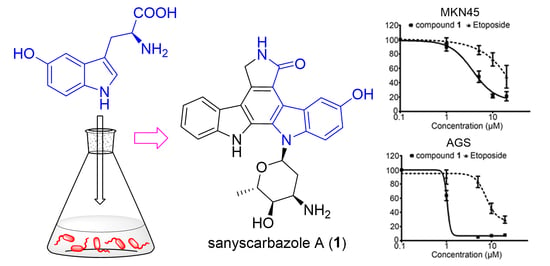
Precursor-Directed Generation of Indolocarbazoles with Topoisomerase IIα Inhibitory Activity
Abstract
:1. Introduction
2. Results and Discussion
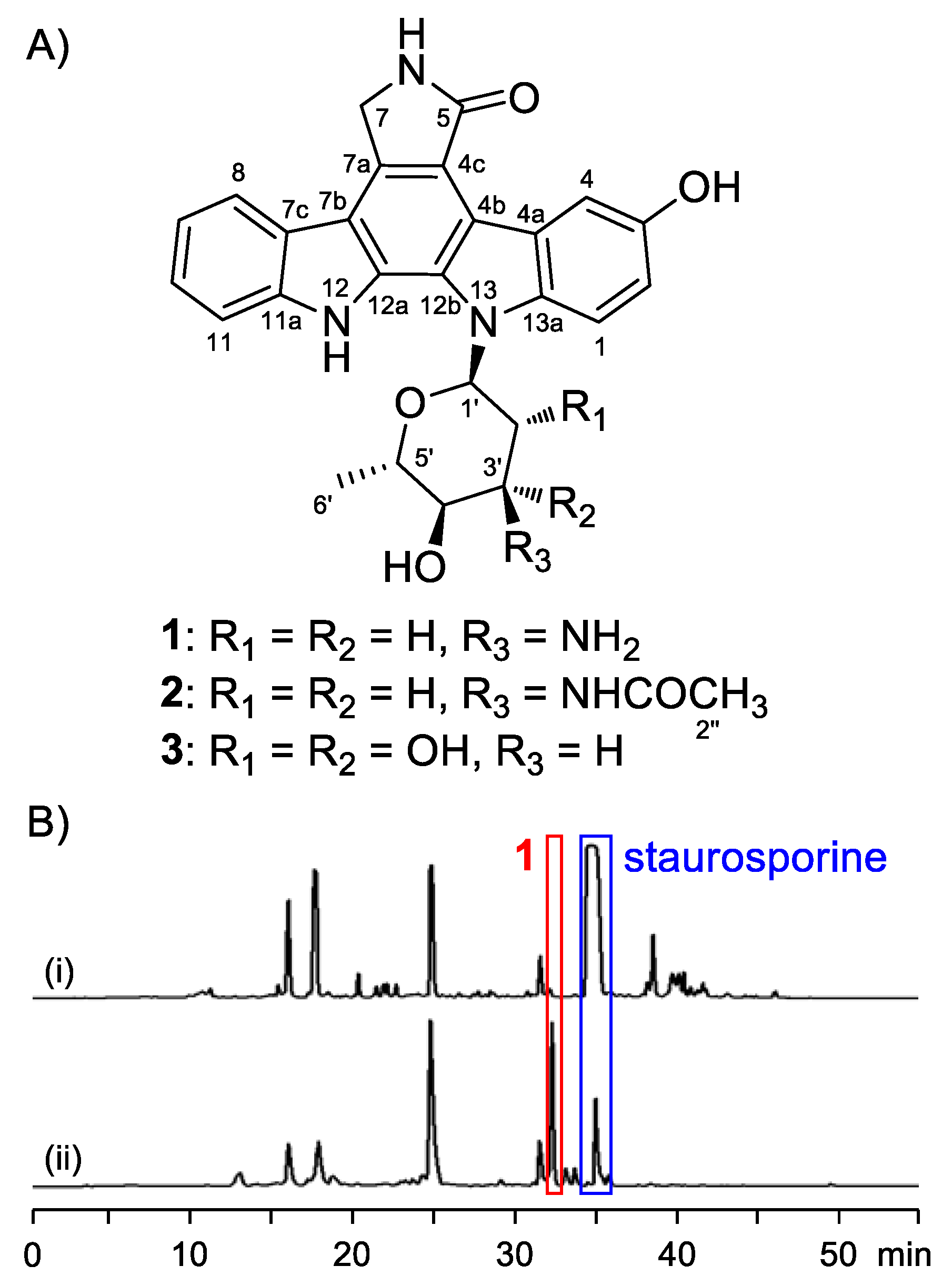
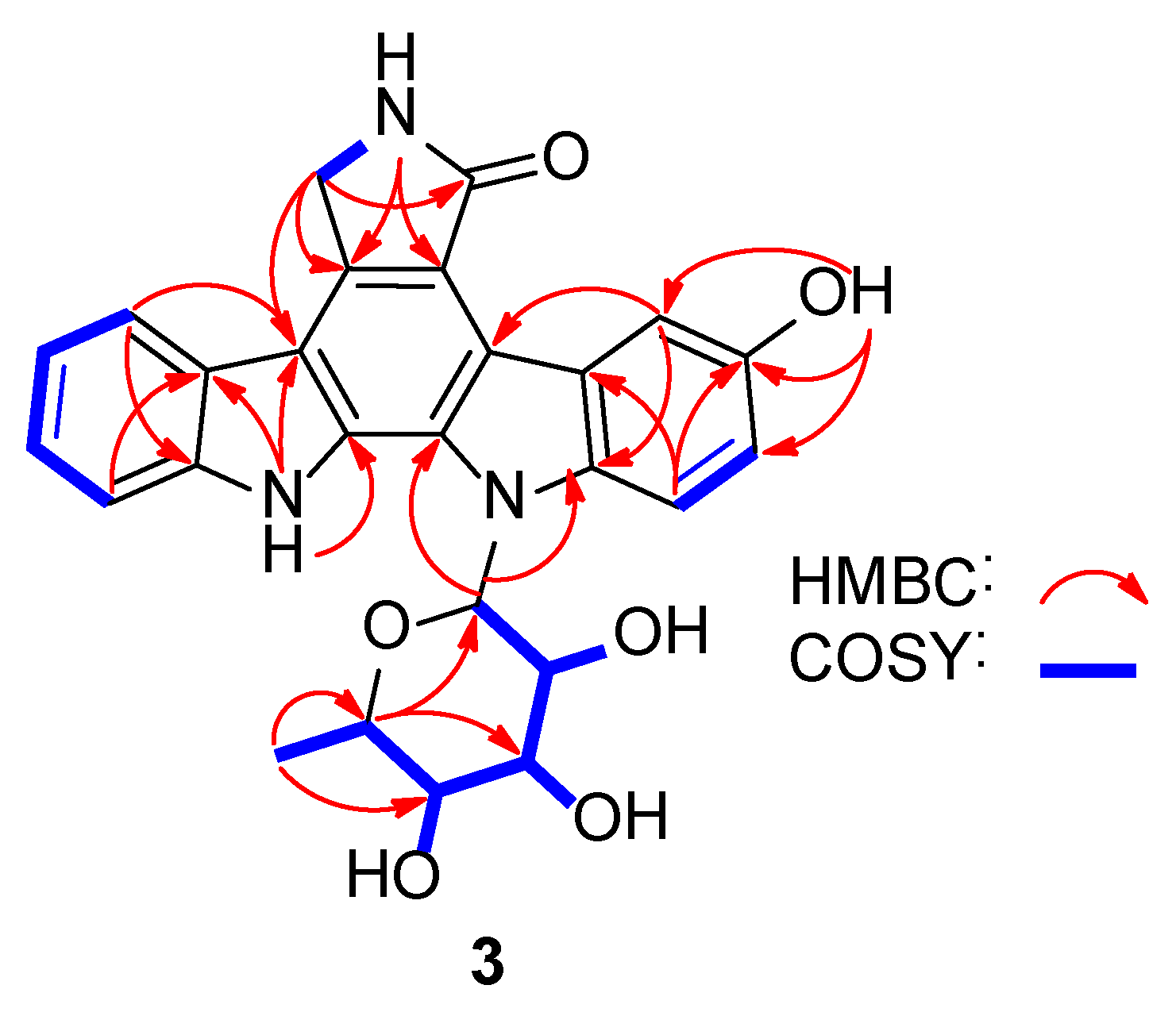
2.1. Structure Elucidation
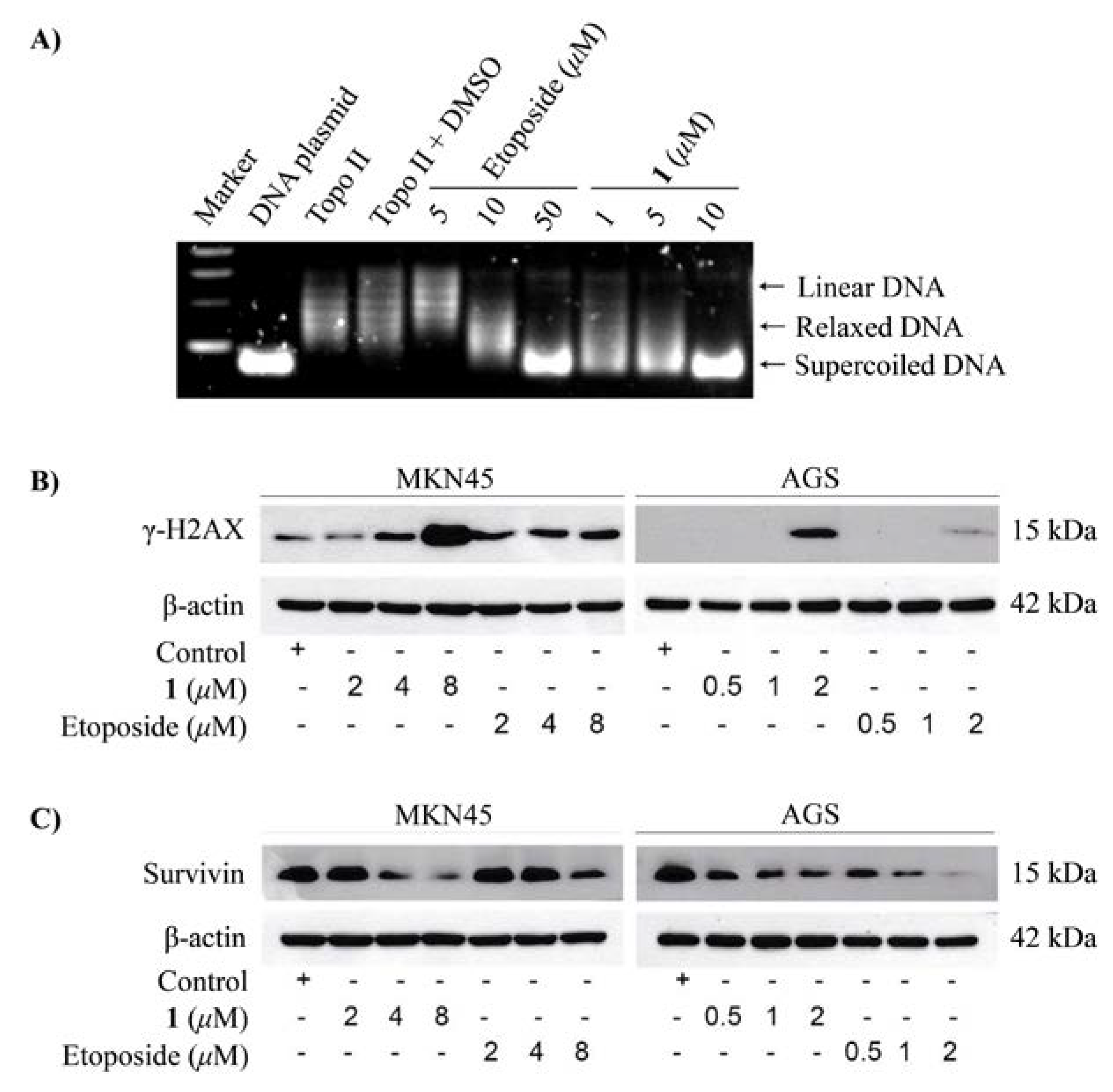
2.2. The Bioactivities of Compounds 1 and 3 from Streptomyces sp. OUCMDZ-3118
3. Experimental Section
3.1. General Experimental Procedures
3.2. Strain OUCMDZ-3118 Material
3.3. Cultivation and Extraction of OUCMDZ-3118
3.4. Purification
3.5. Sugar Analysis for 3
3.6. Supercoiled Plasmid DNA Relaxation Assay
3.7. Apoptosis Analysis
3.8. Western Blotting Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sanchez, C.; Mendez, C.; Salas, J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006, 23, 1007–1045. [Google Scholar] [CrossRef] [PubMed]
- Long, B.H.; Rose, W.C.; Vyas, D.M.; Matson, J.A.; Forenza, S. Discovery of Antitumor Indolocarbazoles: Rebeccamycin, NSC 655649, and Fluoroindolocarbazoles. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchiya, H.; Takahashi, Y.; Masuma, R. A new alkaloid am-2282 of streptomyces origin taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Undevia, S.D.; Vogelzang, N.J.; Mauer, A.M.; Janisch, L.; Mani, S.; Ratain, M.J. Phase I clinical trial of CEP-2563 dihydrochloride, a receptor tyrosine kinase inhibitor, in patients with refractory solid tumors. Investig. New Drugs 2004, 22, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Fuse, E.; Kuwabara, T.; Sparreboom, A.; Sausville, E.A.; Figg, W.D. Review of UCN-01 Development: A Lesson in the Importance of Clinical Pharmacology. J. Clin. Pharmacol. 2005, 45, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W. Becatecarin (Helsinn Healthcare). IDrugs 2005, 8, 838–847. [Google Scholar] [PubMed]
- Bharate, S.B.; Sawant, S.D.; Singh, P.P.; Vishwakarma, R.A. Kinase Inhibitors of Marine Origin. Chem. Rev. 2013, 113, 6761–6815. [Google Scholar] [CrossRef] [PubMed]
- Rasko, J.E.J.; Hughes, T.P. First Approved Kinase Inhibitor for AML. Cell 2017, 171, 981. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cui, C.; Gu, Q.; Zhu, W.; Liu, H.; Gu, J.; Osada, H. ZHD-0501, a novel naturally occurring staurosporine analog from Actinomadura sp. 007. Tetrahedron Lett. 2005, 46, 6137–6140. [Google Scholar] [CrossRef]
- Fu, P.; Zhuang, Y.; Wang, Y.; Liu, P.; Qi, X.; Gu, K.; Zhang, D.; Zhu, W. New Indolocarbazoles from a Mutant Strain of the Marine-Derived Actinomycete Streptomyces fradiae 007M135. Org. Lett. 2012, 14, 6194–6197. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Yang, C.; Wang, Y.; Liu, P.; Ma, Y.; Xu, L.; Su, M.; Hong, K.; Zhu, W. Streptocarbazoles A and B, Two Novel Indolocarbazoles from the Marine-Derived Actinomycete Strain Streptomyces sp. FMA. Org. Lett. 2012, 14, 2422–2425. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwaran, G.G.; Mohanakrishnan, A.K. Synthetic studies on indolocarbazoles: Total synthesis of staurosporine aglycon. Org. Lett. 2011, 13, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mei, X.; Wang, C.; Zhu, W. Biomimetic semi-synthesis of fradcarbazole A and its analogues. Tetrahedron 2015, 71, 7990–7997. [Google Scholar] [CrossRef]
- Varala, R. Scope of Selective Heterocycles from Organic and Pharmaceutical Perspective; InTechOpen: London, UK, 2016; pp. 83–114. [Google Scholar]
- Graziani, E.I.; Ritacco, F.V.; Summers, M.Y.; Zabriskie, T.M.; Yu, K.; Bernan, V.S.; Greenstein, M.; Carter, G.T. Novel sulfur-containing rapamycin analogs prepared by precursor-directed biosynthesis. Org. Lett. 2003, 5, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Kwon, S.J.; Dordick, J.S. In vitro precursor-directed synthesis of polyketide analogues with coenzyme a regeneration for the development of antiangiogenic agents. Org. Lett. 2009, 11, 3806–3809. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Oswald, N.W.; Legako, A.G.; Life, J.M.; Posner, B.A.; MacMillan, J.B. Precursor-directed generation of amidine containing ammosamide analogs: ammosamides E–P. Chem. Sci. 2013, 4, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Zhang, H.J.; Li, J.Q.; Ding, W.J.; Ma, W.J. Bioactive Indolocarbazoles from the Marine-Derived Streptomyces sp. DT-A61. J. Nat. Prod. 2018, 81, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Yasuzawa, T.; Iida, T.; Yoshida, M.; Hirayama, N.; Takahashi, M.; Shirahata, K.; Sano, H. The structures of the novel protein kinase C inhibitors K-252a, b, c and d. J. Antibiot. 1986, 39, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Nateewattana, J.; Saeeng, R.; Kasemsook, S.; Suksen, K.; Dutta, S.; Jariyawat, S.; Chairoungdua, A.; Suksamrarn, A.; Piyachaturawat, P. Inhibition of topoisomerase II α activity and induction of apoptosis in mammalian cells by semi-synthetic andrographolide analogues. Investig. New Drugs 2013, 31, 320–332. [Google Scholar] [CrossRef] [PubMed]

| Compound | 1 | 3 |
|---|---|---|
| A549 | 0.51 ± 0.05 | 1.2 ± 0.05 |
| K562 | 5.0 ± 0.2 | >10 |
| MCF-7 | 7.2 ± 0.6 | 1.6 ± 0.09 |
| Cell Line | Incubation Time (hours) | IC50 (μM) | |
|---|---|---|---|
| Etoposide | 1 | ||
| MKN45 | 24 | >20 | 14.2 ± 1.8 |
| 48 | >20 | 4.3 ± 1.0 | |
| 72 | 16.0 ± 2.3 | 4.7 ± 0.4 | |
| AGS | 24 | >20 | 4.9 ± 1.3 |
| 48 | 8.6 ± 2.4 | 1.7 ± 0.2 | |
| 72 | 2.9 ± 0.5 | 2.1 ± 0.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Monger, A.; Wang, L.; Fu, P.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Precursor-Directed Generation of Indolocarbazoles with Topoisomerase IIα Inhibitory Activity. Mar. Drugs 2018, 16, 168. https://doi.org/10.3390/md16050168
Wang C, Monger A, Wang L, Fu P, Piyachaturawat P, Chairoungdua A, Zhu W. Precursor-Directed Generation of Indolocarbazoles with Topoisomerase IIα Inhibitory Activity. Marine Drugs. 2018; 16(5):168. https://doi.org/10.3390/md16050168
Chicago/Turabian StyleWang, Cong, Adeep Monger, Liping Wang, Peng Fu, Pawinee Piyachaturawat, Arthit Chairoungdua, and Weiming Zhu. 2018. "Precursor-Directed Generation of Indolocarbazoles with Topoisomerase IIα Inhibitory Activity" Marine Drugs 16, no. 5: 168. https://doi.org/10.3390/md16050168
APA StyleWang, C., Monger, A., Wang, L., Fu, P., Piyachaturawat, P., Chairoungdua, A., & Zhu, W. (2018). Precursor-Directed Generation of Indolocarbazoles with Topoisomerase IIα Inhibitory Activity. Marine Drugs, 16(5), 168. https://doi.org/10.3390/md16050168