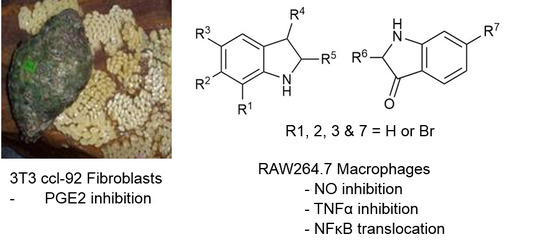
Anti-Inflammatory Activity and Structure-Activity Relationships of Brominated Indoles from a Marine Mollusc
Abstract
:1. Introduction
2. Results
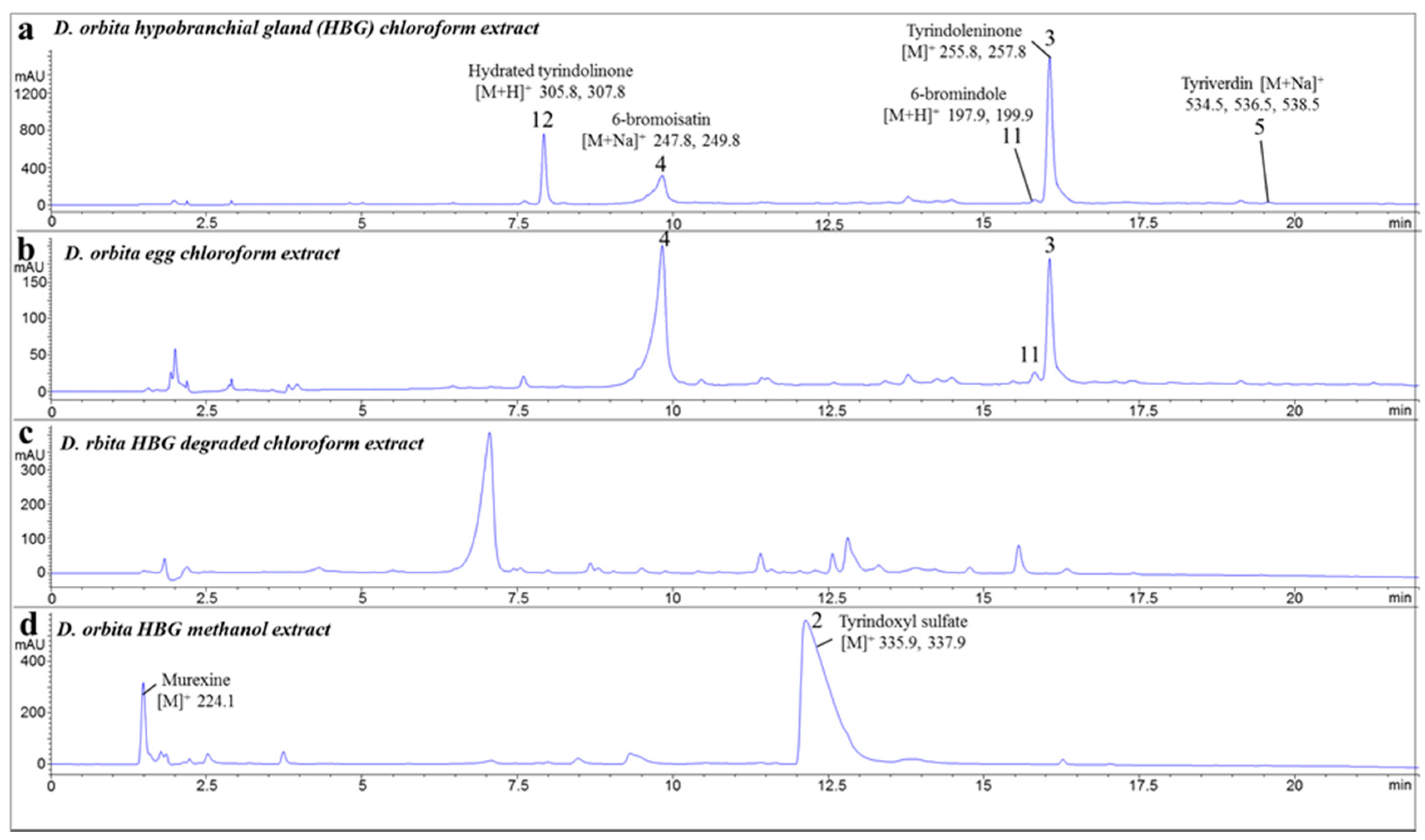
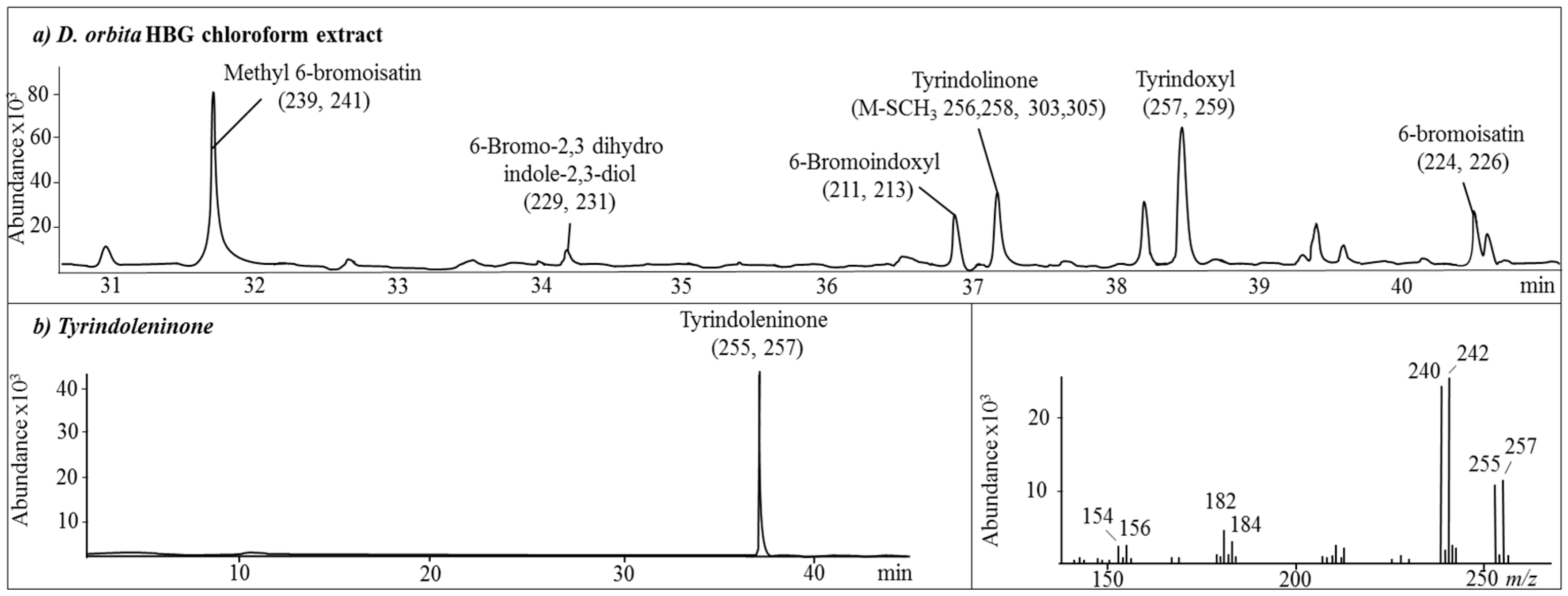
2.1. Chemical Analysis of the Crude Extracts
2.2. Cytotoxicity Assays
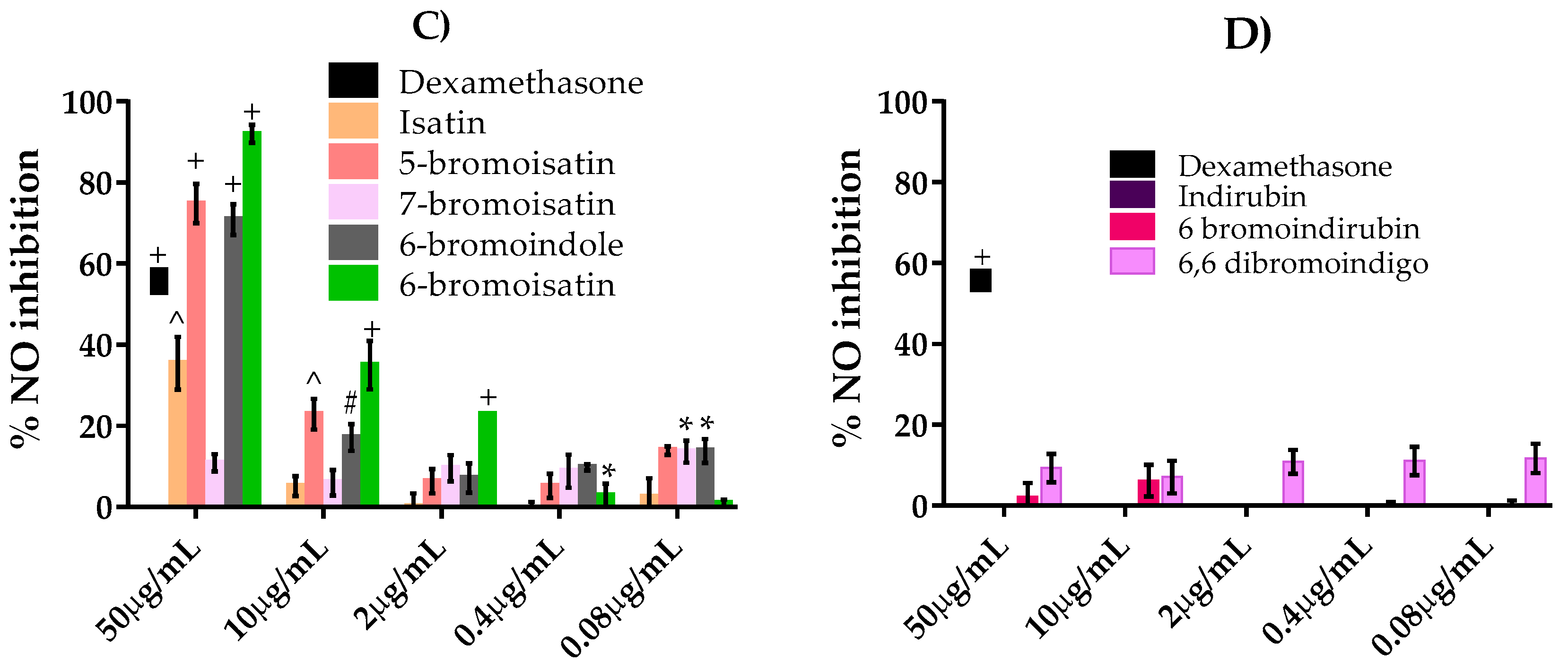
2.3. NO Inhibition Assay
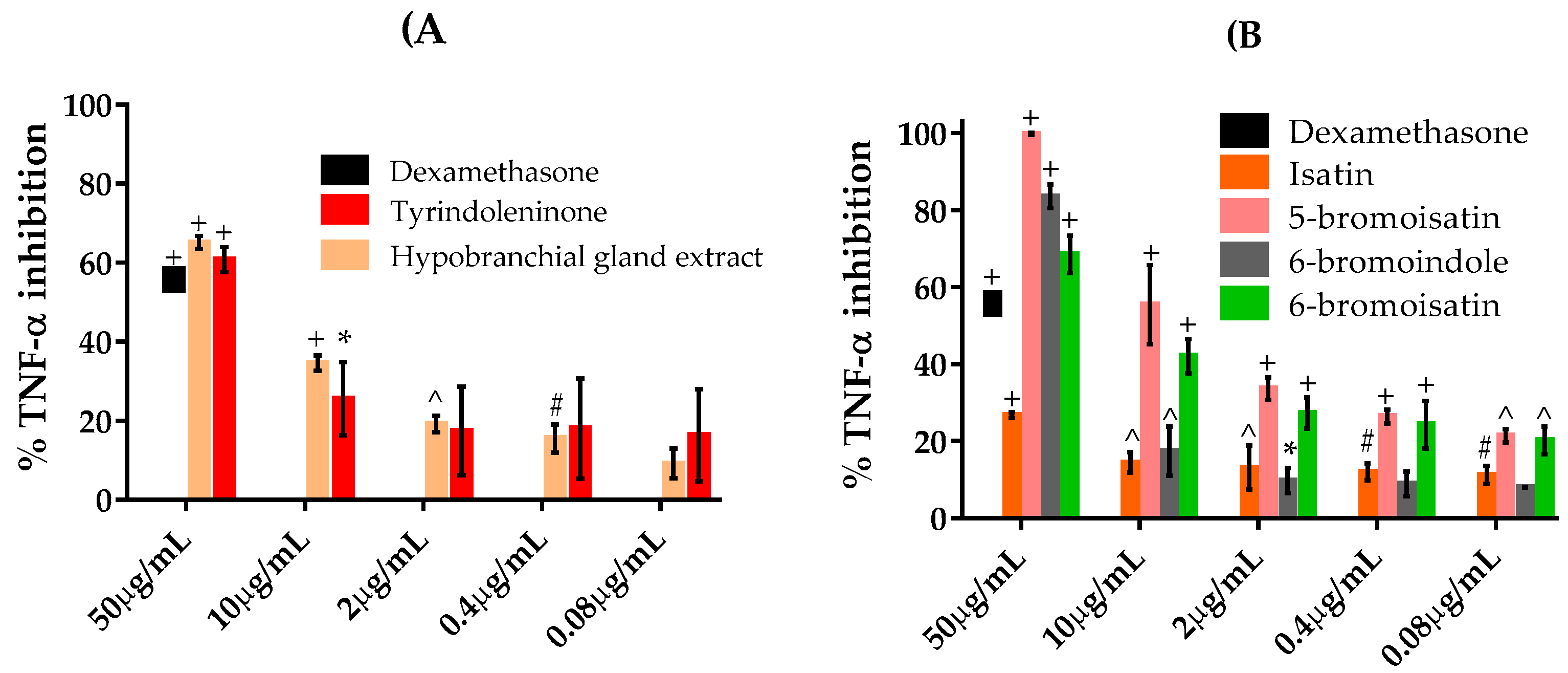
2.4. TNFα Inhibition
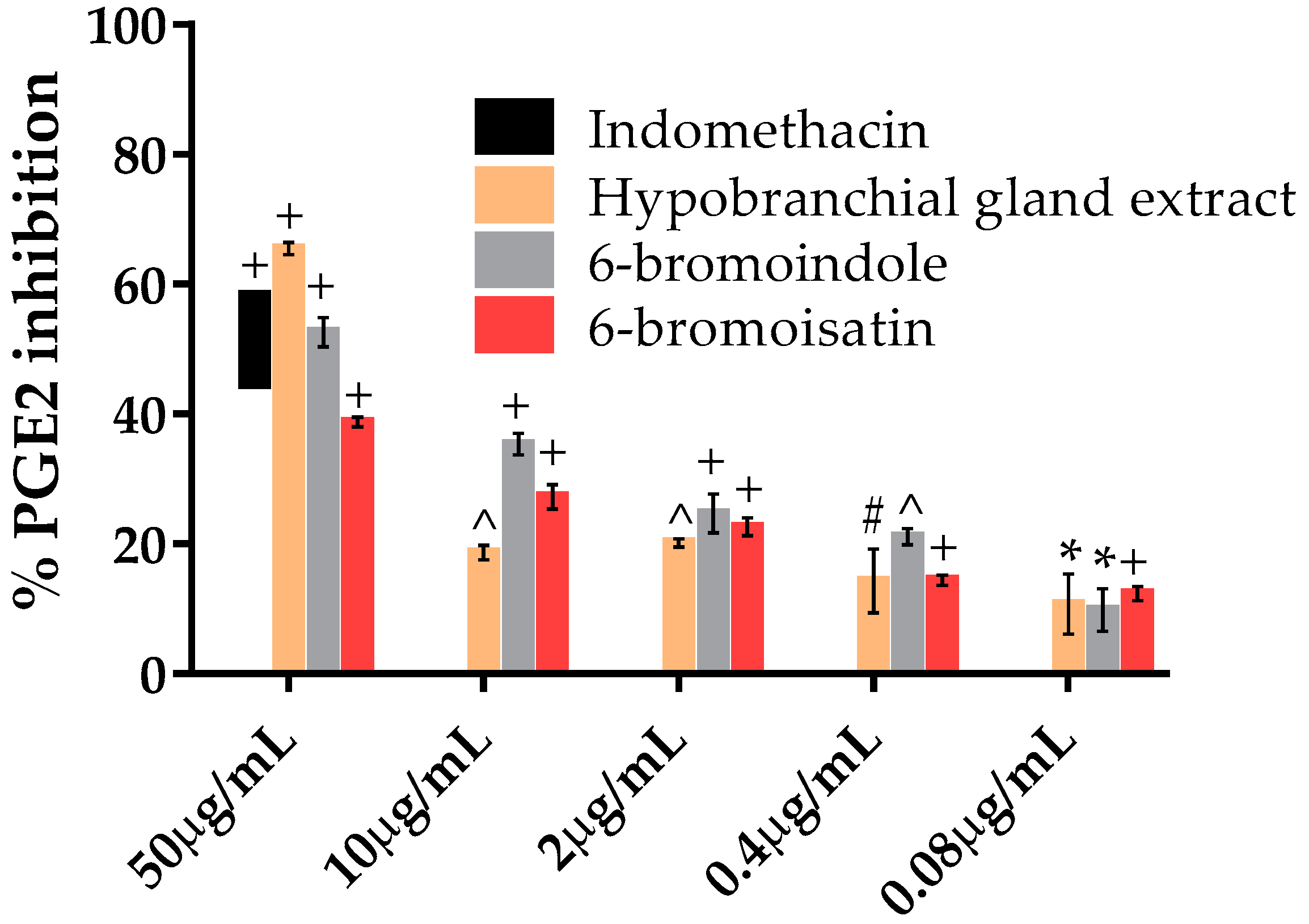
2.5. PGE2 Inhibition Assay
2.6. Assessment of NFκB Translocation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Mollusc Collection, Dissection, and Extraction
4.4. Purification of Brominated Natural Products
4.5. Chemical Analysis of the Extracts and Purified Compounds
4.6. Preparation of Extracts and Compounds
4.7. Cytotoxicity Assay
4.8. NO Inhibition Assay
4.9. TNF-Alpha Inhibition Assay
4.10. PGE2 Inhibition Assay
4.11. Assessment of NFκB Translocation
4.12. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of inflammation: Mechanisms and opportunity for drug development. Pharmacol. Ther. 2013, 139, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. Nf-kappab: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.J.; Chiu, K.C.; Fu, M.; Lo, R.; Helton, S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J. Surg. Res. 1999, 82, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Moraes, V.L.G.; Vargaftig, B.B.; Lefort, J.; Meager, A.; Chignard, M. Effect of cyclo-oxygenase inhibitors and modulators of cyclic AMP formation on lipopolysaccharide-induced neutrophil infiltration in mouse lung. Br. J. Pharmacol. 1996, 117, 1792–1796. [Google Scholar] [CrossRef]
- Wink, D.A.; Grisham, M.B.; Mitchell, J.B.; Ford, P.C. Direct and indirect effects of nitric oxide in chemical reactions relevant to biology. Methods Enzymol. 1996, 268, 12–31. [Google Scholar] [PubMed]
- Tamir, S.; Burney, S.; Tannenbaum, S.R. DNA damage by nitric oxide. Chem. Res. Toxicol. 1996, 9, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Mayer, A.M.S. Marine Pharmaceuticals: The Clinical Pipeline. Available online: http://marinepharmacology.midwestern.edu/clinPipeline.htm (accessed on 9 October 2016).
- Ciavatta, M.L.; Lefranc, F.; Carbone, M.; Mollo, E.; Gavagnin, M.; Betancourt, T.; Dasari, R.; Kornienko, A.; Kiss, R. Marine mollusk-derived agents with antiproliferative activity as promising anticancer agents to overcome chemotherapy resistance. Med. Res. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. Camb. Philos. Soc. 2010, 85, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.; Prescott, P.; Coghlan, B.; Bashir, N.; Lewith, G. Systematic review of the nutritional supplement Perna canaliculus (green-lipped mussel) in the treatment of osteoarthritis. QJM 2008, 101, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Chellaram, C.; Edward, J.K.P. Anti-inflammatory potential of coral reef associated gastropod. Drupa Margarit. Indian J. Sci. Technol. 2009, 2, 75–77. [Google Scholar]
- Santhi, V.; Sivakumar, V.; Thangathirupathi, A.; Thilaga, R.D. Analgesic, anti-pyretic and anti-inflammatory activities of chloroform extract of prosobranch mollusc Purpura persica. Int. J. Pharm. Biol. Sci. 2011, 5, 9–15. [Google Scholar]
- Badiu, D.L.; Balu, A.M.; Barbes, L.; Luque, R.; Nita, R.; Radu, M.; Tanase, E.; Rosoiu, N. Physico-chemical characterisation of lipids from Mytilus galloprovincialis (L.) and Rapana venosa and their healing properties on skin burns. Lipids 2008, 43, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Rudd, D.; Nongmaithem, B.D.; Liu, L.; Young, F.; Edwards, V.; Avila, C.; Abbott, C.A. Are the traditional medical uses of Muricidae molluscs substantiated by their pharmacological properties and bioactive vompounds? Mar. Drugs 2015, 13, 5237–5275. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K. Natural product research in the Australian marine invertebrate Dicathais orbita. Mar. Drugs 2013, 11, 1370–1398. [Google Scholar] [CrossRef] [PubMed]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Benkendorff, K.; Pyne, S.G.; Bremner, J.B. In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 2007, 15, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; McIver, C.M.; Abbott, C.A. Bioactivity of the murex homeopathic remedy and of extracts from an Australian muricid mollusc against human cancer cells. Evid. Based Complement. Altern. Med. 2011, 2011, 879585. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.; Benkendorff, K.; Young, F. Marine compounds selectively induce apoptosis in female reproductive cancer cells but not in primary-derived human reproductive granulosa cells. Mar. Drugs 2012, 10, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelian, B.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. 6-bromoisatin found in muricid mollusc extracts inhibits colon cancer cell proliferation and induces apoptosis, preventing early stage tumor formation in a colorectal cancer rodent model. Mar. Drugs 2014, 12, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelian, B.; Benkendorff, K.; Johnston, M.R.; Abbott, C.A. Purified brominated indole derivatives from Dicathais orbita induce apoptosis and cell cycle arrest in colorectal cancer cell lines. Mar. Drugs 2013, 11, 3802–3822. [Google Scholar] [CrossRef] [PubMed]
- Westley, C.B.; McIver, C.M.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. Enhanced acute apoptotic response to azoxymethane-induced DNA damage in the rodent colonic epithelium by tyrian purple precursors: A potential colorectal cancer chemopreventative. Cancer Biol. Ther. 2010, 9, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelian, B.; Benkendorff, K.; Le Leu, R.; Abbot, C. Simultaneous assessment of the efficacy and toxicity of marine mollusc derived brominated indoles in an in vivo model for early stage colon cancer. Integr. Cancer Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Tyrian purple precursors in the egg masses of the Australian muricid, Dicathais orbita: A possible defensive role. J. Chem. Ecol. 2000, 26, 1037–1050. [Google Scholar] [CrossRef]
- Meijer, L.; Skaltsounis, A.L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R.; et al. Gsk-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Buettner, R.; Turkson, J.; Kim, D.; Cheng, J.Q.; Muehlbeyer, S.; Hippe, F.; Vatter, S.; Merz, K.H.; Eisenbrand, G.; et al. Indirubin derivatives inhibit stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5998–6003. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kim, Y.C.; Kim, S.W.; Lee, S.H.; Min, J.J.; Ahn, S.G.; Yoon, J.H. Antitumor activity of novel indirubin derivatives in rat tumor model. Clin. Cancer Res. 2007, 13, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.-Z.; Mandelkow, E.-M. Indirubins inhibit glycogen synthase kinase-3β and cdk5/p25, two protein kinases involved in abnormal tau phosphorylation in alzheimer’s disease: A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Karabelas, K.; Lepistö, M.; Sjö, P. Protein Kinase Inhibitors. U.S. Patent 6,346,625 B1, 12 February 2002. [Google Scholar]
- Wang, L.; Liu, X.; Chen, R. Derivatives of Isoindigo, Indigo and Indirubin and Methods of Treating Cancer. U.S. Patent 6,933,315 B2, 13 August 2005. [Google Scholar]
- Carson, D.A.; Leoni, L.M.; Cottam, H.B. Indole Compounds Useful for the Treatment of Cancer. U.S. Patent 7,151,100 B1, 19 December 2006. [Google Scholar]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic and clinical issues. J. Natl. Cancer Inst. 2002, 94, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.C.; Sung, P.J.; Duh, C.Y.; Chen, B.W.; Sheu, J.H.; Yang, N.S. Anti-inflammatory activities of natural products isolated from soft corals of taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, G.M. Indirubin-3-monoxime exhibits anti-inflammatory properties by down-regulating nf-kappab and jnk signaling pathways in lipopolysaccharide-treated RAW264.7 cells. Inflamm. Res. 2012, 61, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Matheus, M.E.; Violante, F.A.; Garden, S.J.; Pinto, A.C.; Fernandes, P.D. Isatins inhibit cyclooxygenase-2 and inducible nitric oxide synthase in a mouse macrophage cell line. Eur. J. Pharmacol. 2007, 556, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, Y.; Ito, F.; Stevens, R.W.; Asai, N. Antiallergy and Antiinflammatory Agents. U.S. Patent 5,006,541 A, 9 April 1991. [Google Scholar]
- Stevens, R.W.; Morita, H.; Nakane, M. Indole Derivatives as Antiallergy and Antiinflammatory Agents. U.S. Patent 5,290,788 A, 1 March 1994. [Google Scholar]
- Pelcman, B.; Olofsson, K.; Katkevics, M.; Ozola, V.; Suna, E.; Kalvins, I.; Trapencieris, P.; Katkevica, D.; Schaal, W. Indoles Useful in the Treatment of Inflammation. U.S. Patent 20100197687 A1, 5 August 2010. [Google Scholar]
- Valles-Regino, R.; Mouatt, P.; Rudd, D.; Yee, L.H.; Benkendorff, K. Extraction and quantification of bioactive Tyrian purple precursors: A comparative and validation study from the hypobranchial gland of a muricid Dicathais orbita. Molecules 2016, 21, 1672. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, C.J. Tyrian purple: 6,6′-dibromoindigo and related compounds. Molecules 2001, 6, 736–769. [Google Scholar] [CrossRef]
- Baker, J.; Duke, C. Chemistry of the indoleninones. II. Isolation from the hypobranchial glands of marine molluscs of 6-bromo-2, 2-dimethylthioindolin-3-one and 6-bromo-2-methylthioindoleninone as alternative precursors to Tyrian purple. Aust. J. Chem. 1973, 26, 2153–2157. [Google Scholar] [CrossRef]
- Lee, D.; Long, S.A.; Murray, J.H.; Adams, J.L.; Nuttall, M.E.; Nadeau, D.P.; Kikly, K.; Winkler, J.D.; Sung, C.M.; Ryan, M.D.; et al. Potent and selective nonpeptide inhibitors of caspases 3 and 7. J. Med. Chem. 2001, 44, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D.; et al. Indirubin, the active constituent of a chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [PubMed]
- Yuskaitis, C.J.; Jope, R.S. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal 2009, 21, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, T.; Tatefuji, T.; Aga, H.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharmacol. 2000, 410, 93–100. [Google Scholar] [CrossRef]
- Hamburger, M.; Jahne, E.; Butterweck, V.; Oufir, M.; Eigenmann, D.; Culot, M.; Cecchelli, R.; Walter, F.; Deli, M.; Smiesko, M. Pharmacokinetic, in vitro and in silico assessment of anti-inflammatory alkaloids from Isatis tinctoria L. Planta Med. 2015, 81, 901. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Calvo, T.R.; Colombo, L.L.; Vilegas, W.; Carlos, I.Z. Immunostimulatory and cytotoxic activities of Indigofera suffruticosa (Fabaceae). Nat. Prod. Res. 2011, 25, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Molecular therapeutic targets in inflammation: Cyclooxygenase and NF-κB. Curr. Drug Targets Inflamm. Allergy 2004, 3, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Shearer, J.; Bettayeb, K.; Ferandin, Y. Diversity of intracellular mechanisms underlying the anti-tumor properties of indirubins. In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, A.-L., Eisenbrand, G., Eds.; Life in Progress Editions: Roscoff, France, 2006; pp. 235–246. [Google Scholar]
- Westley, C.; Benkendorff, K. Sex-specific Tyrian purple genesis: Precursor and pigment distribution in the reproductive system of the marine mollusc, Dicathais orbita. J. Chem. Ecol. 2008, 34, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, D.; Shanmugam, K.; Low, M.; Bennett, L.; Govindaraghavan, S.; Head, R.; Ooi, L.; Munch, G. Determination of anti-inflammatory activities of standardised preparations of plant- and mushroom-based foods. Eur. J. Nutr. 2014, 53, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.W.; Macrides, T.A.; Kalafatis, N.; Betts, W.H.; Haynes, D.R.; Broadbent, J. Anti-inflammatory activity of a lipid fraction (lyprinol) from the NZ green-lipped mussel. Inflammopharmacology 1997, 5, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.J.; Kim, J.Y.; Kim, J.B.; Lee, K.W.; Jeong, S.Y.; Park, H.J.; Jung, H.J.; Cho, Y.W.; Yun, K.; Lee, K.T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NK-kappa B inactivation in RAW 264.7 macrophages: Possible involvement of the ikK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Phanse, M.A. In Vivo and in vitro screening of medicinal plants for their anti-inflammatory activity: An overview. J. Appl. Pharm. Sci. 2012, 2, 19–33. [Google Scholar] [CrossRef]
- Kim, D.S.; Shin, M.R.; Kim, Y.S.; Bae, W.J.; Roh, D.H.; Hwang, Y.S.; Kim, E.C. Anti-inflammatory effects of glutamine on LPS-stimulated human dental pulp cells correlate with activation of MKP-1 and attenuation of the MAPK and NF-kappa b pathways. Int. Endod. J. 2015, 48, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I. Inhibition of the NF-κb signaling pathway by the curcumin analog, 3, 5-bis (2-pyridinylmethylidene)-4-piperidone (ef31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of CDK1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]


| Chemical Structure | Molecular Weight 1 | Log p Value 2 | Synthetic vs. Purified | |
|---|---|---|---|---|
| 1 |  | 420.06 | 4.47 | Synthetic |
| 2 |  | 337.18 | −0.346 | Semi-purified (methanol extract) |
| 3 |  | 256.12 | 2.89 | Purified |
| 4 |  | 226.03 | 1.61 | Synthetic |
| 5 |  | 514.25 | 4.66 | Purified |
| 6 |  | 356.18 | 4.08 | Synthetic |
| 7 |  | 262.27 | 2.9 | Synthetic |
| 8 |  | 147.13 | 0.83 | Synthetic |
| 9 |  | 226.03 | 1.61 | Synthetic |
| 10 |  | 226.03 | 1.59 | Synthetic |
| 11 |  | 196.05 | 2.94 | Synthetic |
| 12 |  | 304.22 | 3.00 | Semi-purified (chloroform extract) |
| 13 |  | 258.13 | 3.38 | Semi-purified (chloroform extract) |

| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Cytotoxicity * RAW264.7 | Cytotoxicity * 3T3 ccl-92 | NO IC50 µM | TNFα IC50 µM | PGE2 IC50 µM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6,6 dibromoindigo (1) | H | Br | H | O | Y | X | Br | NA | NA | NA | NT | NT |
| Tyrindoxyl sulfate (2) | H | Br | H | OSO3− | SCH3 | - | - | NA | NA | NA | NT | NT |
| Tyrindoleninone (3) | H | Br | H | O | SCH3 | - | - | >195.22 | NA | 103 | 157 | NT |
| 6-bromoisatin (4) | H | Br | H | O | O | - | - | NA | NA | 120 | 123 | >221.21 |
| Tyriverdin (5) | H | Br | H | O | SCH3, Y | SCH3, X | Br | NA | NA | >97.23 | NT | NT |
| 6-bromoindirubin (6) | H | Br | H | Y | O | X | H | ~140.38 | NT | NA | NT | NT |
| Indirubin (7) | H | H | H | Y | O | X | H | NA | NA | NA | NT | NT |
| Isatin (8) | H | H | H | O | O | - | - | NA | NA | 339.83 | >339.83 | NT |
| 5-bromoisatin (9) | H | H | Br | O | O | - | - | ~221.21 | NA | 152 | 38 | NT |
| 7-bromoisatin (10) | Br | H | H | O | O | - | - | >221.21 | >221.21 | NA | NT | NT |
| 6-bromoindole (11) | H | Br | H | H | H | >255.04 | NA | 187 | 150 | 223 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.B.; Rudd, D.; Smith, J.; Kotiw, M.; Mouatt, P.; Seymour, L.M.; Liu, L.; Benkendorff, K. Anti-Inflammatory Activity and Structure-Activity Relationships of Brominated Indoles from a Marine Mollusc. Mar. Drugs 2017, 15, 133. https://doi.org/10.3390/md15050133
Ahmad TB, Rudd D, Smith J, Kotiw M, Mouatt P, Seymour LM, Liu L, Benkendorff K. Anti-Inflammatory Activity and Structure-Activity Relationships of Brominated Indoles from a Marine Mollusc. Marine Drugs. 2017; 15(5):133. https://doi.org/10.3390/md15050133
Chicago/Turabian StyleAhmad, Tarek B., David Rudd, Joshua Smith, Michael Kotiw, Peter Mouatt, Lisa M. Seymour, Lei Liu, and Kirsten Benkendorff. 2017. "Anti-Inflammatory Activity and Structure-Activity Relationships of Brominated Indoles from a Marine Mollusc" Marine Drugs 15, no. 5: 133. https://doi.org/10.3390/md15050133