Effects of the Combination of Gliotoxin and Adriamycin on the Adriamycin-Resistant Non-Small-Cell Lung Cancer A549 Cell Line
Abstract
:1. Introduction
2. Results
2.1. Establishment of an ADR-Resistant Cell Line
2.2. GTX Overcame ADR Resistance in A549 NSCLC Cells
2.3. GTX Induced Apoptosis in A549/ADR Cells
2.3.1. GTX Induced Cell Cycle Arrest in A549/ADR Cells
2.3.2. Hoechst 33342 Staining of A549/ADR Cells Treated with GTX
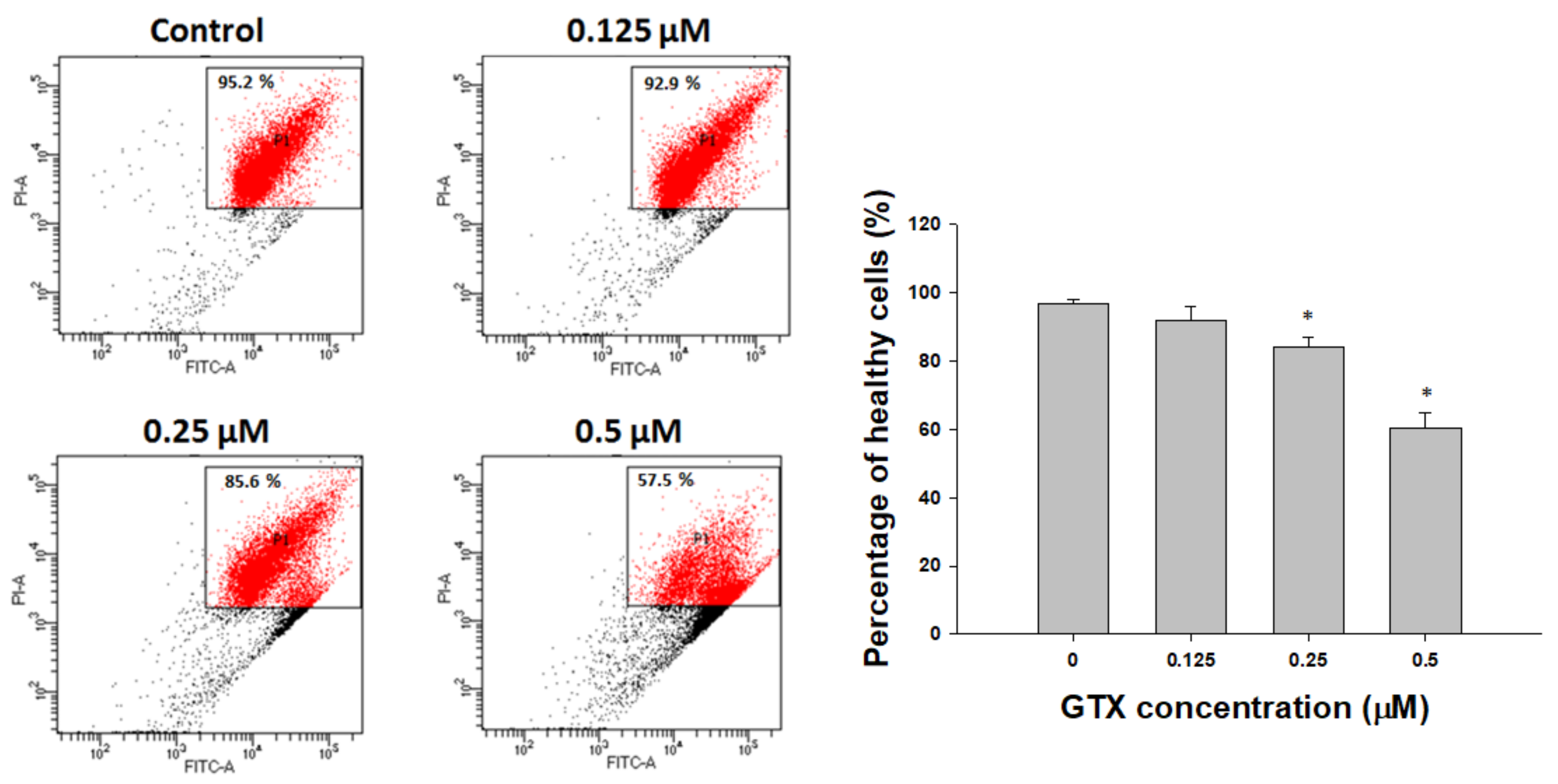
2.3.3. Annexin V/PI Staining
2.4. Disruption of Mitochondrial Membrane Potential by GTX in A549/ADR Cells
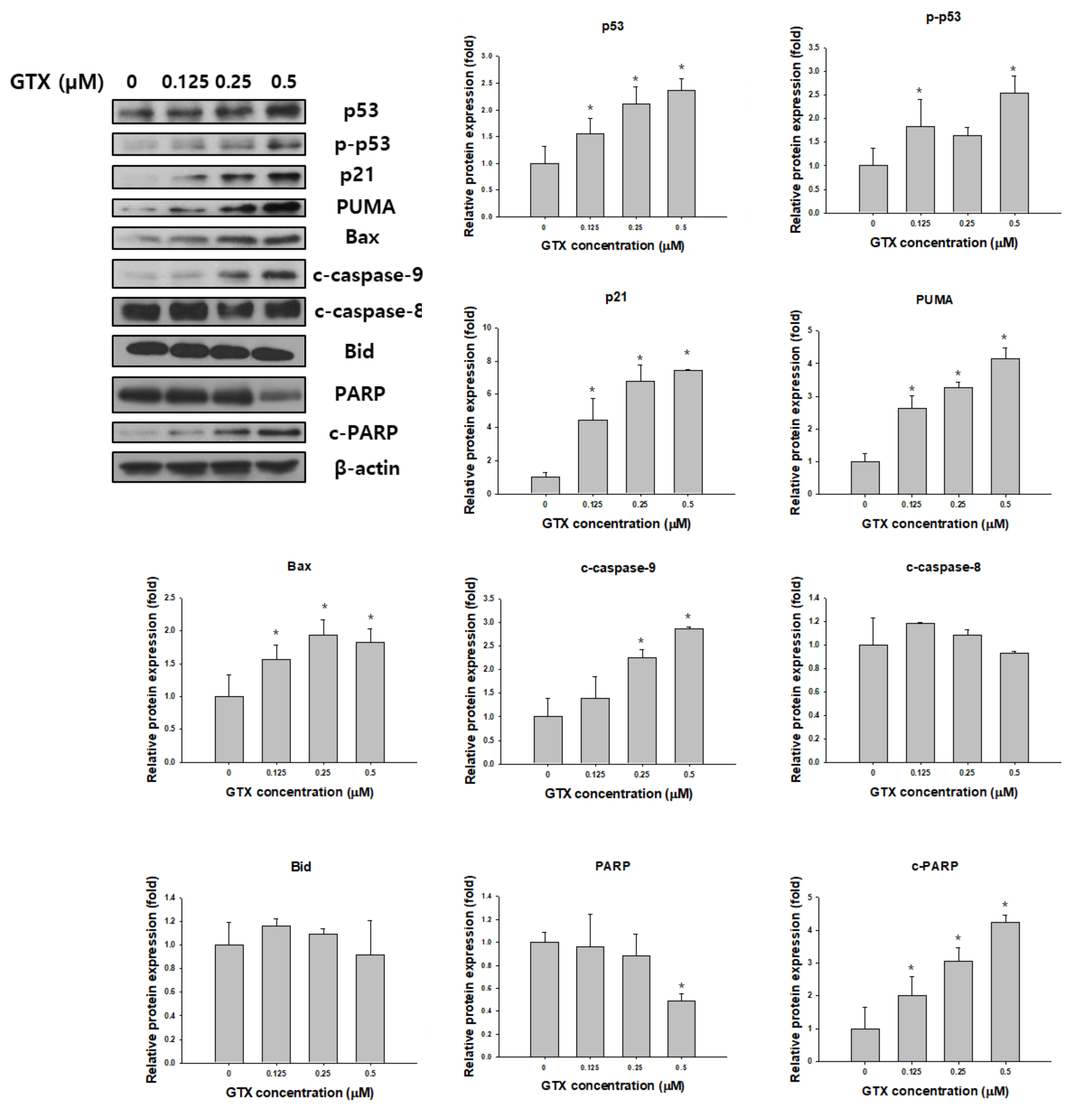
2.5. Effects of GTX on the Expression of Apoptosis-Related Proteins
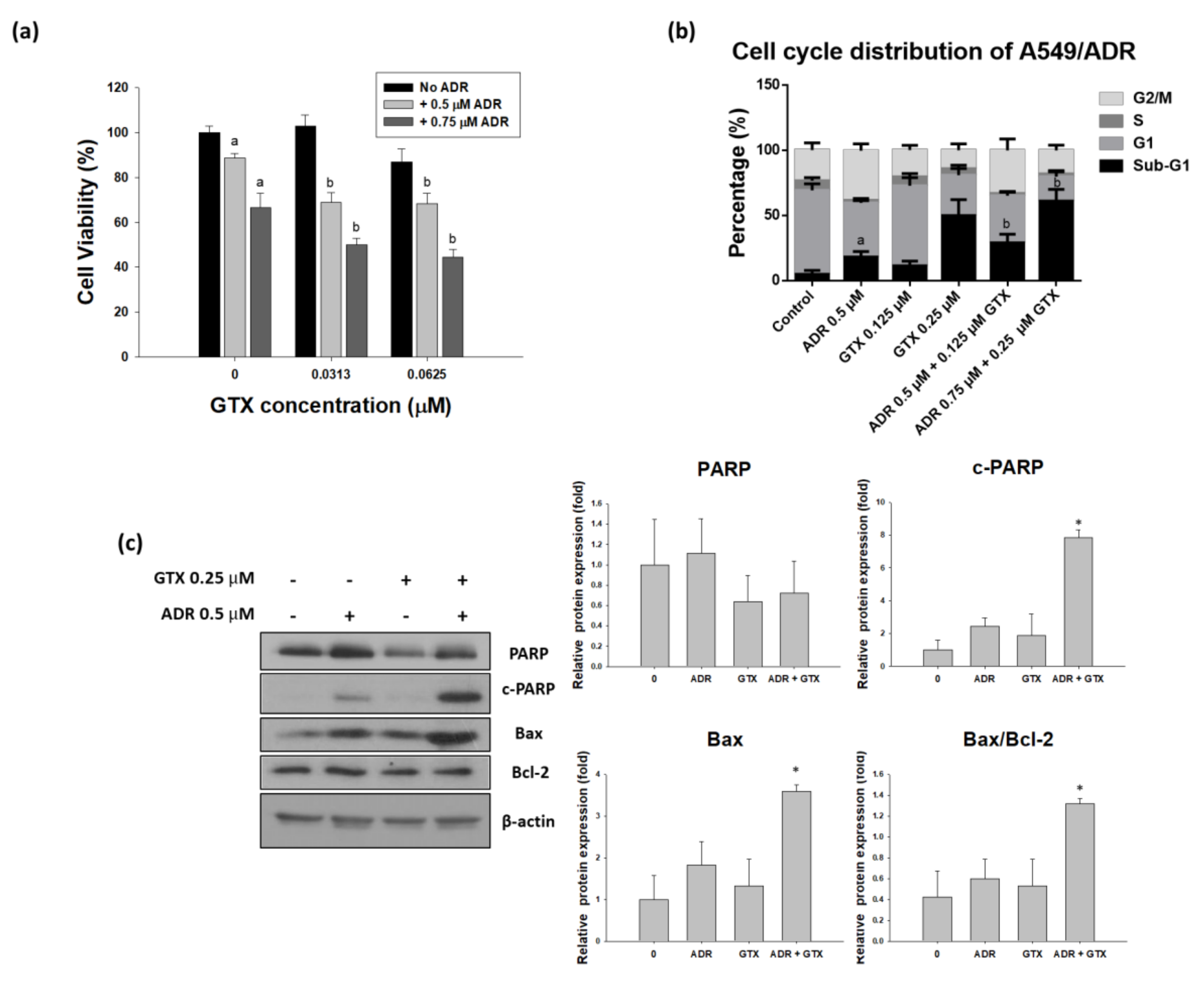
2.6. GTX Potentiates the Effects of ADR on A549/ADR Cells
3. Discussion
4. Materials and Methods
4.1. Cell Line, Reagents, and Chemicals
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Flow Cytometry
4.5. Western Blot Analysis
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sher, T.; Dy, G.K.; Adjei, A.A. Small Cell Lung Cancer. Mayo Clin. Proc. 2008, 83, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; de Marinis, F.; Dediu, M.; Thomas, M.; Pujol, J.-L.; Bidoli, P.; Molinier, O.; Sahoo, T.P.; Laack, E.; Reck, M. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (paramount): A double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012, 13, 247–255. [Google Scholar] [PubMed]
- Tsvetkova, E.; Goss, G. Drug resistance and its significance for treatment decisions in non-small-cell lung cancer. Curr. Oncol. 2012, 19, S45–S51. [Google Scholar] [PubMed]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from s. Peucetius var. Caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Zhang, X.; Rabbani, Z.N.; Liu, Y.; Reddy, S.K.; Su, Z.; Salahuddin, F.K.; Viles, K.; Giangrande, P.H.; Dewhirst, M.W. Rna aptamer-targeted inhibition of nf-κb suppresses non-small cell lung cancer resistance to doxorubicin. Mol. Ther. 2008, 16, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar] [PubMed]
- Choi, Y.K.; Kim, J.; Lee, K.M.; Choi, Y.-J.; Ye, B.-R.; Kim, M.-S.; Ko, S.-G.; Lee, S.-H.; Kang, D.-H.; Heo, S.-J. Tuberatolide B suppresses cancer progression by promoting ros-mediated inhibition of stat3 signaling. Mar. Drugs 2017, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, F.H.; Salvador, L.A.; Law, B.K.; Paul, V.J.; Luesch, H. Kempopeptin c, a novel marine-derived serine protease inhibitor targeting invasive breast cancer. Mar. Drugs 2017, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-C.; Din, Z.-H.; Su, J.-H.; Wu, Y.-J.; Liu, C.-I. Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the pi3k/akt/mtor signaling pathway in human bladder cancer cells. Mar. Drugs 2017, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.M.; Waring, P.; Howlett, B.J. The epipolythiodioxopiperazine (etp) class of fungal toxins: Distribution, mode of action, functions and biosynthesis. Microbiology 2005, 151, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Reilly, H.C.; Schatz, A.; Waksman, S.A. Antifungal properties of antibiotic substances. J. Bacterial. 1945, 49, 585. [Google Scholar]
- McDougall, J. Antiviral action of gliotoxin. Arch. Virol. 1969, 27, 255–267. [Google Scholar] [CrossRef]
- López-Franco, O.; Suzuki, Y.; Sanjuán, G.; Blanco, J.; Hernández-Vargas, P.; Yo, Y.; Kopp, J.; Egido, J.; Gómez-Guerrero, C. Nuclear factor-κb inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am. J. Pathol. 2002, 161, 1497–1505. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Lan, W.; Huang, C.; Lin, M.; Wang, Z.; Liang, W.; Iwamoto, A.; Yang, X.; Liu, H. Gliotoxin inhibits proliferation and induces apoptosis in colorectal cancer cells. Mar. Drugs 2015, 13, 6259–6273. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Lee, J.S.; Qian, Z.-J.; Li, Y.-X.; Kim, K.-N.; Heo, S.-J.; Jeon, Y.-J.; Park, W.S.; Choi, I.-W.; Je, J.-Y. Gliotoxin isolated from marine fungus aspergillus sp. Induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar. Drugs 2013, 12, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, R.; Hilgarth, M.; Schnabl, S.; Ponath, E.; Reiter, M.; Demirtas, D.; Sieghart, W.; Valent, P.; Zielinski, C.; Jäger, U. Gliotoxin is a potent notch2 transactivation inhibitor and efficiently induces apoptosis in chronic lymphocytic leukaemia (CLL) cells. Br. J. Haematol. 2013, 160, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, R.; Sieghart, W.; Schnabl, S.; Araghi, M.; Hilgarth, M.; Reiter, M.; Demirtas, D.; Valent, P.; Zielinski, C.; Jäger, U. Gliotoxin targets nuclear notch2 in human solid tumor derived cell lines in vitro and inhibits melanoma growth in xenograft mouse model. Front. Pharmacol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.; Mirsaidi, N.; Brooke, G.; Sun, C.; Pace, P.; Inman, L.; Moody, C.; Coombes, R. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase i with antitumor activity against breast cancer in vivo. Med. Oncol. 2004, 21, 21–30. [Google Scholar] [CrossRef]
- Li, J.W.-H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lorendeau, D.; Dury, L.; Genoux-Bastide, E.; Lecerf-Schmidt, F.; Simoes-Pires, C.; Carrupt, P.-A.; Terreux, R.; Magnard, S.; Di Pietro, A.; Boumendjel, A. Collateral sensitivity of resistant mrp1-overexpressing cells to flavonoids and derivatives through gsh efflux. Biochem. Pharmacol. 2014, 90, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lorendeau, D.; Dury, L.; Nasr, R.; Boumendjel, A.; Teodori, E.; Gutschow, M.; Falson, P.; Di Pietro, A.; Baubichon-Cortay, H. Mrp1-dependent collateral sensitivity of multidrug-resistant cancer cells: Identifying selective modulators inducing cellular glutathione depletion. Curr. Med. Chem. 2017, 24, 1186–1213. [Google Scholar] [CrossRef] [PubMed]
- De Groot, D.; Van Der Deen, M.; Le, T.; Regeling, A.; De Jong, S.; De Vries, E. Indomethacin induces apoptosis via a mrp1-dependent mechanism in doxorubicin-resistant small-cell lung cancer cells overexpressing mrp1. Br. J. Cancer 2007, 97, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005, 12, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, A.; Shao, M.; Lin, L.; Li, P.; Wang, Y. Schisandrin b reverses doxorubicin resistance through inhibiting p-glycoprotein and promoting proteasome-mediated degradation of survivin. Sci. Rep. 2017, 7, 8419. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Kumar, V.B.; Weng, C.F.; Padma, V.V. Doxorubicin induced apoptosis was potentiated by neferine in human lung adenocarcima, a549 cells. Food Chem. Toxicol. 2014, 68, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, S.A.; Al-Abd, A.M.; Ali, A.A.; Abdel-Naim, A.B. Didox and resveratrol sensitize colorectal cancer cells to doxorubicin via activating apoptosis and ameliorating p-glycoprotein activity. Sci. Rep. 2016, 6, 36855. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Curtin, N. The role of parp in DNA repair and its therapeutic exploitation. Br. J. Cancer 2011, 105, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma u373mg cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manh Hung, L.V.; Song, Y.W.; Cho, S.K. Effects of the Combination of Gliotoxin and Adriamycin on the Adriamycin-Resistant Non-Small-Cell Lung Cancer A549 Cell Line. Mar. Drugs 2018, 16, 105. https://doi.org/10.3390/md16040105
Manh Hung LV, Song YW, Cho SK. Effects of the Combination of Gliotoxin and Adriamycin on the Adriamycin-Resistant Non-Small-Cell Lung Cancer A549 Cell Line. Marine Drugs. 2018; 16(4):105. https://doi.org/10.3390/md16040105
Chicago/Turabian StyleManh Hung, Le Van, Yeon Woo Song, and Somi Kim Cho. 2018. "Effects of the Combination of Gliotoxin and Adriamycin on the Adriamycin-Resistant Non-Small-Cell Lung Cancer A549 Cell Line" Marine Drugs 16, no. 4: 105. https://doi.org/10.3390/md16040105



