 2008, 28, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.F.; Laposata, M.; Njoroge, S.W.; Umunakwe, O.C.; Katrangi, W.; Seegmiller, A.C. Increased elongase 6 and Delta9-desaturase activity are associated with n-7 and n-9 fatty acid changes in cystic fibrosis. Lipids 2011, 46, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, S.W.; Laposata, M.; Katrangi, W.; Seegmiller, A.C. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. J. Lipid Res. 2012, 53, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernandez, C.; Destaillats, F.; Thakkar, S.K.; Goulet, L.; Wynn, E.; Grathwohl, D.; Roessle, C.; de Giorgi, S.; Tappy, L.; Giuffrida, F.; et al. Monoacylglycerol-enriched oil increases EPA/DHA delivery to circulatory system in humans with induced lipid malabsorption conditions. J. Lipid Res. 2016, 57, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Fortin, S.; Cantin, A.M.; Sirois, M.; Sirois, C.; Rizcallah, E.; Rousseau, É. Anti-cancer effects of a new docosahexaenoic acid monoacylglyceride in lung adenocarcinoma. Recent Pat. Anticancer Drug Discov. 2013, 8, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Fortin, S.; Cantin, A.M.; Rousseau, E. Docosahexaenoic acid derivative prevents inflammation and hyperreactivity in lung: Implication of PKC-Potentiated inhibitory protein for heterotrimeric myosin light chain phosphatase of 17 kD in asthma. J. Respir. Cell Mol. Biol. 2011, 45, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Panchaud, A.; Sauty, A.; Kernen, Y.; Decosterd, L.A.; Buclin, T.; Boulat, O.; Hug, C.; Pilet, M.; Roulet, M. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: A randomized, crossover placebo-controlled trial. Clin. Nutr. 2006, 25, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Eiserich, J.P.; Cross, C.E.; Morrissey, B.M.; Hammock, B.D. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic. Biol. Med. 2012, 53, 160–171. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Study Groups | |
|---|---|---|
| Placebo (n = 5) | MAG-DHA (n = 6) | |
| Mean ± SEM | Mean ± SEM | |
| Age, years | 24.4 ± 3.3 | 32.7 ± 4.6 |
| BMI (kg/m2) | 20.0 ± 0.5 | 20.1 ± 0.5 |
| FEV1/FVC | 67.7 ± 7.5 | 63.8 ± 4.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, C.; Cantin, A.M.; Vézina, F.-A.; Fortin, S. The Efficacy of MAG-DHA for Correcting AA/DHA Imbalance of Cystic Fibrosis Patients. Mar. Drugs 2018, 16, 184. https://doi.org/10.3390/md16060184
Morin C, Cantin AM, Vézina F-A, Fortin S. The Efficacy of MAG-DHA for Correcting AA/DHA Imbalance of Cystic Fibrosis Patients. Marine Drugs. 2018; 16(6):184. https://doi.org/10.3390/md16060184
Chicago/Turabian StyleMorin, Caroline, André M. Cantin, Félix-Antoine Vézina, and Samuel Fortin. 2018. "The Efficacy of MAG-DHA for Correcting AA/DHA Imbalance of Cystic Fibrosis Patients" Marine Drugs 16, no. 6: 184. https://doi.org/10.3390/md16060184




