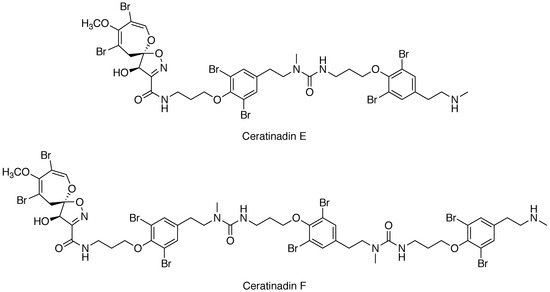
Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp.
Abstract
:1. Introduction
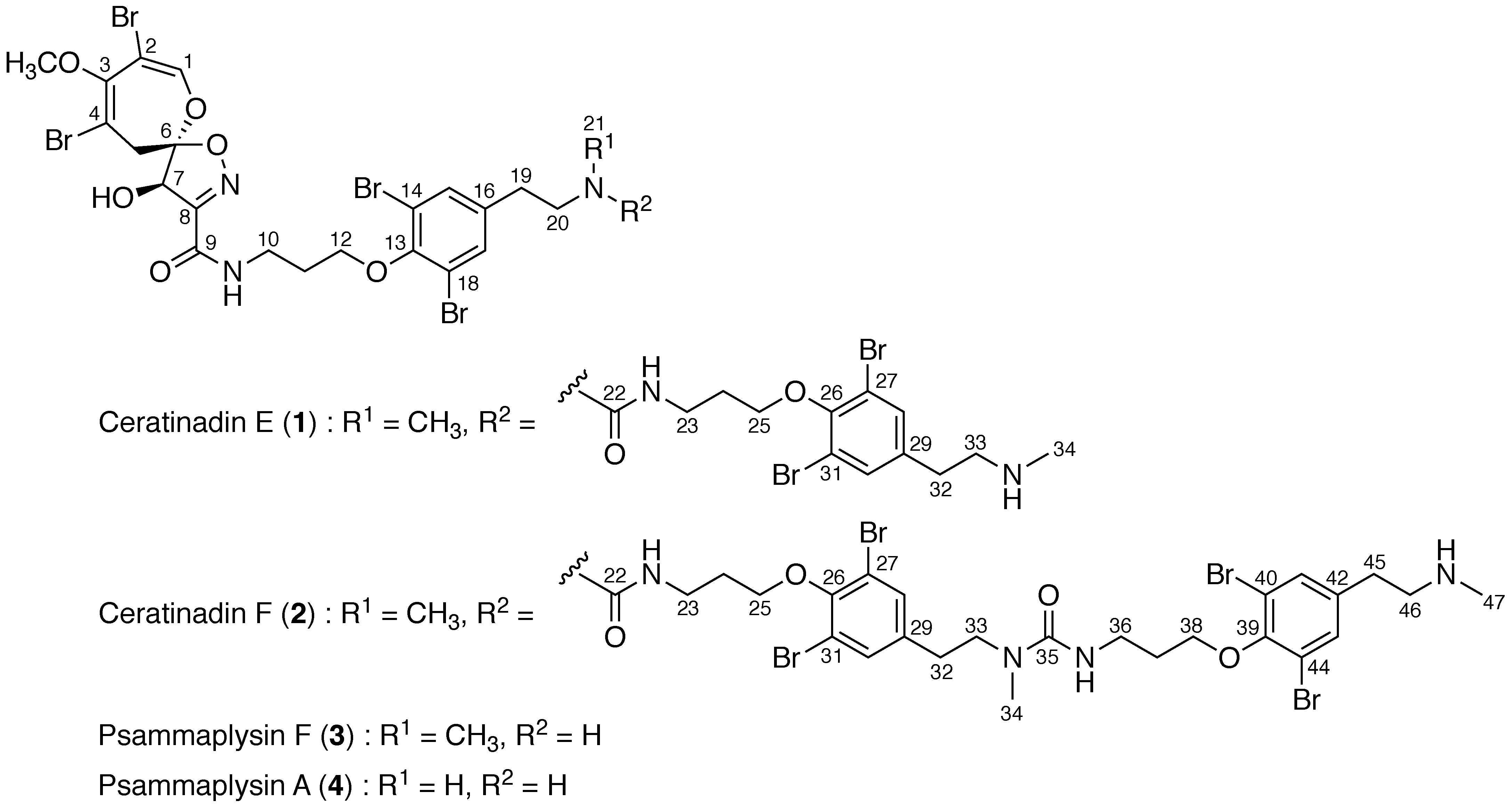
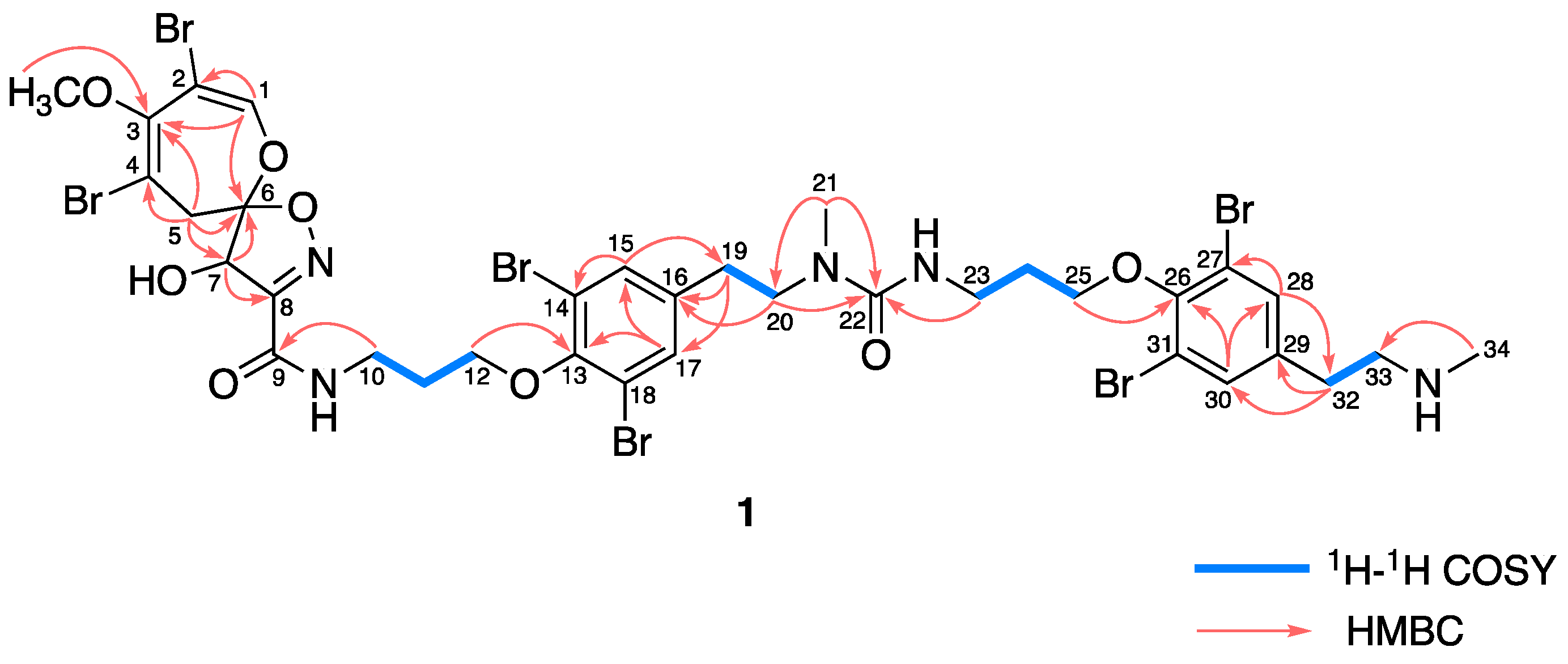
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction and Isolation
4.3. Ceratinadin E (1)
4.4. Ceratinadin F (2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2017; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Fu, X.; Schmitz, F.J.; Kelly-Borges, M. Psammaplysin F, a new bromotyrosine derivative from a sponge, Aplysinella sp. J. Nat. Prod. 1997, 60, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial Bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Andrews, K.T.; Birell, G.W.; Tran, T.L.; Camp, D.; Davis, R.A.; Quinn, R.J. Psammaplysin H, a new antimalarial bromotyrosine alkaloid from a marine sponge of the genus Pseudoceratina. Bioorg. Med. Chem. Lett. 2011, 21, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strangylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kubota, T.; Tsuda, M.; Watanabe, K.; Fromont, J.; Kobayashi, J. Ma’edamines A and B, cytotoxic bromotyrosine alkaloids with a unique 2(1H)pyrazinone ring from sponge Suberea sp. Tetrahedron 2000, 56, 8107–8110. [Google Scholar] [CrossRef]
- Mukai, H.; Kubota, T.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Tyrokeradines A and B, new bromotyrosine alkaloids with an imidazolyl-quinolinone moiety from a Verongid sponge. Bioorg. Med. Chem. Lett. 2009, 19, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Kon, Y.; Kubota, T.; Shibazaki, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Ceratinadins A-C, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Bioorg. Med. Chem. Lett. 2010, 20, 4569–4572. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watase, S.; Mukai, H.; Fromont, J.; Kobayashi, J. Tyrokeradines C-F, new bromotyrosine alkaloids from the Verongid sponges. Chem. Pharm. Bull. 2012, 60, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watase, S.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J. Tyrokeradines G and H, new bromotyrosine alkaloids from an Okinawan Verongid sponge. Bioorg. Med. Chem. Lett. 2015, 25, 5221–5223. [Google Scholar] [CrossRef] [PubMed]
- Rotem, M.; Carmely, S.; Kashman, Y. Two new antibiotics from the red sea sponge Psammaplysilla purpurea: Total 13C-NMR line assignment of psammaplysins A and B and aerothionin. Tetrahedron 1983, 39, 667–676. [Google Scholar] [CrossRef]
- Roll, D.M.; Chang, C.W.J.; Scheuer, P.J.; Gray, G.A.; Shoolery, J.M.; Matsumoto, G.K.; Duyne, G.D.V.; Clardy, J. Structure of the psammaplysins. J. Am. Chem. Soc. 1985, 107, 2916–2920. [Google Scholar] [CrossRef]
- Mándi, A.; Mudianta, I.W.; Kurtán, T.; Garson, M.J. Absolute configuration and conformational study of psammaplysins A and B from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2015, 78, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kobayashi, H.; van Soest, R.W.M.; Fusetani, N. Novel bromotyrosine derivatives that inhibit growth of the fish pathogenic bacterium Aeromonas hydrophila, from a marine sponge Hexadella sp. J. Org. Chem. 2005, 70, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Kohana, A.; Manabe, C.; Ishiyama, A.; Ui, H.; Shiomi, K.; Yamada, H.; Ōmura, S. Potent antimalarial activities of polyether antibiotic, X-206. J. Antibiot. 2001, 54, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Tymiak, A.A.; Rinehert, K.L., Jr. Biosynthesis of dibromotyrosine-derived antimicrobial compounds by the marine sponge Aplysina fistularis (Verongia aurea). J. Am. Chem. Soc. 1981, 103, 6763–6765. [Google Scholar] [CrossRef]

| Ceratinadin E (1) | Ceratinadin F (2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | δH a | Multi (J in Hz) | δC b | Multi | Position | δH a | Multi (J in Hz) | δC b | Multi |
| 1 | 7.18 | s | 147.6 | d | 1 | 7.17 | s | 147.6 | d |
| 2 | − | 105.1 | s | 2 | − | 105.1 | s | ||
| 3 | − | 150.7 | s | 3 | − | 150.7 | s | ||
| 3-OCH3 | 3.69 d | s | 60.1 | q | 3-OCH3 | 3.69 d | s | 60.1 | q |
| 4 | - | 105.3 | s | 4 | - | 105.3 | s | ||
| 5a | 3.40 | me | 39.1 | t | 5a | 3.41 | d (16.4) | 39.1 | t |
| 5b | 3.11 | d (15.7) | 5b | 3.10 | d (16.4) | ||||
| 6 | - | 121.7 | s | 6 | - | 121.6 | s | ||
| 7 | 5.03 | s | 81.2 | d | 7 | 5.02 | s | 81.2 | d |
| 8 | - | 159.5 | s | 8 | - | 159.5 | s | ||
| 9 | - | 161.5 | s | 9 | - | 161.5 | s | ||
| 10 | 3.65 c | td (6.6, 1.2) | 38.8 | t | 10 | 3.65 c | td (6.6, 2.7) | 38.8 | t |
| 11 | 2.15 c | dt (6.6, 6.6) | 31.3 | t | 11 | 2.15 c | dt (6.6, 6.6) | 31.3 | t |
| 12 | 4.08 c | me | 72.9 | t | 12 | 4.08 c | me | 72.9 | t |
| 13 | - | 153.5 | s | 13 | - | 153.5 | s | ||
| 14, 18 | - | 119.7 | s | 14, 18 | - | 119.8 | s | ||
| 15, 17 | 7.49 | s | 135.2 | d | 15, 17 | 7.49 | s | 135.2 | d |
| 16 | - | 140.8 | s | 16 | - | 140.8 | s | ||
| 19 | 2.81 c | t (7.1) | 34.6 | t | 19 | 2.81 c | me | 34.8 | t |
| 20 | 3.52 c | t (7.1) | 51.7 | t | 20 | 3.51 c | me | 51.7 | t |
| 21 | 2.88 d | s | 35.8 | q | 21 | 2.88 d | s | 35.8 | q |
| 22 | - | 161.2 | s | 22 | - | 161.2 | s | ||
| 23 | 3.45 c | me | 40.1 | t | 23 | 3.45 c | me | 40.2 | t |
| 24 | 2.06 c | dt (6.4, 6.4) | 32.4 | t | 24 | 2.05 c | me | 32.5 | t |
| 25 | 4.09 c | me | 73.7 | t | 25 | 4.08 c | me | 73.7 | t |
| 26 | - | 154.4 | s | 26 | - | 153.6 | s | ||
| 27, 31 | - | 120.3 | s | 27, 31 | - | 119.8 | s | ||
| 28, 30 | 7.59 | s | 135.2 | d | 28, 30 | 7.49 | s | 135.2 | d |
| 29 | - | 138.0 | s | 29 | - | 140.7 | s | ||
| 32 | 2.98 | br t (7.6) | 33.0 | t | 32 | 2.81 | me | 34.8 | t |
| 33 | 3.27 | br t (7.6) | 51.9 | t | 33 | 3.52 | me | 51.7 | t |
| 34 | 2.70 d | s | 34.7 | q | 34 | 2.88 d | s | 35.8 | q |
| 35 | - | 161.2 | s | ||||||
| 36 | 3.44 c | me | 40.2 | t | |||||
| 37 | 2.05 c | me | 32.4 | t | |||||
| 38 | 4.05 c | me | 73.6 | t | |||||
| 39 | - | 154.5 | s | ||||||
| 40, 44 | - | 120.3 | s | ||||||
| 41, 43 | 7.56 | s | 135.2 | d | |||||
| 42 | - | 138.0 | s | ||||||
| 45 | 2.93 | br t (7.8) | 33.0 | t | |||||
| 46 | 3.18 | me | 51.9 | t | |||||
| 47 | 2.70 d | s | 34.7 | q | |||||
| Antimalarial Activity a | Cytotoxicity a | Selectivity Index | |||
|---|---|---|---|---|---|
| K1 b | FCR3 c | MRC-5d | MRC-5/K1 | MRC-5/FCR3 | |
| Psammaplysin F (3) | 3.77 | 2.45 | 12.65 | 3.4 | 5.2 |
| Ceratinadin E (1) | 1.03 | 0.77 | 15.99 | 15.5 | 20.8 |
| Ceratinadin F (2) | >12.5 | -e | >50 | >4 | -e |
| Chloroquine f | 0.34 | 0.035 | >25.80 | >75.9 | >737.1 |
| Artemisinin f | 0.010 | 0.0088 | >14.12 | >1412 | >1604.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurimoto, S.-i.; Ohno, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Ōmura, S.; Kobayashi, J.; Kubota, T. Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Mar. Drugs 2018, 16, 463. https://doi.org/10.3390/md16120463
Kurimoto S-i, Ohno T, Hokari R, Ishiyama A, Iwatsuki M, Ōmura S, Kobayashi J, Kubota T. Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Marine Drugs. 2018; 16(12):463. https://doi.org/10.3390/md16120463
Chicago/Turabian StyleKurimoto, Shin-ichiro, Taito Ohno, Rei Hokari, Aki Ishiyama, Masato Iwatsuki, Satoshi Ōmura, Jun’ichi Kobayashi, and Takaaki Kubota. 2018. "Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp." Marine Drugs 16, no. 12: 463. https://doi.org/10.3390/md16120463