Developments of Cyanobacteria for Nano-Marine Drugs: Relevance of Nanoformulations in Cancer Therapies
Abstract
:1. Introduction
2. Microalgal Uniqueness: Recent Paths to Anti-Cancer Compound Discovery
3. Current Trends in Toxicity and Safety Concerns of Microalgae/Cyanobacteria
4. Status of Microalgal Anti-Cancer Drugs in Commercialized Platforms
5. Strategies to Address Difficulties: Development of High Potency Drug Delivery Formula
5.1. Possibility of Peptide-Based Microalgal Drug Development
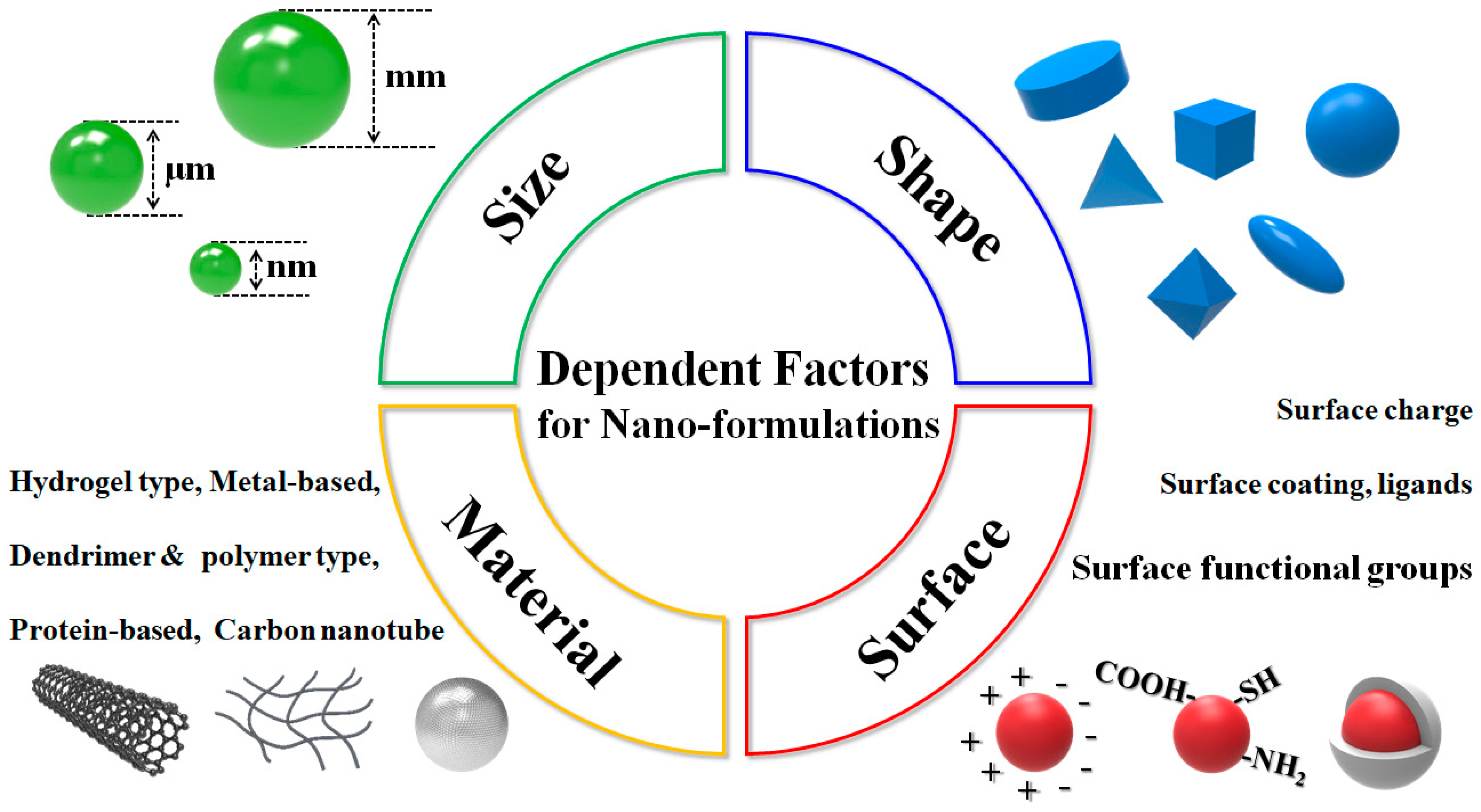
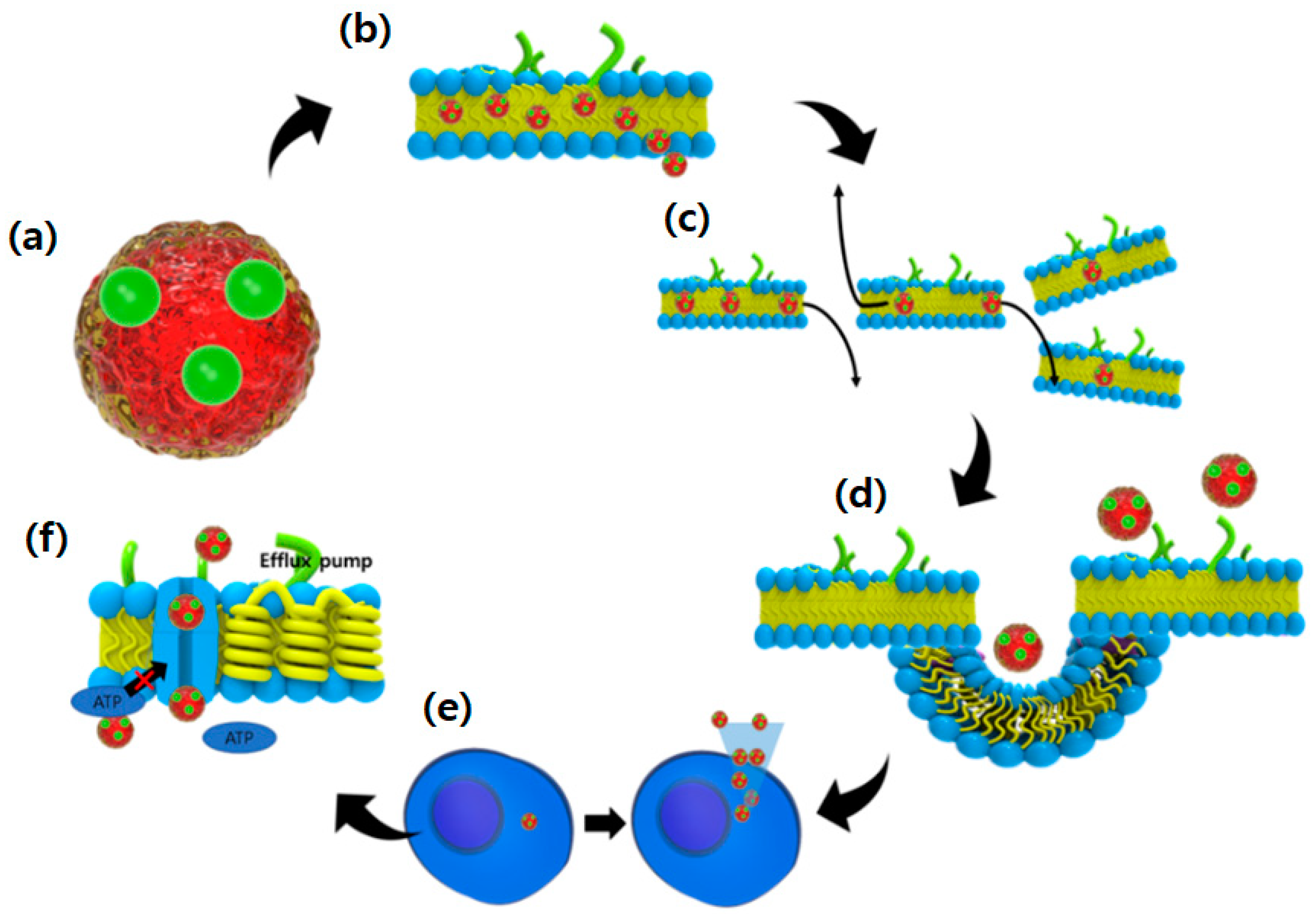
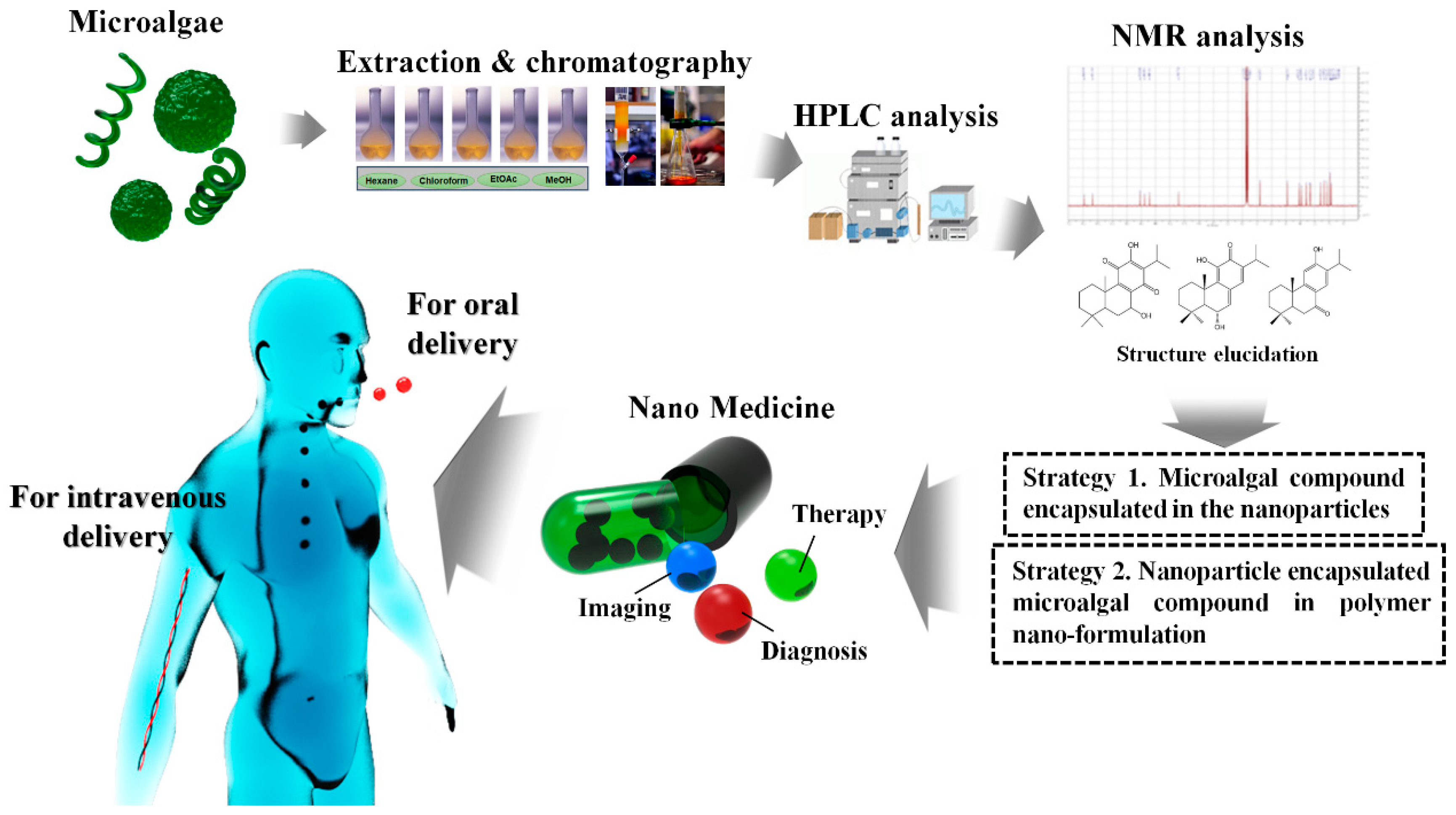
5.2. Possibilities and Facts Related to Nano-Formulated Microalgal Drug Development
5.3. Interest in Albumin-Based Nanoparticles: Useful Tool for Nanoformulation
5.4. Microalgal Polysaccharide-Based Nanoformulation
6. Facts of the Commercial Market
7. Concluding Remarks and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torresa, F.A.E.; Passalacquaa, T.G.; Velasqueza, A.M.A.; de Souz, R.A.; Colepicolo, P.; Graminha, M.A.S. New drugs with antiprotozoal activity from marine algae: A review. Rev. Bras. Farmacogn. 2014, 24, 265–276. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Understanding Cancer. Available online: https://www. cancer.gov/about-cancer/understanding (accessed on 22 May 2018).
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef]
- Prasad, V.; De Jesús, K.; Mailankody, S. The high price of anticancer drugs: Origins, implications, barriers, solutions. Nat. Rev. Clin. Oncol. 2017, 14, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ju, D.T.; Chang, C.F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, e23. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Souza, C.B.; Lhullier, C.; Falkenberg, M.; Schenkel, E.P.; Ribeiro-do-Valle, R.M.; Siqueira, J.M. Anti-tumour effects of elatol, a marine derivative compound obtained from red algae Laurencia microcladia. J. Pharm. Pharmacol. 2012, 64, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Butt, G.; Razzaq, Z. Algae extracts and methyl jasmonate anti-cancer activities in prostate cancer: Choreographers of the dance macabre. Cancer Cell Int. 2012, 12, e50. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Virginia Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Pyne, S.K.; Bhattacharjee, P.; Srivastav, P.K. Microalgae (Spirulina platensis) and its bioactive molecules: Review. Indian J. Nutr. 2017, 4, e160. [Google Scholar]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Simanek, E.E. Cancer therapies utilizing the camptothecins: A review of in vivo literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Zhang, Y.N.; Zhang, W. Cancer-on-a-hip systems at the frontier of nanomedicine. Drug Discov. Today 2017, 22, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y. Nanomaterials at work in biomedical research. Nat. Mater. 2008, 7, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, G.; Mai, J.; Deng, X.; Segura-Ibarra, V.; Wu, S. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 2016, 34, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [PubMed]
- Marcazzana, S.; Varonib, E.M.; Blancoa, E.; Lodib, G.; Ferraria, M. Nanomedicine, an emerging therapeutic strategy for oral cancer therapy. Oral Oncol. 2018, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Tandon, P.; Jin, Q.; Huang, L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Fact. 2017, 16, e219. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Venkatesan, J.; Manivasagan, P.; Kim, S.K. Marine Microalgae Biotechnology: Present Trends and Future Advances. Handbook of Marine Microalgae: Biotechnology Advances; Academic Press: Cambridge, MA, USA, 2015; pp. 1–9. [Google Scholar]
- Boopathy, N.S.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, e214186. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Ravenm, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Grace, K.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. Scytonemin—A marine natural product inhibitor of kinases key in hyperproliferative inflammatory diseases. Inflam. Res. 2002, 51, 112–114. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.H.; Chai, C.L.; Heath, G.A.; Mahon, P.J.; Smith, G.D.; Waring, P.; Wilkes, B.A. Synthesis, electrochemistry, and bioactivity of the cyanobacterial calothrixins and related quinones. J. Med. Chem. 2004, 47, 4958–4963. [Google Scholar] [CrossRef] [PubMed]
- Hatae, N.; Satoh, R.; Chiba, H.; Osaki, T.; Nishiyama, T.; Ishikura, M.; Abe, T.; Hibino, S.; Choshi, T.; Okada, C. N-Substituted calothrixin B derivatives inhibited the proliferation of HL-60 promyelocytic leukemia cells. Med. Chem. Res. 2014, 23, 4956–4961. [Google Scholar] [CrossRef]
- Kwan, J.C.; Teplitski, M.; Gunasekera, S.P.; Paul, V.J.; Luesch, H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Santarsiero, B.D.; Kim, H.; Krunic, A.; Shen, Q.; Swanson, S.M.; Chai, H.; Kinghorn, A.D.; Orjala, J. Merocyclophanes A and B, antiproliferative cyclophanes from the cultured terrestrial cyanobacterium Nostoc sp. Phytochemistry 2012, 79, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Leao, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of hierridin B, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PLoS ONE 2013, 8, e69562. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Pavlick, A.C.; Johnson, D.B.; Hart, L.L.; Infante, J.R.; Luke, J.J.; Lutzky, J.; Rothschild, N.; Spitler, L.; Cowey, C.L. A phase 2 study of glembatumumab vedotin (GV), an antibody-drug conjugate (ADC) targeting gpNMB, in advanced melanoma. Ann. Oncol. 2016, 27, e1147. [Google Scholar] [CrossRef]
- Luesch, H.; Chanda, S.K.; Raya, R.M. A functional genomics approach to the mode of action of apratoxin A. Nat. Chem. Biol. 2006, 2, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Law, B.K.; Luesch, H. Apratoxin A reversibly inhibits the secretory pathway by preventing cotranslational translocation. Mol. Pharmacol. 2009, 76, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Koníčková, R.; Vaňková, K.; Vaníková, J.; Váňová, K.; Muchová, L.; Subhanová, I.; Zadinová, M.; Zelenka, J.; Dvořák, A.; Kolář, M.; et al. Anti-cancer effects of blue-green alga Spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann. Hepatol. 2014, 13, 273–283. [Google Scholar] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Goeger, D.E.; Hills, P. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Tan, L.T.; Sitachitta, N. Nitrogen-containing metabolites from marine cyanobacteria. In The Alkaloids; Cordell, G., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 75–184. [Google Scholar]
- Banker, R.; Carmeli, S. Tenuecyclamides A-D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J. Nat. Prod. 1998, 61, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.S. New dimensions in natural products research: Cultured marine microorganisms. Curr. Opin. Biotechnol. 1995, 6, 284–291. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Camacho, R.; González-Jasso, E.; Ferriz-Martínez, R.; Villalón-Corona, B.; Loarca-Piña, G.F.; Salgado, L.M.; Ramos-Gomez, M. Dietary supplementation of lutein reduces colon carcinogenesis in DMH-treated rats by modulating K-ras, PKB, and β-catenin proteins. Nutr. Cancer 2011, 63, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Voračova, K.; Hajek, J.; Mares, J.; Urajova, P.; Kuzma, M.; Cheel, J. The cyanobacterial metabolite nocuolin A is a natural oxadiazine that triggers apoptosis in human cancer cells. PLoS ONE 2017, 12, e0172850. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Asati, V.; Singh, J.; Roy, P.P. 1,3,4-Oxadiazoles: An emerging scaffold to target growth factors, enzymes and kinases as anticancer agents. Eur. J. Med. Chem. 2015, 97, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Semenov, S.G.; Makarova, M.V. 1,2,3-Oxadiazole rings in the aromatic compounds: A quantum-chemical investigation. Russ. J. Gen. Chem. 2011, 81, 1555–1557. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Hegarty, A.F.; Elguero, J. Can 1,2,3-oxadiazole be stable. Angew. Chem. Int. Ed. 1985, 24, 713–715. [Google Scholar] [CrossRef]
- Tondres, Z.V. Chalcogenadiazoles: Chemistry and Applications, 1st ed.; Tondres, Z.V., Ed.; CRC Press: New York, NY, USA, 2012; p. 310. [Google Scholar]
- Mohareb, R.M.; Schatz, J. Anti-tumor and anti-leishmanial evaluations of 1,3,4-oxadiazine, pyran derivatives derived from cross-coupling reactions of beta-bromo-6H-1,3,4-oxadiazine derivatives. Bioorg. Med. Chem. 2011, 19, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Pisconti, S.; Scarpati, G.D.V. P53 mutations and cancer: a tight linkage. Ann. Trans. Med. 2016, 4, e522. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Gogoi, S.K.; Sanpui, P.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf. B Biointerfaces 2010, 77, 240–245. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Janicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.E.; Badawi, M.H.; Mostafa, S.S.; Higazy, A.M. Human anticancer and antidiabetic activities of the cyanobacterium Fischerella sp. BS1-EG isolated from River Nile, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3473–3485. [Google Scholar] [CrossRef]
- Samydurai, S.; Namasivayam, S.K.R.; Pandey, V.K. Influence of algal based protein nanoparticles loading on antibacterial activity, in vitro drug release and cytotoxicity of cephalosporin derivatives. Asian J. Pharm. 2016, 10, 693–699. [Google Scholar]
- Hess, P. Requirements for screening and confirmatory methods for the detection and quantification of marine biotoxins in end-product and official control. Anal. Bioanal. Chem. 2010, 397, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, Y.; Gao, Q.; Tam, N.F.; Zhu, W. Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 2010, 80, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Teneva, I.; Dzhambazov, B.; Koleva, L.; Mladenov, R.; Schirmer, K. Toxic potential of five freshwater Phormidium species (Cyanoprokaryota). Toxicon 2005, 45, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Blaha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms—Toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Los Angeles Times. Toxins from Algal Blooms May Cause Alzheimer’s-Like Brain Changes. Available online: http://www.latimes.com/science/sciencenow/la-sci-sn-algal-blooms-alzheimers-brain-changes-20160119-story.html (accessed on 11 February 2018).
- El-Baz, F.K.; Aly, H.F.; Khalil, W.K.; Booles, H.F.; Ali, G.H. Antineurodegenerative activity of microalgae Dunaliella salina in rats with Alzheimer’s disease. Asian J. Pharm. Clin. Res. 2017, 10, 134–139. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites: A short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Hinder, S.L.; Hays, G.C.; Brooks, C.J.; Davies, A.P.; Edwards, M.; Walne, A.W.; Gravenor, M.B. Toxic marine microalgae and shellfish poisoning in the British Isles: History, review of epidemiology, and future implications. Environ. Health 2011, 10, e54. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.K.; Pandey, R.; Jha, S.K.; Singh, J.; Sodhi, K.S. Spirulina: The single cell protein. Indo Am. J. Pharm. Res. 2014, 4, 221–2217. [Google Scholar]
- Mishra, P.; Singh, V.P.; Prasad, S.M. Spirulina and its nutritional importance: A possible approach for development of functional food. Biochem. Pharmacol. 2014, 3, e171. [Google Scholar] [CrossRef]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Zhang, X.; Jarjoura, D.; Moy, F.J.; Orjala, J.; Kinghorn, A.D.; Pearce, C.J.; Oberlies, N.H. Chemical diversity of metabolites from fungi, cyanobacteria, and plants relative to FDA-approved anticancer agents. ACS Med. Chem. Lett. 2012, 3, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Clarens, A.; Resurreccion, E.; White, M.; Colosi, L. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Norsker, N.; Barbosa, M.; Vermue, M.; Wijffels, R. Microalgal production-a close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Iconomou, D.; Sotiroudis, T.; Israilides, C.; Muylaert, K. Exploration of using stripped ammonia and ash from poultry litter for the cultivation of the cyanobacterium Arthrospira platensis and the green microalga Chlorella vulgaris. Bioresour. Technol. 2015, 196, 459–468. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, e00056. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Kobayashi, K.; Suenaga, K.; Kigoshi, H. Phormidinines A and B, novel 2-alkylpyridine alkaloids from the cyanobacterium Phormidium sp. Tetr. Lett. 2005, 46, 4001–4003. [Google Scholar] [CrossRef]
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J. 2012, 2012, e179782. [Google Scholar] [CrossRef]
- Advani, R.H.; Lebovic, D.; Chen, A.; Brunvand, M.; Goy, A.; Chang, J.E.; Hochberg, E.; Yalamanchili, S.; Kahn, R.; Lu, D. Phase I study of the anti-CD22 antibody-drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma. Clin. Cancer Res. 2016, 23, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- De Goeij, B.E.; Lambert, J.M. New developments for antibody-drug conjugate-based therapeutic approaches. Curr. Opin. Immunol. 2016, 40, 14–23. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.M.; Monie, A.; Wu, A.; Mao, C.P.; Hung, C.F. Treatment with LL-37peptide enhances anti-tumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum. Gene Ther. 2009, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides–challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Wan, L.; Cheng, J.; Lu, X. Penetratin-mediated delivery enhances the antitumor activity of the cationic antimicrobial peptide magainin, II. Cancer Biother. Radiopharm. 2013, 28, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.; Pechon, P.; Tartar, A.; Dunn, M.K. Report Summary: Development Trends for Peptide Therapeutics; Peptide Therapeutics Foundation: San Diego, CA, USA, 2010; pp. 1–11. [Google Scholar]
- Mendoza, F.J.; Espino, P.S.; Cann, K.L.; Bristow, N.; McCrea, K.; Los, M. Anti-tumor chemotherapy utilizing peptide-based approaches—Apoptotic pathways, kinases, and proteasome as targets. Arch. Immunol. Ther. Exp. 2005, 53, 47–60. [Google Scholar]
- Ramasubburayana, R.; Sumathi, S.; Prakash, S.; Ramkumar, V.S.; Titus, S.; Immanuel, G.; Palavesam, A. Synthesis of nano silver by a marine epibiotic bacterium Bacillus vallismortis and its potent ecofriendly antifouling properties. Environ. Nanotechnol. Monitor. Manag. 2017, 8, 112–120. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Prakash, S.; Ramasubburayan, R.; Pugazhendhi, A.; Gopalakrishnane, K.; Kannapiran, E.; Rajendran, R.B. Seaweeds: A resource for marine bionanotechnology. Enzym. Microb. Technol. 2016, 95, 45–57. [Google Scholar]
- Ahila, N.K.; Sri Ramkumar, V.; Prakasha, S.; Manikandan, B.; Ravindran, J.; Dhanalakshmi, P.K.; Kannapirana, E. Synthesis of stable nanosilver particles (AgNPs) by the proteins of seagrass Syringodium isoetifolium and its biomedicinal properties. Biomed. Pharmacother. 2016, 84, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Elghazawy, N.H.; Hefnawy, A.; Sedky, N.K.; El-Sherbiny, I.M.; Araf, R.K. Preparation and nanoformulation of new quinolone scaffold-based anticancer agents: Enhancing solubility for better cellular delivery. Eur. J. Pharm. Sci. 2017, 105, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.R.; Kathiresan, K.; Anandhan, S. A review on marine based nanoparticles and their potential applications. Afr. J. Biotechnol. 2015, 14, 1525–1532. [Google Scholar]
- Ibrahim, S.; Tagami, T.; Kishi, T.; Ozeki, T. Curcumin marinosomes as promising nanodrug delivery system for lung cancer. Int. J. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, N.; Cansell, M.; Denizot, A. Marinosomes®, marine lipid-based liposomes: Physical characterization and potential application in cosmetics. Int. J. Pharm. 2002, 242, 361–365. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Biological significance of essential fatty acids. J. Assoc. Phys. India 2006, 54, 309–319. [Google Scholar]
- Peng, J.; Larondelle, Y.; Pham, D.; Ackman, R.G.; Rollin, X. Polyunsaturated fatty acid profiles of whole body phospholipids and triacylglycerols in anadromous and landlocked Atlantic salmon (Salmo salar L.) fry. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 335–348. [Google Scholar] [CrossRef]
- Winther, B.; Hoem, N.; Berge, K.; Reubsaet, L. Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids 2011, 46, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Alaarg, A.; Ordan, N.; Verhoef, J.; Metselaar, J.; Storm, G.; Kok, R. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K/Akt pathway. Lipids Health Dis. 2017, 16, e87. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.G.; Navin Thomas, C.K.; Prakash, S.; Sathish Kumar, C. Biosynthesis of silver nano-particles by marine sediment fungi for a dose dependent cytotoxicity against HEp2 cell lines. Biocat. Agric. Biotechnol. 2015, 4, 150–157. [Google Scholar] [CrossRef]
- Palaniappan, P.; Sathishkumar, G.; Sankar, R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim. Acta A 2015, 138, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural characterization antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R. Br.) Asch. & Magnus mediated silver nanoparticles. J. Photochem. Photobiol. B 2015, 153, 145–152. [Google Scholar] [PubMed]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Toxicity study of silver nanoparticles synthesized from Suaeda monoica on Hep-2 cell line. Avicenna J. Med. Biotechnol. 2012, 4, 35–39. [Google Scholar] [PubMed]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, e43. [Google Scholar] [CrossRef]
- Rocha, T.L.; Gomes, T.; Cardoso, C.; Letendre, J.; Pinheiro, J.P.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Immunocytotoxicity, cytogenotoxicity and genotoxicity of cadmium-based quantum dots in the marine mussel Mytilus galloprovincialis. Mar. Environ. Res. 2014, 101, 29–37. [Google Scholar] [CrossRef] [PubMed]
- An, F.F.; Zhang, X.H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, H.; Wang, J.; Wu, J.; Ma, X.; Li, L.; Wei, X.; Ling, Q.; Song, P.; Zhou, L.; et al. Self-assembling prodrugs by precise programming of molecular structures that contribute distinct stability, pharmacokinetics, and antitumor efficacy. Adv. Funct. Mater. 2015, 25, 4956–4965. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Gao, M. Remotely controlled red blood cell carriers for cancer targeting and near-infrared light-triggered drug release in combined photothermal-chemotherapy. Adv. Funct. Mater. 2015, 25, 2386–2394. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, C.; Wang, C. An imagable and photothermal “Abraxane-like” nanodrug for combination cancer therapy to treat subcutaneous and metastatic breast tumors. Adv. Mater. 2015, 27, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Youn, Y.S. Albumin-based potential drugs: Focus on half-life extension and nanoparticle preparation. J. Pharm. Investig. 2016, 46, 305–315. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.Y.; Li, J.; Tai, J.N. Avasimibe encapsulated in human serum albumin blocks cholesterol esterification for selective cancer treatment. ACS Nano 2015, 9, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, A.C.; Buckle, T.; Bendle, G. Tracer-cocktail injections for combined pre- and intraoperative multimodal imaging of lymph nodes in a spontaneous mouse prostate tumor model. J. Biomed. Opt. 2011, 16, 016004. [Google Scholar] [CrossRef] [PubMed]
- Tessa, B.; Anne, C.V.L.; Patrick, T.K.C. A self-assembled multimodal complex for combined pre- and intraoperative imaging of the sentinel lymph node. Nanotechnology 2010, 21, e355101. [Google Scholar] [CrossRef]
- Manivasagana, P.; Oh, P. Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manivasagan, P.; Bharathiraja, S.; Santha Moorthy, M.; Oh, Y.O.; Seo, H.; Oh, J. Marine biopolymer-based nanomaterials as a novel platform for theranostic applications. Polym. Rev. 2017, 57, 631–667. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Lee, J.B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.D.; Kuang, X.C.; Pang, H.; Tang, G.F.; Yu, Y.H. The effects of polysaccharide from Spirulina platensis on proliferation of BEL 7404 cells. Guangxi Med. J. 2008, 30, 1122–1124. [Google Scholar]
- Akao, Y.; Ebihara, T.; Masuda, H.; Saeki, Y.; Akazawa, T.; Hazeki, K. Enhancement of antitumor natural killer cell activation by orally administered Spirulina extract in mice. Cancer Sci. 2009, 100, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.J.; Costa de Morais, R.M.S.; Bernardo de Morais, A.M.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Müller-Feuga, A. The role of microalgae in aquaculture: Situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Sinha, V.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, e515. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. The high energy radiation protectant extracellular sheath pigment scytonemin and its reduced counterpart in the cyanobacterium Scytonema sp. R77DM. Bioresour. Technol. 2014, 71, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Halpern, A.C.; Kopp, L.J. Awareness, knowledge and attitudes to nonmelanoma skin cancer and actinic keratosis among the general public. Int. J. Dermatol. 2005, 44, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. Sunscreens as a preventative measure in melanoma: An evidence-based approach or the precautionary principle? Br. J. Dermatol. 2009, 161, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Hedges, J.I. Photodegradation and photosensitization of mycosporine-like amino acids. J. Photochem. Photobiol. B Biol. 2005, 80, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Rocca, J.R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Total structure determination of grassypeptolide, a new marine cyanobacterial cytotoxin. Org. Lett. 2008, 10, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; De Pascale, D.; Andersen, J.; Reyes, F.; Crawford, A.; Ianora, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef]
- Life Science News. Available online: http://www.iflscience.com/health-and-medicine/scientists-transform-algae-cancer-killing-drug-delivery-systems/ (accessed on 15 February 2018).



| Natural Compounds/Drugs | Source | Company Launched | Status after 2015 Food and Drug Administration (FDA)/European Medicines Evaluation Agency (EMEA) |
|---|---|---|---|
| Brentuximab vedotin 63 (Adcetris™) | Cyanobacteria: Symploca hydnoides and Lyngbya majuscula | Seattle Genetics (Bothell, WA, USA) | In market with antibody-drug conjugates |
| Glembatumumab vedotin | Cyanobacterium: Lyngbya sp. | Celldex Therapeutics | Phase II |
| DMMC (Cyclic depsipeptide) | Cyanobacterium: Lyngbya majuscula | - | Preclinical |
| Largazole | Cyanobacterium: Symploca sp. | - | Preclinical |
| Apratoxin A | Cyanobacterium: Lyngbya boulloni | - | Preclinical |
| Cryptophycin 1 | Cyanobacterium: Nostoc sp. GSV 224 | Merck Pvt. | In market |
| Tasipeptins A–B | Cyanobacterium: Symploca sp. | - | Preclinical |
| Coibamide A | Cyanobacterium: Leptolyngbya sp. | - | Preclinical |
| Marine Resources | Name of the Species | Nanoparticles/Size (nm) | Activity | References |
|---|---|---|---|---|
| Seagrass | Cymodocea serrulata (Aqueous extract) | Ag/5–25 | Anticancer | [107] |
| Cymodocea serrulata (Aqueous extract) | Ag/17–29 | Anticancer and cytotoxicity | [108] | |
| Salt marshes | Suaeda monoica (Leaf extract) | Ag/30–31 | Anticancer | [109] |
| Sand dune | Citrullus colosynthis (Callus extract) | Ag/85–100 | Anticancer | [110] |
| Marine fungi | Aspergillus flavus, Trichoderma gamsii, Talaromyces flavus, and Aspergillus oryzae (Cell-free filtrate) | Ag/20–60 | Anticancer | [106] |
| Marine mussel | Mytilus galloprovincialis (Muscle tissue) | Cadmium-based quantum dots/6–10 | Immunocytotoxicity, cytogenotoxicity and genotoxicity | [111] |
| Marine cocktail | Marine poly unsaturated fatty acids (PUFAs) (Curcumin) | Lipid nanoparticles/100–200 | Anticancer | [98] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajpai, V.K.; Shukla, S.; Kang, S.-M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.-K. Developments of Cyanobacteria for Nano-Marine Drugs: Relevance of Nanoformulations in Cancer Therapies. Mar. Drugs 2018, 16, 179. https://doi.org/10.3390/md16060179
Bajpai VK, Shukla S, Kang S-M, Hwang SK, Song X, Huh YS, Han Y-K. Developments of Cyanobacteria for Nano-Marine Drugs: Relevance of Nanoformulations in Cancer Therapies. Marine Drugs. 2018; 16(6):179. https://doi.org/10.3390/md16060179
Chicago/Turabian StyleBajpai, Vivek K., Shruti Shukla, Sung-Min Kang, Seung Kyu Hwang, Xinjie Song, Yun Suk Huh, and Young-Kyu Han. 2018. "Developments of Cyanobacteria for Nano-Marine Drugs: Relevance of Nanoformulations in Cancer Therapies" Marine Drugs 16, no. 6: 179. https://doi.org/10.3390/md16060179





