How Environmental Factors Affect the Production of Guanidine Alkaloids by the Mediterranean Sponge Crambe crambe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Temperature and Light Experiment
2.2. Nutrient Experiment
3. Results
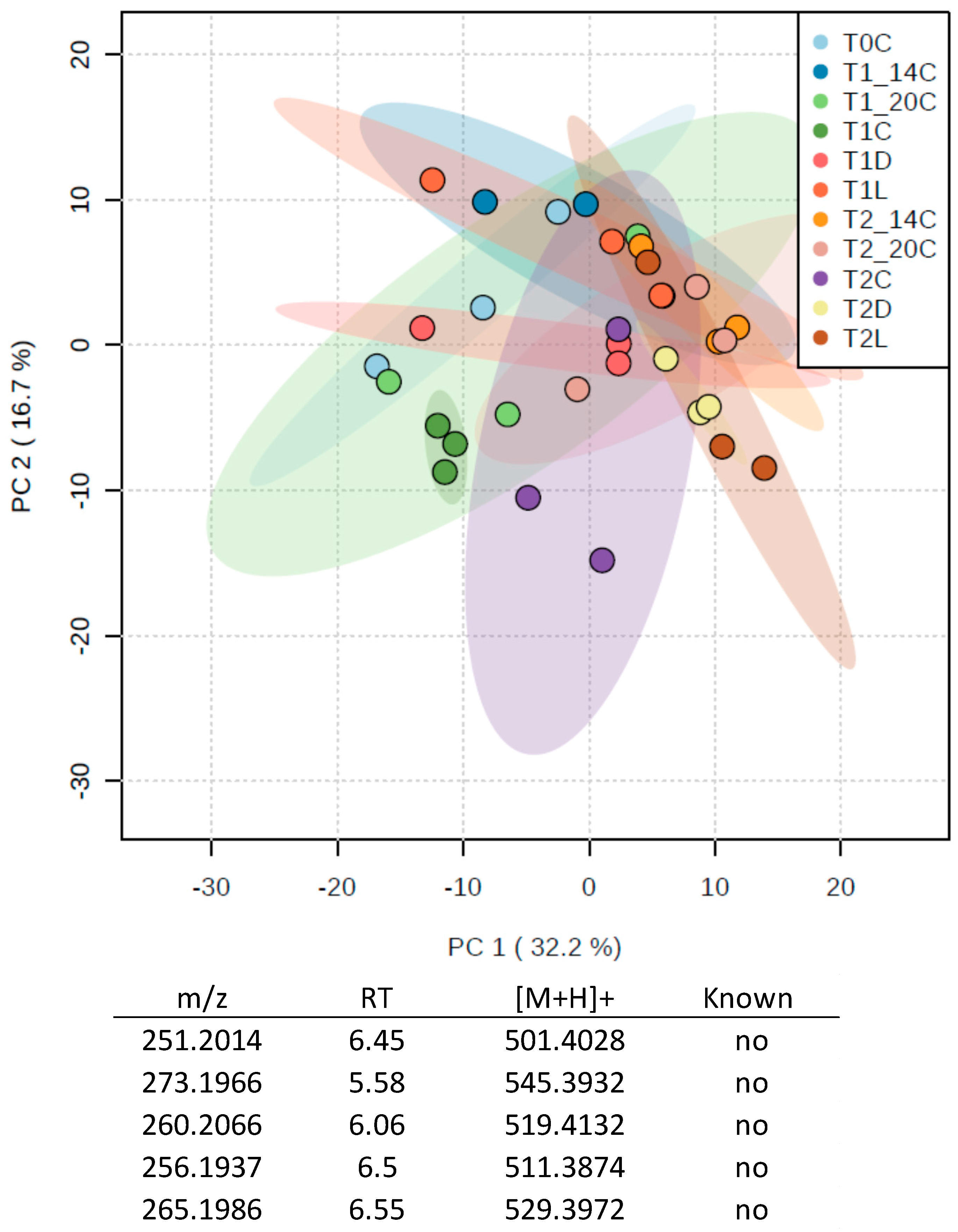
3.1. Temperature and Light Experiments
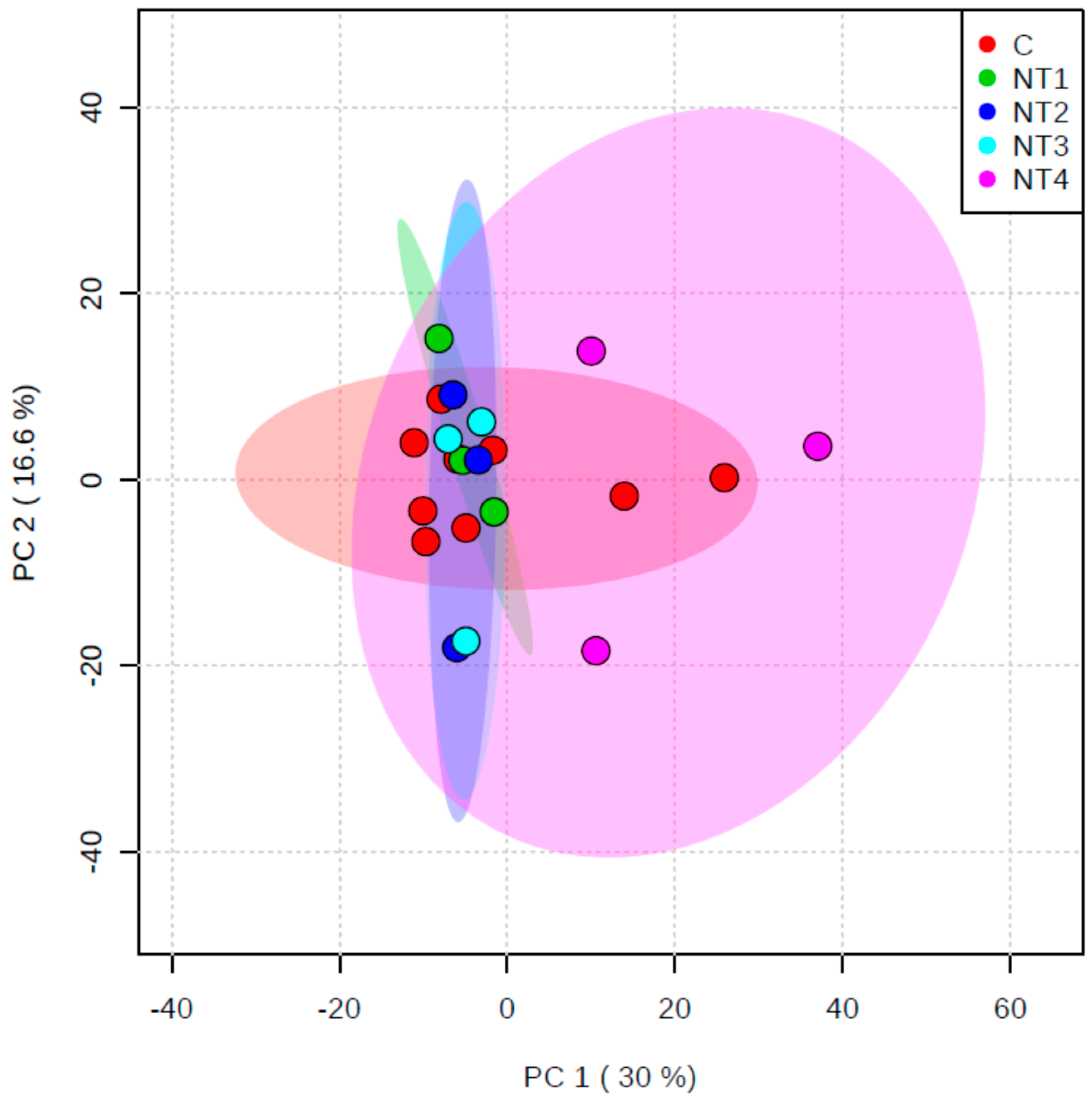
3.2. Nutrient Experiment
3.2.1. Nutrient Concentrations
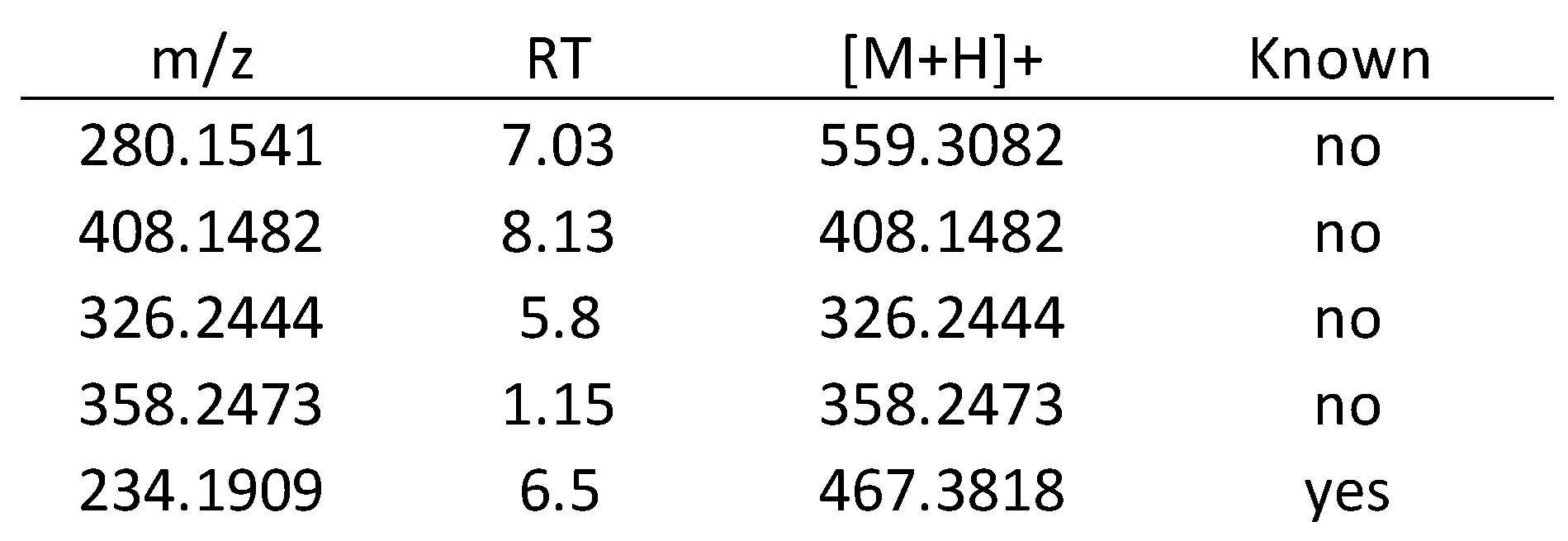
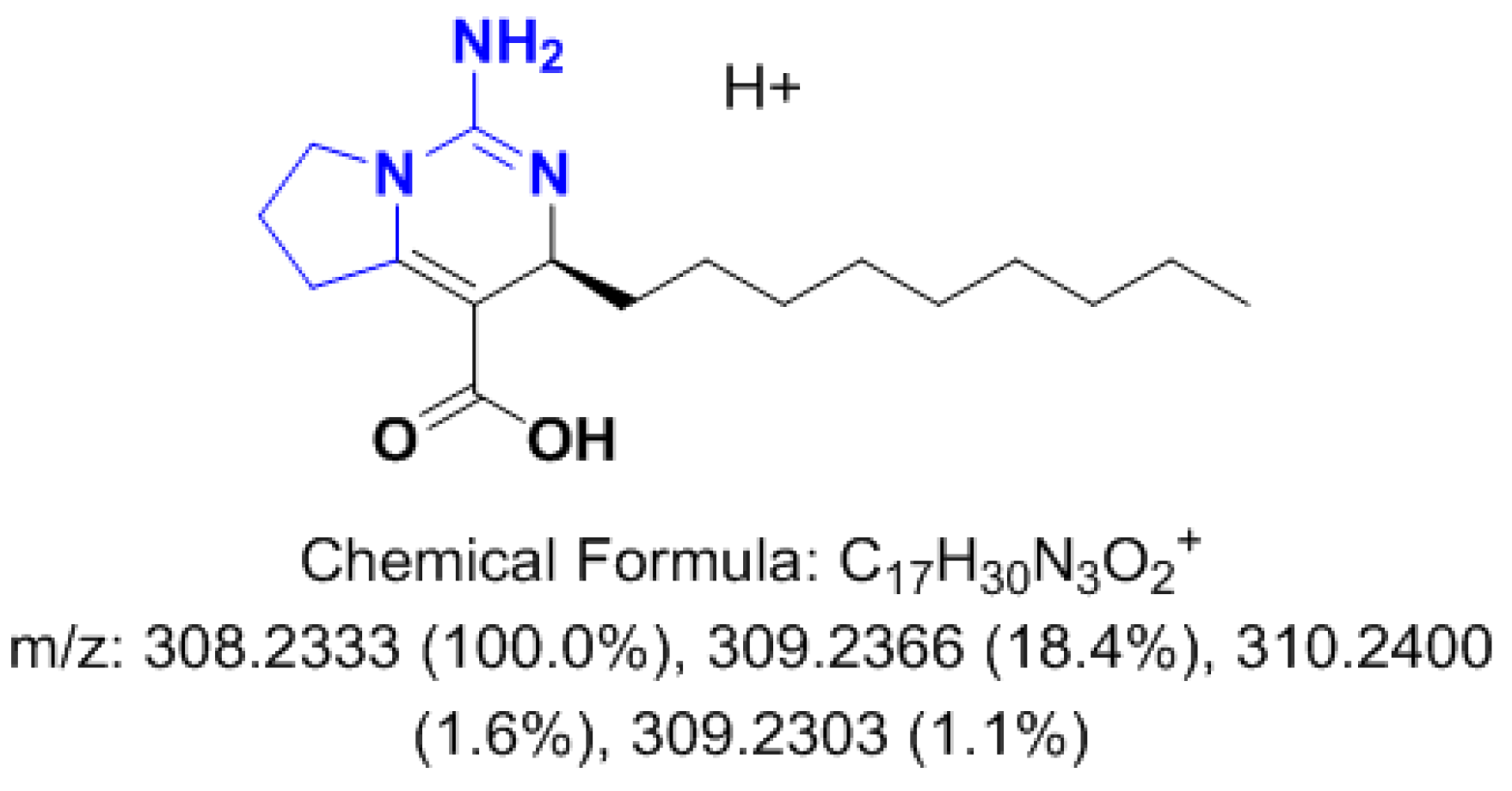
3.2.2. Metabolomics Study
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Becerro, M.A.; Turon, X.; Uriz, M.J. Multiple Functions for Secondary Metabolites in Encrusting Marine Invertebrates. J. Chem. Ecol. 1997, 23, 1527–1547. [Google Scholar] [CrossRef]
- Assmann, M.; Lichte, E.; Pawlik, J.R.; Kck, M. Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera. Mar. Ecol. Prog. Ser. 2000, 207, 255–262. [Google Scholar] [CrossRef]
- Engel, S.; Pawlik, J.R. Allelopathic activities of sponge extracts. Mar. Ecol. Prog. Ser. 2000, 207, 273–281. [Google Scholar] [CrossRef]
- Loh, T.-L.; Pawlik, J.R. Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs. Proc. Natl. Acad. Sci. USA 2014, 111, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Genta-Jouve, G.; Croué, J.; Weinberg, L.; Cocandeau, V.; Holderith, S.; Bontemps, N.; Suzuki, M.; Thomas, O.P. Two-dimensional ultra high pressure liquid chromatography quadrupole/time-of-flight mass spectrometry for semi-targeted natural compounds identification. Phytochem. Lett. 2014, 10, 318–323. [Google Scholar] [CrossRef]
- Ternon, E.; Zarate, L.; Chenesseau, S.; Croué, J.; Dumollard, R.; Suzuki, M.T.; Thomas, O.P. Spherulization as a process for the exudation of chemical cues by the encrusting sponge C. crambe. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Braekman, J.-C.; Daloze, D. Chemical and biological aspects of sponge secondary metabolites. Phytochem. Rev. 2004, 3, 275–283. [Google Scholar] [CrossRef]
- Wijffels, R.H. Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol. 2008, 26, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Sipkema, D.; Franssen, M.C.R.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine Sponges as Pharmacy. Mar. Biotechnol. 2005, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, M.; Martens, D.; Wijffels, R.H. Towards Commercial Production of Sponge Medicines. Mar. Drugs 2009, 7, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Sipkema, D.; Yosef, N.; Adamczewski, M.; Osinga, R.; Mendola, R.; Tramper, J.; Wijffels, R.H. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis? Biotechnol. Bioeng. 2005, 90, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.; Battershill, C. Sponge aquaculture for the production of biologically active metabolites: The influence of farming protocols and environment. Aquaculture 2003, 221, 311–329. [Google Scholar] [CrossRef]
- Page, M.; West, L.; Northcote, P.; Battershill, C.; Kelly, M. Spatial and Temporal Variability of Cytotoxic Metabolites in Populations of the New Zealand Sponge Mycale hentscheli. J. Chem. Ecol. 2005, 31, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, J.; Thomas, O.P.; Pedel, L.; Pénez, N.; Ereskovsky, A.; Culioli, G.; Pérez, T. Biochemical Trade-Offs: Evidence for Ecologically Linked Secondary Metabolism of the Sponge Oscarella balibaloi. PLoS ONE 2011, 6, e28059. [Google Scholar] [CrossRef] [PubMed]
- De Caralt, S.; Agell, G.; Uriz, M.-J. Sources of Secondary Metabolite Variation in Dysidea avara (Porifera: Demospongiae): The Importance of Having Good Neighbors. Mar. Drugs 2013, 11, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Becerro, M.A.; Turon, X.; Uriz, M.J. Natural variation of toxicity in encrusting sponge Crambe crambe (Schmidt) in relation to size and environment. J. Chem. Ecol. 1995, 21, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Becerro, M.A.; Uriz, M.J.; Turon, X. Chemically-mediated interactions in benthic organisms: The chemical ecology of Crambe crambe (Porifera, Poecilosclerida). In Interactions and Adaptation Strategies of Marine Organisms; Naumov, A.D., Hummel, H., Sukhotin, A.A., Ryland, J.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 77–89. [Google Scholar] [CrossRef]
- Luter, H.M.; Duckworth, A.R. Influence of size and spatial competition on the bioactivity of coral reef sponges. Biochem. Syst. Ecol. 2010, 38, 146–153. [Google Scholar] [CrossRef]
- Luter, H.M.; Duckworth, A.R.; Syms, C. Cytotoxic and anti-microbial activity of the sponge Iotrochota sp. as a function of size and spatial competitors. Mar. Biol. Res. 2007, 3, 312–318. [Google Scholar] [CrossRef]
- Abdo, D.A.; Motti, C.A.; Battershill, C.N.; Harvey, E.S. Temperature and Spatiotemporal Variability of Salicylihalamide A in the Sponge Haliclona sp. J. Chem. Ecol. 2007, 33, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, C.; Vacca, S.; De Ciucis, C.; Marengo, B.; Duckworth, A.R.; Manconi, R.; Pronzato, R.; Domenicotti, C. Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar. Ecol. 2009, 30, 327–336. [Google Scholar] [CrossRef]
- Turon, X.; Martí, R.; Uriz, M.J. Chemical bioactivity of sponges along an environmental gradient in a Mediterranean cave. Sci. Mar. 2009, 73, 387–397. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Pérez, T.; Ereskovsky, A.V.; Barnathan, G.; Thomas, O.P. Lysophospholipids in the Mediterranean Sponge Oscarella tuberculata: Seasonal Variability and Putative Biological Role. J. Chem. Ecol. 2011, 37, 537. [Google Scholar] [CrossRef] [PubMed]
- Turon, X.; Tarjuelo, I.; Uriz, M.J. Growth dynamics and mortality of the encrusting sponge Crambe crambe (Poecilosclerida) in contrasting habitats: Correlation with population structure and investment in defence. Funct. Ecol. 1998, 12, 631–639. [Google Scholar] [CrossRef]
- Leong, W.; Pawlik, J. Evidence of a resource trade-off between growth and chemical defenses among Caribbean coral reef sponges. Mar. Ecol. Prog. Ser. 2010, 406, 71–78. [Google Scholar] [CrossRef]
- Becerro, M.A.; Paul, V.J. Effects of depth and light on secondary metabolites and cyanobacterial symbionts of the sponge Dysidea granulosa. Mar. Ecol. Prog. Ser. 2004, 280, 115–128. [Google Scholar] [CrossRef]
- Sacristán-Soriano, O.; Banaigs, B.; Becerro, M.A. Temporal Trends in the Secondary Metabolite Production of the Sponge Aplysina aerophoba. Mar. Drugs 2012, 10, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.; West, L.; Vansach, T.; Stubler, A.; Hardt, M. Effects of water temperature and pH on growth and metabolite biosynthesis of coral reef sponges. Mar. Ecol. Prog. Ser. 2012, 462, 67–77. [Google Scholar] [CrossRef]
- Thoms, C.; Horn, M.; Wagner, M.; Hentschel, U.; Proksch, P. Monitoring microbial diversity and natural product profiles of the sponge Aplysina cavernicola following transplantation. Mar. Biol. 2003, 142, 685–692. [Google Scholar] [CrossRef]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient Fluxes through Sponges: Biology, Budgets, and Ecological Implications; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Kuhlisch, C.; Pohnert, G. Metabolomics in chemical ecology. Nat. Prod. Rep. 2015, 32, 937–955. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.; Karu, K.; Hornshaw, M.; Woffendin, G.; Wang, Y. Metabolomics and metabolite profiling: Past heroes and future developments. Eur. J. Mass Spectrom. 2007, 13, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 5, 3. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Erythroid Differentiation in K562 Chronic Myelogenous Cells Induced by Crambescidin 800, a Pentacyclic Guanidine Alkaloid. Anticancer Res. 2004, 24, 2325–2330. [Google Scholar] [PubMed]
- Aron, Z.D.; Pietraszkiewicz, H.; Overman, L.E.; Valeriote, F.; Cuevas, C. Synthesis and anticancer activity of side chain analogs of the crambescidin alkaloids. Bioorg. Med. Chem. Lett. 2004, 14, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Ferri, S.; Spampinato, S.; Speroni, E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. J. Nat. Prod. 1993, 56, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Bondu, S.; Genta-Jouve, G.; Leiros, M.; Vale, C.; Guigonis, J.-M.; Botana, L.M.; Thomas, O.P. Additional bioactive guanidine alkaloids from the Mediterranean sponge Crambe crambe. RSC Adv. 2012, 2, 2828–2835. [Google Scholar] [CrossRef]
- Martín, V.; Vale, C.; Bondu, S.; Thomas, O.P.; Vieytes, M.R.; Botana, L.M. Differential Effects of Crambescins and Crambescidin 816 in Voltage-Gated Sodium, Potassium and Calcium Channels in Neurons. Chem. Res. Toxicol. 2013, 26, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Ternon, E.; Lopez-Alonso, H.; Thomas, O.P.; Vega, F.V.; Vieytes, M.R.; Botana, L.M. Crambescidin-816 Acts as a Fungicidal with More Potency than Crambescidin-800 and -830, Inducing Cell Cycle Arrest, Increased Cell Size and Apoptosis in Saccharomyces cerevisiae. Mar. Drugs 2013, 11, 4419–4434. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Sakai, R.; Rinehart, K.L. Crambescidins: New antiviral and cytotoxic compounds from the sponge Crambe crambe. J. Org. Chem. 1991, 56, 5712–5715. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Ingrum, A.L.; Carney, J.R.; Rinehart, K.L.; Sakai, R. Polycyclic guanidine-containing compounds from the mediterranean sponge Crambe crambe: The structure of 13,14,15-isocrambescidin 800 and the absolute stereochemistry of the pentacyclic guanidine moieties of the crambescidins. J. Org. Chem. 1993, 58, 4805–4808. [Google Scholar] [CrossRef]
- Berlinck, R.G.; Braekman, J.C.; Daloze, D.; Bruno, I.; Ruccio, R.; Rogeau, D.; Amade, P. Crambines C1 and C2: Two further ichthyotoxic guanidine alkaloids from the sponge Crambe crambe. J. Nat. Prod. 1992, 55, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Tipologie di Impianto Modulare per la Spongicoltura Subacquea Anche in Policoltura U.S.A.M.A. (Underwater Sponge Aquaculture Modular System). Available online: https://iris.unige.it/handle/11567/243308#.WUNwWmdLeUk (accessed on 16 June 2017).
- Perino, E. Sustainable Production of Bioactive Compounds from Sponges. Ph.D. Thesis, Universita’ degli studi di Genova, Genova, Italy, 2014; p. 187. [Google Scholar]
- Pérez-López, P.; Ternon, E.; Gonzalez-Garcia, S.; Genta-Jouve, G.; Feijoo, G.; Thomas, O.P.; Moreira, M.T.L. Environmental solutions for the sustainable production of bioactive natural products from the marine sponge Crambe crambe. Sci. Total Environ. 2014, 475, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Tedetti, M.; Longhitano, R.; Garcia, N.; Guigue, C.; Ferretto, N.; Goutx, M. Fluorescence properties of dissolved organic matter in coastal Mediterranean waters influenced by a municipal sewage effluent (Bay of Marseilles, France). Environ. Chem. 2012, 9, 438–449. [Google Scholar] [CrossRef]
- Kerouel, R.; Aminot, A. Procédure Optimisée Hors-contaminations pour l’Analyse des eléments nutritifs dissous dans l’eau de mer. Mar. Environ. Res. 1987, 22, 19–32. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Tautenhahn, R.; Siuzdak, G. Meta-analysis of untargeted metabolomic data from multiple profiling experiments. Nat. Protoc. 2012, 7, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Lévy, M. Mesoscale variability of phytoplankton and of new production: Impact of the large-scale nutrient distribution. J. Geophys. Res. Oceans 2003, 108, 3358. [Google Scholar] [CrossRef]
- Lafuente, J.G.; Garcia, A.; Mazzola, S.; Quintanilla, L.; Delgado, J.; Cuttita, A.; Patti, B. Hydrographic phenomena influencing early life stages of the Sicilian Channel anchovy. Fish. Oceanogr. 2002, 11, 31–44. [Google Scholar] [CrossRef]
- Bosc, E.; Bricaud, A.; Antoine, D. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Glob. Biogeochem. Cycles 2004, 18, GB1005. [Google Scholar] [CrossRef]
- Ivanišević, J.; Thomas, O.P.; Lejeusne, C.; Chevaldonné, P.; Pérez, T. Metabolic fingerprinting as an indicator of biodiversity: Towards understanding inter-specific relationships among Homoscleromorpha sponges. Metabolomics 2011, 7, 289–304. [Google Scholar] [CrossRef]
- López-Legentil, S.; Turon, X. Population genetics, phylogeography and speciation of Cystodytes (Ascidiacea) in the western Mediterranean Sea. Biol. J. Linn. Soc. 2006, 88, 203–214. [Google Scholar] [CrossRef]
- Paul, V.J.; Arthur, K.E.; Ritson-Williams, R.; Ross, C.; Sharp, K. Chemical Defenses: From Compounds to Communities. Biol. Bull. 2007, 213, 226–251. [Google Scholar] [CrossRef] [PubMed]
- López-Legentil, S.; Turon, X. How do morphotypes and chemotypes relate to genotypes? The colonial ascidian Cystodytes (Polycitoridae). Zool. Scr. 2005, 34, 3–14. [Google Scholar] [CrossRef]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Seasonal Patterns of Toxicity in Benthic Invertebrates: The Encrusting Sponge Crambe crambe (Poecilosclerida). Oikos 1996, 75, 33–40. [Google Scholar] [CrossRef]
- Coma, R.; Ribes, M. Seasonal energetic constraints in Mediterranean benthic suspension feeders: Effects at different levels of ecological organization. Oikos 2003, 101, 205–215. [Google Scholar] [CrossRef]
- Jiménez, E.; Ribes, M. Sponges as a source of dissolved inorganic nitrogen: Nitrification mediated by temperate sponges. Limnol. Oceanogr. 2007, 52, 948–958. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Net Primary Productivity in Coral Reef Sponges. Science 1983, 219, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Ribes, M.; Jimenez, E.; Yahel, G.; Lopez-Sendino, P.; Diez, B.; Massana, R.; Sharp, J.H.; Coma, R. Functional convergence of microbes associated with temperate marine sponges. Environ. Microbiol. 2012, 14, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Blasiak, L.C.; Karolin, J.O.; Powell, R.JJ.; Geddes, C.D.; Hill, R.T. Phosphorus sequestration in the form of polyphosphate by microbial symbionts in marine sponges. Proc. Natl. Acad. Sci. USA 2015, 112, 4381–4386. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J. Ind. Microbiol. Biotechnol. 2006, 33, 545. [Google Scholar] [CrossRef] [PubMed]
- Croué, J.; West, N.J.; Escande, M.-L.; Intertaglia, L.; Lebaron, P.; Suzuki, M. A single betaproteobacterium dominates the microbial community of the crambescidine-containing sponge Crambe crambe. Sci. Rep. 2013, 3, 2583. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ternon, E.; Perino, E.; Manconi, R.; Pronzato, R.; Thomas, O.P. How Environmental Factors Affect the Production of Guanidine Alkaloids by the Mediterranean Sponge Crambe crambe. Mar. Drugs 2017, 15, 181. https://doi.org/10.3390/md15060181
Ternon E, Perino E, Manconi R, Pronzato R, Thomas OP. How Environmental Factors Affect the Production of Guanidine Alkaloids by the Mediterranean Sponge Crambe crambe. Marine Drugs. 2017; 15(6):181. https://doi.org/10.3390/md15060181
Chicago/Turabian StyleTernon, Eva, Erica Perino, Renata Manconi, Roberto Pronzato, and Olivier P. Thomas. 2017. "How Environmental Factors Affect the Production of Guanidine Alkaloids by the Mediterranean Sponge Crambe crambe" Marine Drugs 15, no. 6: 181. https://doi.org/10.3390/md15060181
APA StyleTernon, E., Perino, E., Manconi, R., Pronzato, R., & Thomas, O. P. (2017). How Environmental Factors Affect the Production of Guanidine Alkaloids by the Mediterranean Sponge Crambe crambe. Marine Drugs, 15(6), 181. https://doi.org/10.3390/md15060181






