Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Skin By-Products
2.1.1. Proximate Composition
2.1.2. Hydroxyproline (HPro) Content
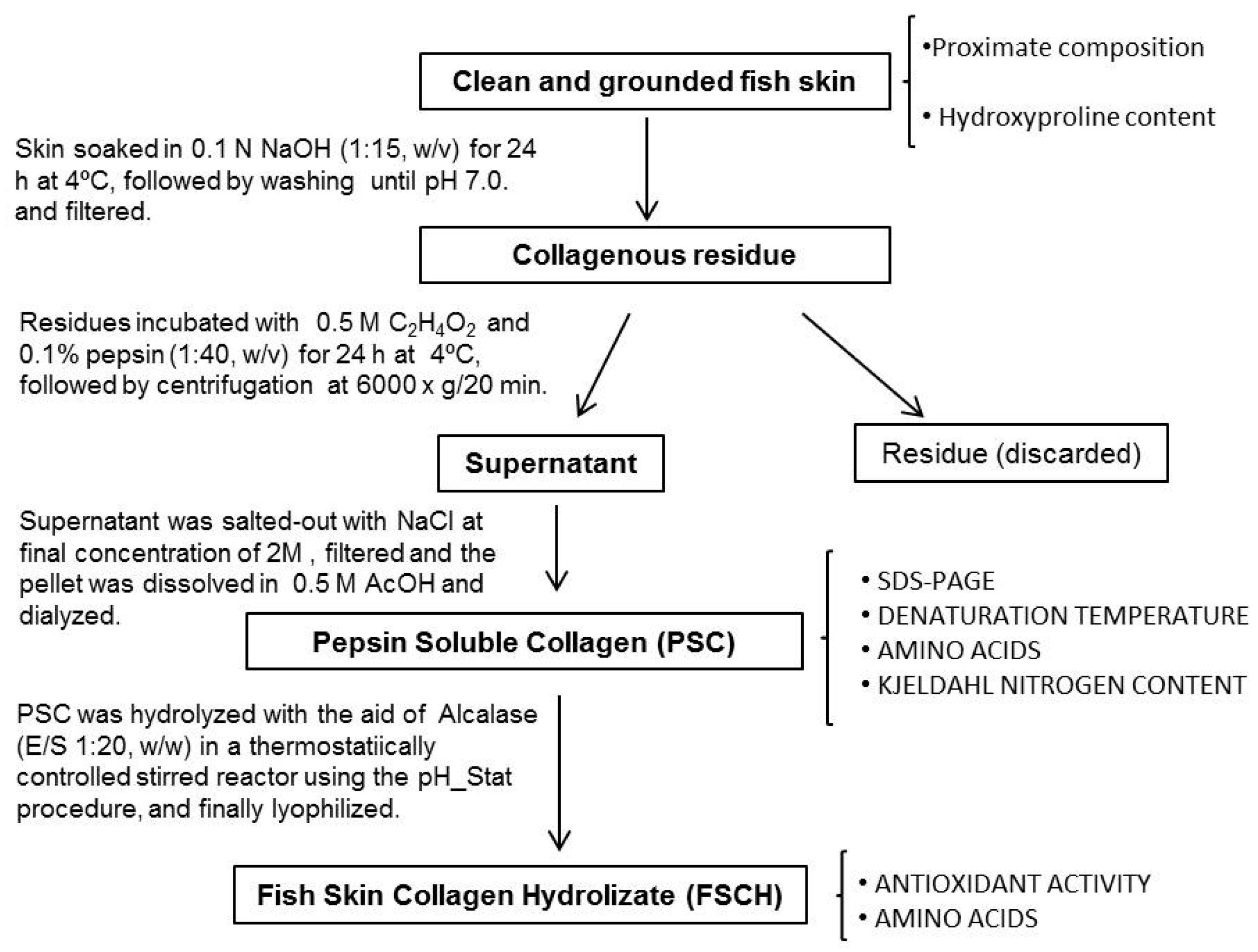
2.2. Extraction of Collagen
2.2.1. Yield of PSC
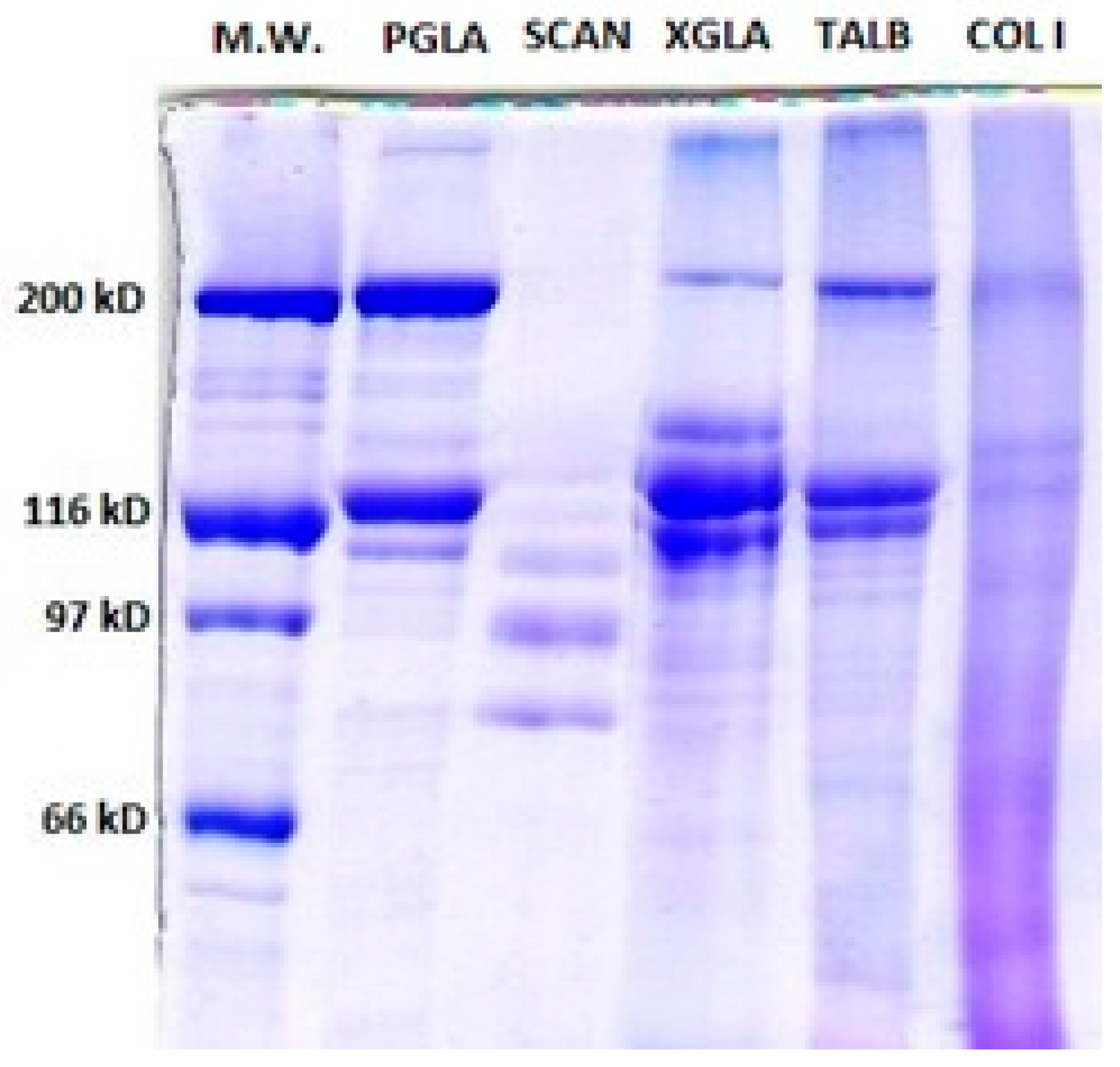
2.2.2. Characterization of PSC
Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Amino Acid Content
Determination of Denaturation Temperature
2.3. Enzymatic Hydrolysis of PSC
2.3.1. Degree of Hydrolysis
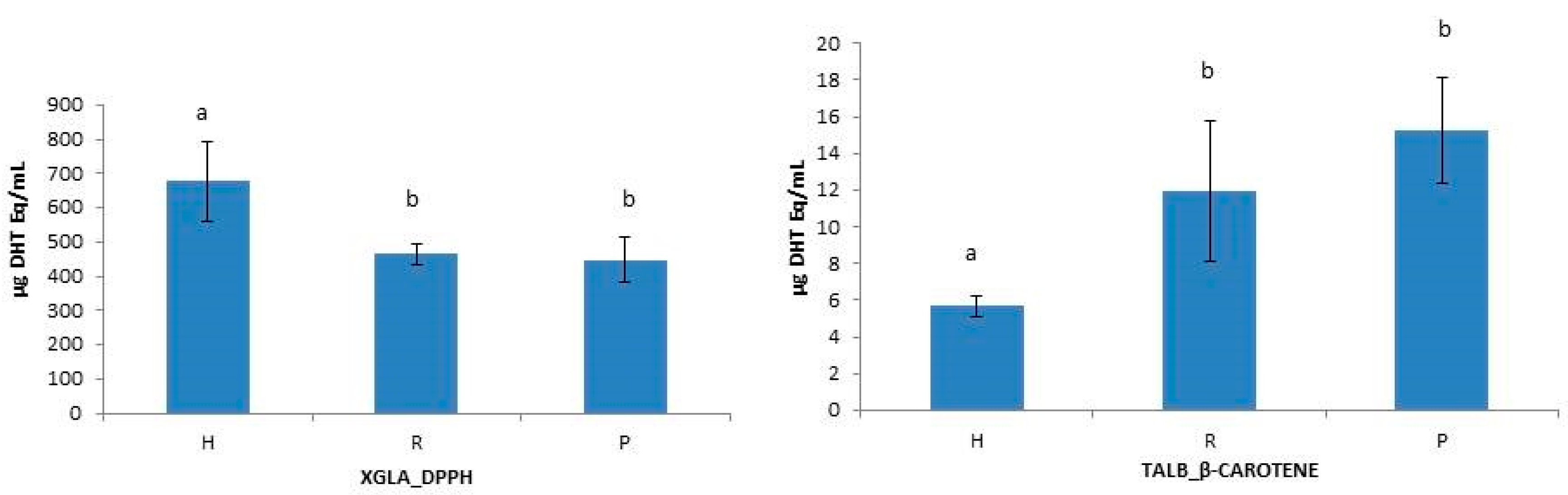
2.3.2. Antioxidant Activities in Hydrolysates
2.3.3. Amino Acid Content
3. Experimental Section
3.1. Raw Material
3.1.1. Proximate Composition
3.1.2. Hydroxyproline Content
3.2. Extraction of Pepsin Soluble Collagen (PSC) from Skin
3.3. Characterization of Pepsin Soluble Collagen (PSC) from Skin
3.3.1. Polyacrylamide Gel Electrophoresis
3.3.2. Differential Scanning Calorimetry
3.3.3. Nitrogen Content
3.3.4. Amino Acid Composition
3.4. Enzymatic Hydrolysis of Pepsin Soluble Collagen
Degree of Hydrolysis
3.5. Antioxidant Capacity of Pepsin Soluble Collagen Hydrolysates
3.5.1. Ultrafiltration
3.5.2. Antioxidant Activity Determinations
β-Carotene Bleaching Method
1,1-Diphenyl-2-Picryhydrazyl (DPPH) Radical-Scavenging Capacity
ABTS Bleaching Method
3.5.3. Amino Acid Composition
3.5.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization. El Estado Mundial de la Pesca y la Acuicultura; FAO: Roma, Italy, 2016. [Google Scholar]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of Chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs 2015, 13, 3287–3308. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Turnay, J.; Férnandez-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Chatziantoniou, S.E.; Zotos, A. Effects of selected process parameters on physical and sensorial properties of yellowfin tuna (Thunnus albacares) skin gelatin. J. Food Process Eng. 2014, 37, 461–473. [Google Scholar] [CrossRef]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [PubMed]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydrolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Nam, K.A.; You, S.G.; Kim, S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008, 73, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.G.; Kim, S.K. Purification and characterization of angiotensis I converting enzyme (ACE) inhibitory peptides from Alaska Pollack (Theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Kumar, B.D.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysate: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, Y.; Lu, J.; Chen, A.; Li, Y.; Zheng, G. Enzymatic hydrolysis of Alaska Pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010, 90, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R.I. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly process. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Autoridad Portuaria de Vigo. Memoria Anual 2015; Autoridad Portuaria de Vigo: Pontevedra, Spain, 2015. [Google Scholar]
- Blanco, M. Valorización de Descartes y Subproductos de Pintarroja (Scyliorhinus canicula). Ph.D. Thesis, Universidad de Vigo, Pontevedra, Spain, December 2015. [Google Scholar]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolyste of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Theodore, A.E.; Raghavan, S.; Kristinsson, H.G. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J. Agric. Food Chem. 2008, 56, 7459–7466. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Pricanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Edwards, C.A.; O’Brien, W.D., Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin. Chim. Acta 1980, 104, 161–167. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Nalinanon, S. Compositional and physicochemical characteristics of acid solubilized collagen extracted from the skin of unicorn leatherjacket (Aluterus monoceros). Food Hydrocoll. 2010, 24, 588–594. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos-Ariza, P.; Pérez-Martín, R.I. Characterization of collagen from different discarded fish species of the West coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2015, 25, 388–399. [Google Scholar] [CrossRef]
- Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T. Extraction and characterisation of pepsin-solubilised collagens from the skin of bigeye snapper (Priacanthus tayenus and Prianthus macracanthus). J. Sci. Food Agric. 2010, 90, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Kunii, S.; Hamano, K.; Tonomura, B. Preparation and structural analysis of actinidain-processed atelocollagen of yellowfin tuna (Thunnus albacares). Biosci. Biotechnol. Biochem. 2004, 68, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Isolation and characterization of collagen from bigeye snapper (Priacanthus macracanthus) skin. J. Sci. Food Agric. 2005, 85, 1203–1210. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Isolation and characterization of acid and pepsin-solubilised collagens from the skin of browntripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Zelechowska, E.; Sadowska, M.; Turk, M. Isolation and some properties of collagen from the backbone of Baltic cod (Gadus morhua). Food Hydrocoll. 2010, 24, 325–329. [Google Scholar] [CrossRef]
- Borderías, A.J.; Montero, P. Changes in fish muscle collagen during frozen storage. In Storage Lives of Chilled and Frozen Fish and Fish Products; International Institute of Refrigeration: Hong Kong, China, 1985; pp. 85–91. [Google Scholar]
- Badij, F.; Howell, N. Elucidation of the effect of formaldehyde and lipids on frozen stored cod collagen by FT-Raman spectroscopy and differential scanning calorimetry. J. Agric. Food Chem. 2003, 51, 1440–1446. [Google Scholar]
- Foegeding, E.A.; Lanier, T.C.; Hultin, H.O. Characteristics of edible muscle tissues. In Food Chemistry; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 902–906. [Google Scholar]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and characteridsation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Woo, J.; Yu, S.; Cho, S.; Lee, Y.; Kim, S. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocoll. 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Love, R.M.; Yamaguchi, K.; Créach, Y.; Lavéty, J. The connective tissues and collagens of cod during starvation. Comp. Biochem. Physiol. B 1976, 55, 487–492. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kolakowska, A.; Pan, B.S. The nutritive composition of the major groups of marine food organisms. In Seafood: Resources, Nutritional Composition and Preservation; Sikorski, Z.E., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 29–54. [Google Scholar]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhow, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Nishimoto, M.; Sakamoto, R.; Mizuta, S.; Yoshinaka, R. Identification and characterization of molecular species of collagen in ordinary muscle and skin of the Japanese flounder Paralichthys olivaceus. Food Chem. 2005, 90, 151–156. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Preparation and partial characterization of collagen from paper nautilus (Argonauta argo, Linnaeus) uter skin. Food Chem. 2002, 76, 149–153. [Google Scholar] [CrossRef]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterization of collagen extracted from the skin of the striped catfish (Pangasianodon hypophtalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Nomura, Y.; Toki, S.; Ishii, Y.; Shirai, K. The physicochemical property of shark type I collagen gel and membrane. J. Agric. Food Chem. 2000, 48, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.S. Mechanism and Theory in Food Chemistry; Van Nostrand Reinhold: New York, NY, USA, 1989. [Google Scholar]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Quian, Z.-J.; Jung, W.K.; Kim, S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Harman, L.S.; Mottley, C.; Mason, R. Free radical metabolites of L-cysteine oxidation. J. Boil. Chem. 1984, 259, 5606–5611. [Google Scholar]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-F.; Wang, B.; Deng, Y.Y.; Wang, Y.M.; Deng, S.G.; Ma, J.Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolsates of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Vélez-Alavez, M. Evaluación de los Indicadores de Estrés Oxidativo Asociados a las Características de Nado en Elasmobranquios y Teleósteos. Ph.D. Thesis, Centro de Investigaciones Biológicas del Noroeste, S.C., La Paz, Mexico, 2015. [Google Scholar]
- Lundblad, R. Techniques in Protein Modification; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Barth, D.; Kyrieleis, O.; Frank, S.; Renner, C.; Moroder, L. The role of cystine knots in collagen folding and stability, part II. Conformational properties of (Pro-Hyp-Gly)n model trimers with N- and C-terminal collagen type III cystine knots. Chemistry 2003, 9, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Perez-Martin, R.I.; Sotelo, C.G. Identification of Shark Species in Seafood Products by Forensically Informative Nucleotide Sequencing (FINS). J. Agric. Food Chem. 2008, 56, 9868–9874. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemistry. Methods of Analysis, 15th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1997. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phsiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of Young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Eastoe, J.; Eastoe, B. A method for the determination of total nitrogen in proteins. Br. Gel. Glue Res. Assoc. Res. Rep. 1952, 5, 1–17. [Google Scholar]
- Adler-Nissen, J. Control of the proteolytic reaction and of the level of bitterness in protein hydrolysis processes. J. Chem. Technol. Biotechnol. 1984, 34, 215–222. [Google Scholar] [CrossRef]
- Camacho, F.; González-Tello, P.; Páez-Dueñas, M.P.; Guadix, E.M.; Guadix, A. Correlation of base consumption with the degree of hydrolysis in enzymic protein hydrolysis. J. Dairy Res. 2001, 68, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Imporoved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vázquez, J.A. An efficient methodology for quantification of synergy and antagonismin single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Amado, I.R.; González, M.P.; Murado, M.A.; Vázquez, J.A. Shrimp wastewater as a source of astaxanthin and bioactive peptides. J. Chem. Technol. Biotechnol. 2016, 91, 793–805. [Google Scholar] [CrossRef]

| Species | Composition (%) | |||
|---|---|---|---|---|
| Moisture | Protein | Lipids | Ash | |
| PGLA | 76.03 ± 0.83 | 20.14 ± 0.97 | 0.24 ± 0.03 | 4.24 ± 0.24 |
| SCAN | 61.5 ± 0.79 | 22.09 ± 0.96 | 0.36 ± 0.01 | 14.01 ± 0.5 |
| XGLA | 42.87 ± 0.54 | 16.28 ± 2.21 | 30.53 ± 1.99 | 2.49 ± 0.21 |
| TALB | 62.57 ± 2.4 | 26.96 ± 2.04 | 3.22 ± 0.72 | 0.67 ± 0.14 |
| Hydroxyproline Content in Skin (%) | Collagen Content (%) | PSC1 Yield (%) | PSC2 Yield (%) | |
|---|---|---|---|---|
| PGLA | 1.23 ± 0.11 | 9.84 ± 0.88 | 5.87 ± 0.49 | 61.17 ± 5.15 |
| SCAN | 1.85 ± 0.14 | 14.8 ± 1.14 | 4.89 ± 0.85 | 33.00 ± 5.25 |
| XGLA | 1.08 ± 0.16 | 8.64 ± 1.28 | 2.59 ± 0.22 | 31.33 ± 5.55 |
| TALB | 2.69 ± 0.26 | 21.53 ± 2.09 | 2.97 ± 0.98 | 14.16 ± 6.14 |
| Amino Acid | PSC | CALF | |||
|---|---|---|---|---|---|
| PGLA | SCAN | TALB | XGLA | ||
| Hydroxyproline | 84.62 ± 0.98 | 88.28 ± 0.62 | 87.38 ± 0.60 | 76.55 ± 0.87 | 94 |
| Aspartic acid | 46.58 ± 0.42 | 52.16 ± 0.43 | 55.40 ± 0.54 | 61.32 ± 0.46 | 45 |
| Serine | 35.98 ± 0.42 | 54.02 ± 0.14 | 35.53 ± 0.25 | 39.89 ± 0.74 | 33 |
| Gultamic acid | 92.02 ± 1.00 | 92.10 ± 0.47 | 97.89 ± 0.43 | 94.64 ± 0.96 | 75 |
| Glycine | 214.80 ± 2.92 | 234.69 ± 1.36 | 217.22 ± 1.32 | 210.20 ± 3.22 | 330 |
| Histidine | 15.80 ± 0.20 | 17.35 ± 0.10 | 12.70 ± 0.05 | 15.67 ± 0.34 | 5 |
| Arginine | 111.50 ± 1.09 | 91.26 ± 1.08 | 92.16 ± 2.97 | 89.54 ± 2.26 | 50 |
| Threonine | 33.59 ± 0.16 | 33.41 ± 0.44 | 40.00 ± 1.81 | 42.89 ± 1.60 | 18 |
| Alanine | 108.57 ±0.87 | 89.79 ± 0.97 | 111.78 ± 2.58 | 105.20 ± 2.39 | 119 |
| Proline | 107.68 ± 0.76 | 95.22 ± 0.29 | 114.86 ± 0.45 | 121.89 ± 1.30 | 121 |
| Cystine | 0.88 ± 0.01 | 0.31 ± 0.00 | 0.07 ± 0.00 | 0.61 ± 0.01 | 0 |
| Tyrosine | 3.39 ± 0.05 | 1.36 ± 0.00 | 4.42 ± 0.07 | 6.45 ± 0.15 | 3 |
| Valine | 27.77 ± 0.39 | 34.13 ± 0.12 | 25.64 ± 0.15 | 26.95 ± 0.40 | 21 |
| Methionine | 13.51 ± 0.33 | 14.06 ± 0.20 | 6.29 ± 0.13 | 3.53 ± 0.15 | 6 |
| Lysine | 33.48 ± 0.36 | 37.78 ± 0.13 | 35.37 ± 0.23 | 31.52 ± 0.43 | 26 |
| Isoleucine | 24.62 ± 0.30 | 18.29 ± 0.02 | 14.26 ± 0.15 | 20.47 ± 0.38 | 11 |
| Leucine | 25.97 ± 0.36 | 27.30 ± 0.07 | 28.28 ± 0.21 | 31.19 ± 0.68 | 23 |
| Phenylalanine | 19.25 ± 0.22 | 18.49 ± 0.01 | 20.75 ± 0.15 | 21.50 ± 0.47 | 3 |
| Iminoacids | 192.3 | 183.5 | 202.24 | 198.44 | 215 |
| % hydroxylation of proline | 44.00 | 48.10 | 43.20 | 38.57 | 44 |
| Species | Fraction | DPPH (mg BHT Eq/mL) | ABTS (mg BHT q/mL) | β-Carotene (mg BHT Eq/mL) |
|---|---|---|---|---|
| XGLA | H | 677.20 ± 114.42 | 253.77 ± 1.85 | 7.59 ± 1.93 |
| TALB | H | 578.87 ± 57.81 | 199.57 ± 37.54 | 5.67 ± 0.61 |
| SCAN | H | 494.17 ± 210.3 | 159.17 ± 30.78 | 20.86 ± 3.53 |
| PGLA | H | 405.30 ± 9.89 | 151.20 ± 43.49 | 15.26 ± 5.02 |
| XGLA | R | 465.63 ± 30.47 | 247.27 ± 10.70 | 5.91 ± 1.04 |
| TALB | R | 435.97 ± 85.54 | 174.10 ± 70.05 | 11.94 ± 3.86 |
| SCAN | R | 603.40 ± 30.88 | 143.57 ± 29.80 | 7.38 ± 11.69 |
| PGLA | R | 422.97 ± 41.32 | 124.90 ± 35.76 | 19.18 ± 1.92 |
| XGLA | P | 448.0 ± 66.45 | 264.87 ± 18.86 | 8.08 ± 0.33 |
| TALB | P | 457.67 ± 95.61 | 192.83 ± 56.66 | 15.26 ± 2.91 |
| SCAN | P | 601.70 ± 175.33 | 209.70 ± 53.71 | 12.40 ± 9.14 |
| PGLA | P | 416.03 ± 18.88 | 134.87 ± 26.76 | 17.03 ± 2.64 |
| Amino Acid | HYDROLYSATES | |||
|---|---|---|---|---|
| PGLA | SCAN | TALB | XGLA | |
| Hydroxyproline | 84.65 ± 0.80 | 87.50 ± 1.22 | 86.97 ± 0.54 | 75.15 ± 0.36 |
| Aspartic acid | 48.56 ± 0.45 | 53.33 ± 0.77 | 53.08 ± 0.24 | 59.39 ± 0.34 |
| Serine | 36.39 ± 0.34 | 52.45 ± 0.65 | 34.81 ± 0.20 | 38.83 ± 0.19 |
| Gultamic acid | 92.49 ± 0.89 | 90.97 ± 1.27 | 90.69 ± 0.42 | 92.02 ± 0.43 |
| Glycine | 230.71 ± 2.10 | 227.17 ± 2.96 | 215.82 ± 0.66 | 211.01 ± 1.06 |
| Histidine | 16.53 ± 0.13 | 16.49 ± 0.18 | 11. 18 ± 0.12 | 14.91 ± 0.03 |
| Arginine | 93.64 ± 0.98 | 93.00 ± 1.08 | 90.92 ± 0.65 | 76.46 ± 0.16 |
| Threonine | 27.99 ± 0.32 | 36.62 ± 0.59 | 40.00 ± .035 | 39.00 ± 0.26 |
| Alanine | 105.81 ±1.11 | 93.50 ± 1.27 | 108.72 ± 0.74 | 97.97 ± 0.62 |
| Proline | 106.47 ± 1.14 | 89.31 ± 1.26 | 100.22 ± 0.77 | 99.87 ± 0.61 |
| Cystine | 8.93 ±0.16 | 8.29 ± 0.33 | 31.91 ± 0.33 | 53.03 ± 0.16 |
| Tyrosine | 2.17 ± 0.01 | 1.68 ± 0.02 | 1.84 ± 0.02 | 2.24 ± 0.00 |
| Valine | 27.84 ± 0.28 | 34.12 ± 0.42 | 26.17 ± 0.17 | 27.61 ± 0.12 |
| Methionine | 13.68 ± 0.15 | 17.06 ± 0.26 | 15.19 ± 0.24 | 12.39 ± 0.09 |
| Lysine | 34.16 ± 0.32 | 37.55 ± 0.48 | 33.88 ± 0.14 | 32.70 ± 0.17 |
| Isoleucine | 24.65 ± 0.26 | 17.45 ± 0.20 | 13.05 ± 0.10 | 19.15 ± 0.09 |
| Leucine | 26.11 ± 0.25 | 25.95 ± 0.27 | 26.20 ± 0.13 | 28.58 ± 0.07 |
| Phenylalanine | 19.23 ± 0.19 | 17.56 ± 0.17 | 19.34 ± 0.10 | 19.67 ± 0.04 |
| Iminoacids | 191.12 | 176.81 | 187.19 | 175.02 |
| % hydroxylation of prol | 44.29 | 49.48 | 46.45 | 42.93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. https://doi.org/10.3390/md15050131
Blanco M, Vázquez JA, Pérez-Martín RI, Sotelo CG. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Marine Drugs. 2017; 15(5):131. https://doi.org/10.3390/md15050131
Chicago/Turabian StyleBlanco, María, José Antonio Vázquez, Ricardo I. Pérez-Martín, and Carmen G. Sotelo. 2017. "Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts" Marine Drugs 15, no. 5: 131. https://doi.org/10.3390/md15050131