The Inhibitory Activity of Luzonicosides from the Starfish Echinaster luzonicus against Human Melanoma Cells
Abstract
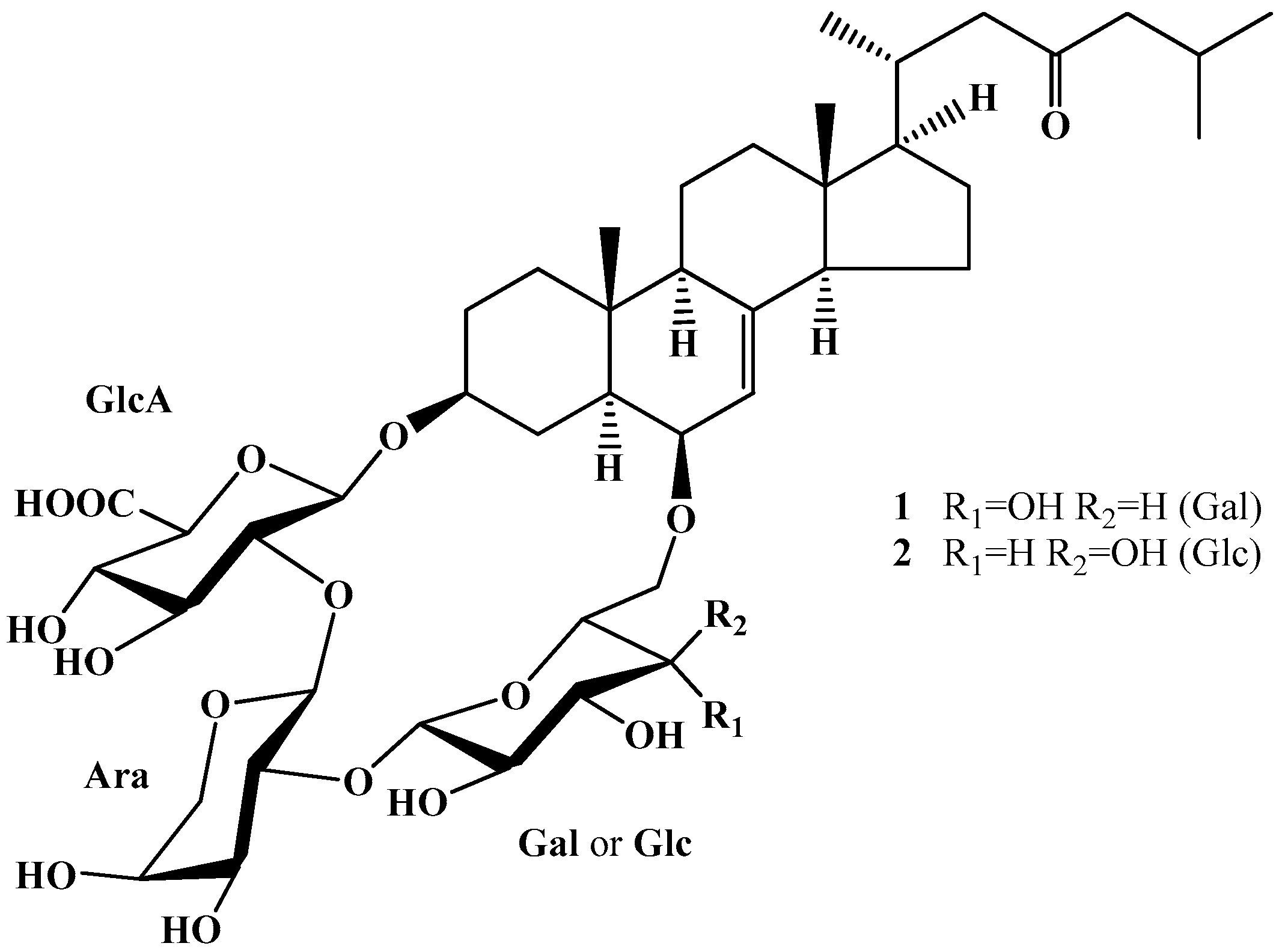
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity and Inhibition of Malignant Melanoma Cell Proliferation by Luzonicosides A and D
2.2. The Cell Cycle Regulation and Induction of Apoptosis of Malignant Melanoma Cells by Luzonicosides A and D
2.3. The Molecular Mechanism of the Pro-Apoptotic Effect of Luzonicosides A and D
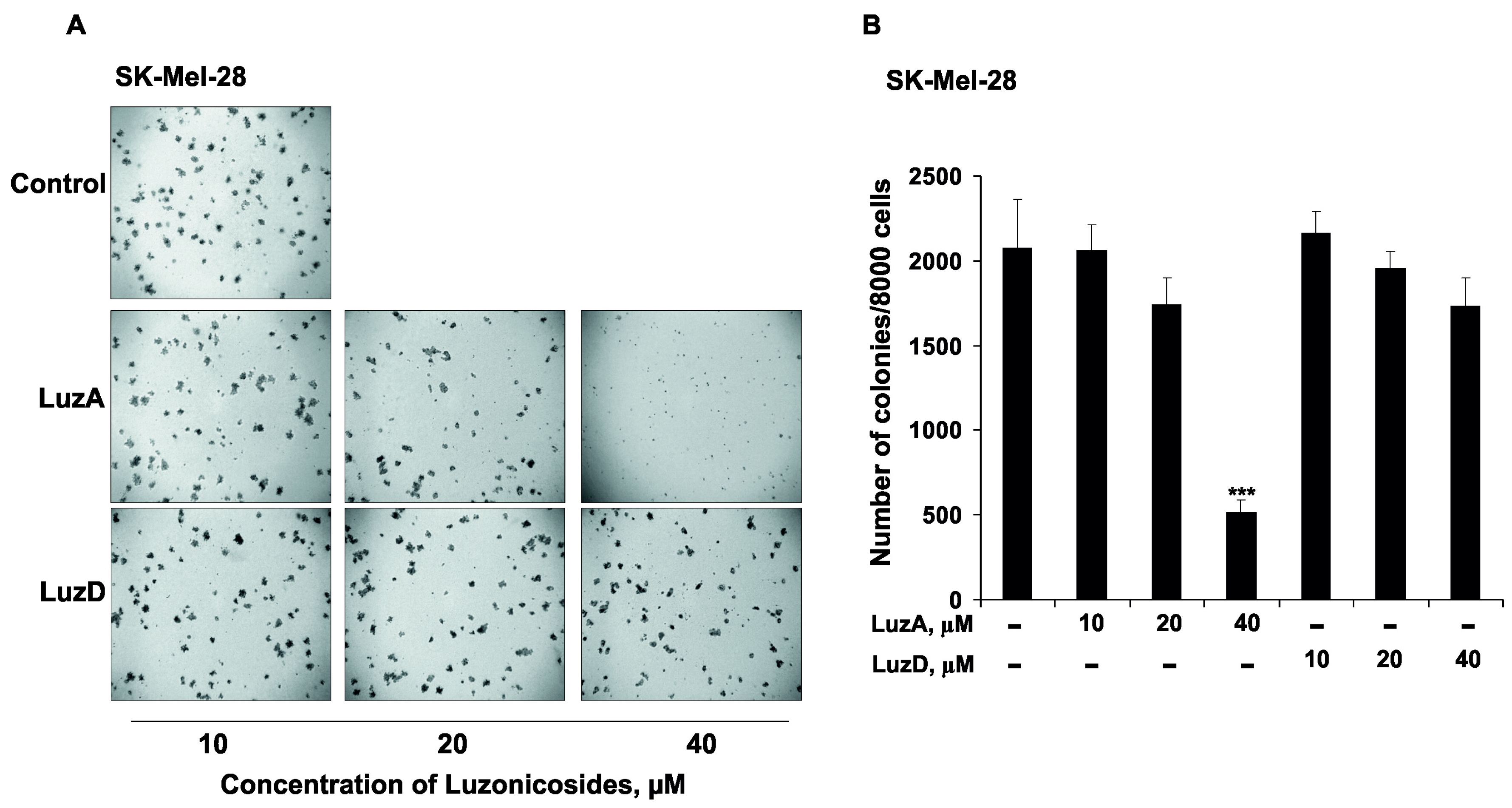
2.4. The Inhibition of Malignant Melanoma Cell Colony Formation by Luzonicosides A and D
2.5. The Inhibition of Malignant Melanoma Cell Migration by Luzonicosides A and D
3. Materials and Methods
3.1. Reagents and Drugs
3.2. Cell Culture
3.3. Cytotoxicity Assay
3.4. Cell Proliferation Assay
3.5. Cell Cycle Analysis and Detection of Apoptotic Cells
3.6. Western Blot Analysis
3.7. Soft Agar Clonogenic Assay
3.8. In Vitro Scratch Assay
3.9. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. Gold from the sea: Marine compounds as inhibitors of the hallmarks of cancer. Biotechnol. Adv. 2011, 29, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Xu, T.; Yang, B.; Lin, X.; Zhou, X.; Yang, X.; Liu, Y. Chemical constituents and bioactivities of starfish. Chem. Biodivers. 2011, 8, 740–791. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Stonik, V.A. Recent studies of polar steroids from starfish: Structures, biological activities and biosynthesis. In Adwances in Natural Products Discovery; Gomes, A.R., Rocha-Santos, T., Duarte, A., Eds.; Nova Science Publishers, Inc: Hauppauge, NY, USA, 2017; Volume Chapter 6, pp. 191–224. [Google Scholar]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Minale, L.; Riccio, R.; Zollo, F. Steroidal oligoglycosides and polyhydroxysteroids from echinoderms. Fortschr. Chem. Org. Naturst. 1993, 62, 75–308. [Google Scholar] [PubMed]
- Tian, X.; Tang, H.; Lin, H.; Cheng, G.; Wang, S.; Zhang, X. Saponins: The potential chemotherapeutic agents in pursuing new anti-glioblastoma drugs. Mini Rev. Med. Chem. 2013, 13, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, G.; Tang, H.F.; Zhang, X. Novaeguinoside II inhibits cell proliferation and induces apoptosis of human brain glioblastoma U87MG cells through the mitochondrial pathway. Brain Res. 2011, 1372, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, C.; Li, X.; Zhang, Z.; Yuan, Y.; Ni, Y.; Liu, T.; Deng, S.; Zhao, J.; Wang, Y. Asterosaponin 1 induces endoplasmic reticulum stress-associated apoptosis in A549 human lung cancer cells. Oncol. Rep. 2011, 26, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Tang, H.F.; Qiu, F.; Lin, H.W.; Tian, X.R.; Yao, M.N. Polyhydroxysteroidal glycosides from the starfish Anthenea chinensis. J. Nat. Prod. 2010, 73, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Ivanchina, N.V.; Huong, T.T.; Kalinovsky, A.I.; Dmitrenok, P.S.; Fedorov, S.N.; Dyshlovoy, S.A.; Long, P.Q.; Stonik, V.A. Two new asterosaponins, archasterosides A and B, from the Vietnamese starfish Archaster typicus and their anticancer properties. Bioorg. Med. Chem. Lett. 2010, 20, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.; Kim, E.J.; Kang, H.K.; Kim, S.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. Asterosaponins from the starfish Astropecten monacanthus suppress growth and induce apoptosis in HL-60, PC-3, and SNU-C5 human cancer cell lines. Biol. Pharm. Bull. 2014, 37, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kalinovsky, A.I.; Kicha, A.A.; Malyarenko, T.V.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Two new asterosaponins from the Far Eastern starfish Lethasterias fusca. Nat. Prod. Commun. 2012, 7, 853–858. [Google Scholar] [PubMed]
- Kicha, A.A.; Kalinovsky, A.I.; Ivanchina, N.V.; Malyarenko, T.V.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Four new asterosaponins, hippasteriosides A-D, from the Far Eastern starfish Hippasteria kurilensis. Chem. Biodivers. 2011, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Vishchuk, O.S.; Stonik, V.A. Asterosaponins from the Far Eastern starfish Leptasterias ochotensis and their anticancer activity. Steroids 2014, 87, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Ermakova, S.P.; Stonik, V.A. Cariniferosides A-F and other steroidal biglycosides from the starfish Asteropsis carinifera. Steroids 2011, 76, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Ivanchina, N.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Yurchenko, E.A.; Pislyagin, E.A.; Aminin, D.L.; Huong, T.T.; et al. Cyclic steroid glycosides from the starfish Echinaster luzonicus: Structures and immunomodulatory activities. J. Nat. Prod. 2015, 78, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Unusual steroid constituents from the tropical starfish Leiaster sp. Nat. Prod. Commun. 2016, 11, 1251–1252. [Google Scholar]
- Riccio, R.; De Simone, F.; Dini, A.; Minale, L.; Pizza, C.; Senatore, F.; Zollo, F. Starfish saponins 6. Unique 22,23-epoxysteroidal cyclic glycosides, minor constituents from Echinaster sepositus. Tetrahedron Lett. 1981, 22, 1557–1560. [Google Scholar] [CrossRef]
- Malyarenko, T.V.; Malyarenko (Vishchuk), O.S.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Kicha, A.A. Four new sulfated polar steroids from the far eastern starfish Leptasterias ochotensis: Structures and activities. Mar. Drugs 2015, 13, 4418–4435. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.; Nurse, P. Animal cell cycles and their control. Annu. Rev. Biochem. 1992, 61, 441–470. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Kirby, R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care 2008, 572–585. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, X.; Tang, H.F.; Zhang, Y.; Zhang, X.H.; Cao, W.D.; Gao, D.K.; Wang, X.L.; Jin, B.Q. Asterosaponin 1, a cytostatic compound from the starfish Culcita novaeguineae, functions by inducing apoptosis in human glioblastoma U87MG cells. J. Neurooncol. 2006, 79, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Karuppusamy Rathinam, M.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Hu, D.; Tu, L.; Zhou, X.; Lu, F.; Wen, B.; Wu, W.; Lin, Y.; Zhou, Z.; Qu, J. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Sun, H.; Wang, Z.; Ermakova, S.P.; Xiao, J.; Lu, T.; Xue, P.; Zvyagintseva, T.N.; Xiong, H.; Shao, C.; et al. PDZ-binding kinaze /TLAK cell-originated protein kinase is a target of the fucoidan from brown alga Fucus evanescens in the prevention of EGF-induced neoplastic cell transformation and colon cancer growth. Oncotarget 2016, 7, 18763–18773. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: isolation, structural characteristics and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Naeth, I.; Venz, S.; Preukschas, M.; Sievert, H.; Jacobsen, C.; Shubina, L.K.; Gesell Salazar, M.; Scharf, C.; Walther, R.; et al. Proteomic profiling of germ cell cancer cells treated with aaptamine, a marine alkaloid with antiproliferative activity. J. Proteome Res. 2012, 11, 2316–2330. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014, 96, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Fedorov, S.N.; Kalinovsky, A.I.; Shubina, L.K.; Bokemeyer, C.; Stonik, V.A.; Honecker, F. Mycalamide A shows cytotoxic properties and prevents EGF-induced neoplastic transformation through inhibition of nuclear factors. Mar. Drugs 2012, 10, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Usoltseva, R.V.; Shevchenko, N.M.; Isakov, V.V.; Zvyagintseva, T.N.; Ermakova, S.P. In vitro anticancer activity of the laminarans from Far Eastern brown seaweeds and their sulfated derivatives. J. Appl. Phycol. 2017, 29, 543–553. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyarenko, O.S.; Dyshlovoy, S.A.; Kicha, A.A.; Ivanchina, N.V.; Malyarenko, T.V.; Carsten, B.; Gunhild, V.A.; Stonik, V.A.; Ermakova, S.P. The Inhibitory Activity of Luzonicosides from the Starfish Echinaster luzonicus against Human Melanoma Cells. Mar. Drugs 2017, 15, 227. https://doi.org/10.3390/md15070227
Malyarenko OS, Dyshlovoy SA, Kicha AA, Ivanchina NV, Malyarenko TV, Carsten B, Gunhild VA, Stonik VA, Ermakova SP. The Inhibitory Activity of Luzonicosides from the Starfish Echinaster luzonicus against Human Melanoma Cells. Marine Drugs. 2017; 15(7):227. https://doi.org/10.3390/md15070227
Chicago/Turabian StyleMalyarenko, Olesya S., Sergey A. Dyshlovoy, Alla A. Kicha, Natalia V. Ivanchina, Timofey V. Malyarenko, Bokemeyer Carsten, Von Amsberg Gunhild, Valentin A. Stonik, and Svetlana P. Ermakova. 2017. "The Inhibitory Activity of Luzonicosides from the Starfish Echinaster luzonicus against Human Melanoma Cells" Marine Drugs 15, no. 7: 227. https://doi.org/10.3390/md15070227








