Diverse Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces cejpii F31-1
Abstract
:1. Introduction
2. Results and Discussion
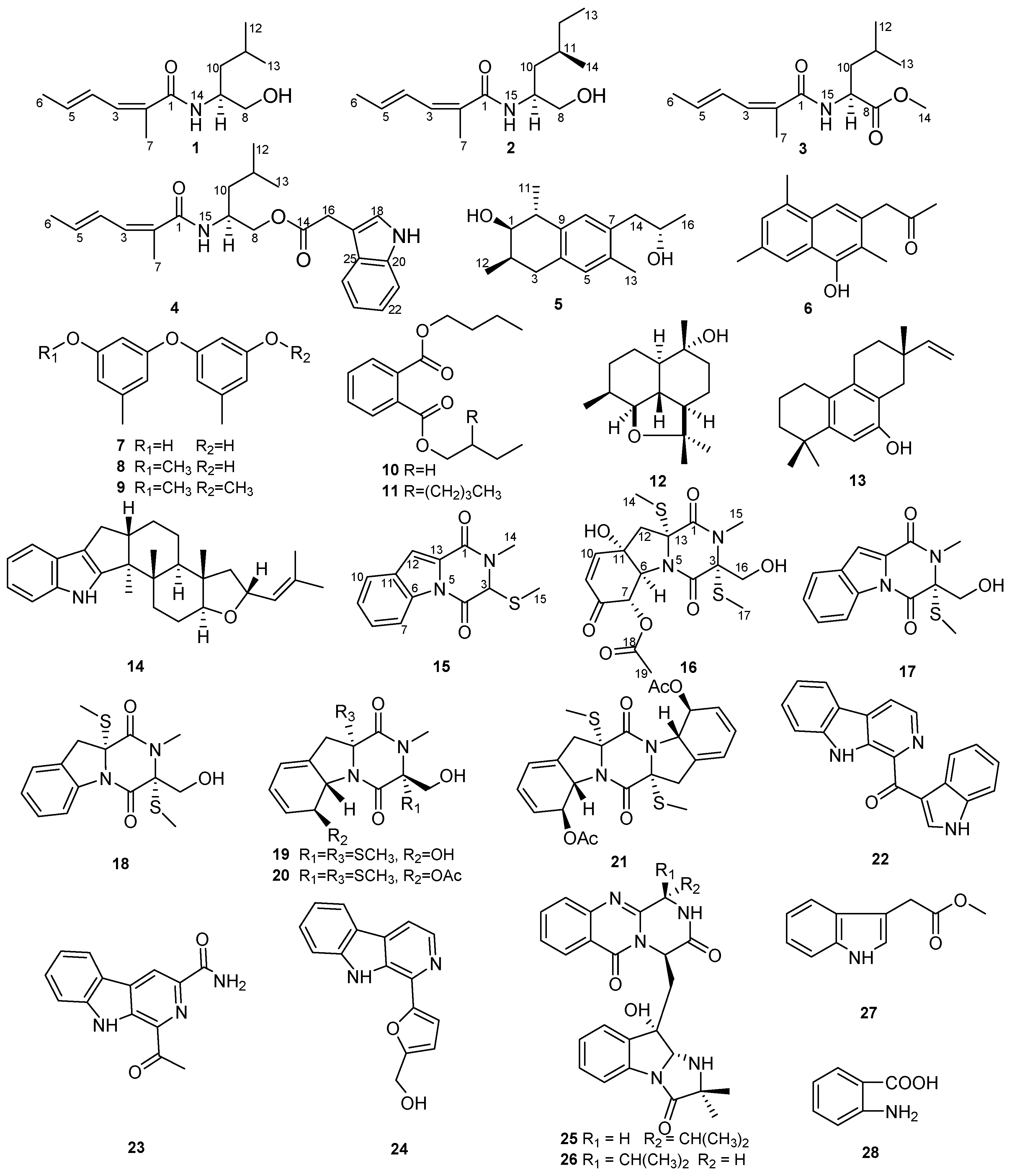
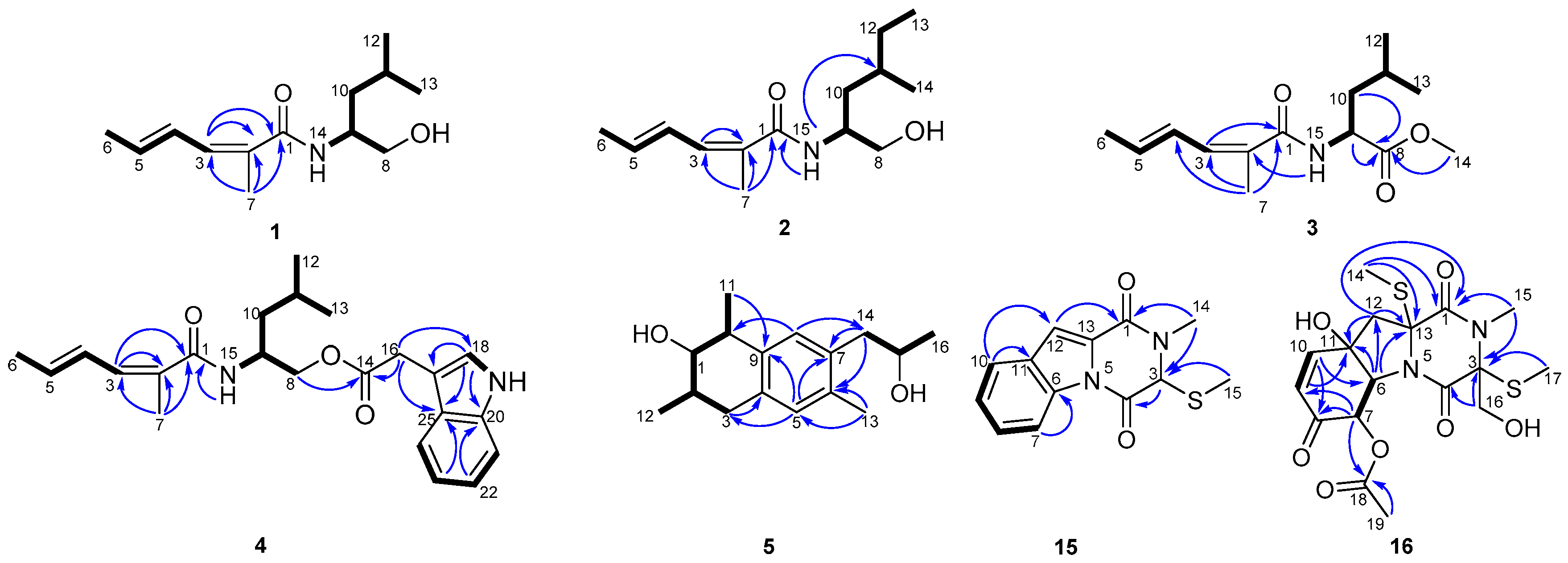
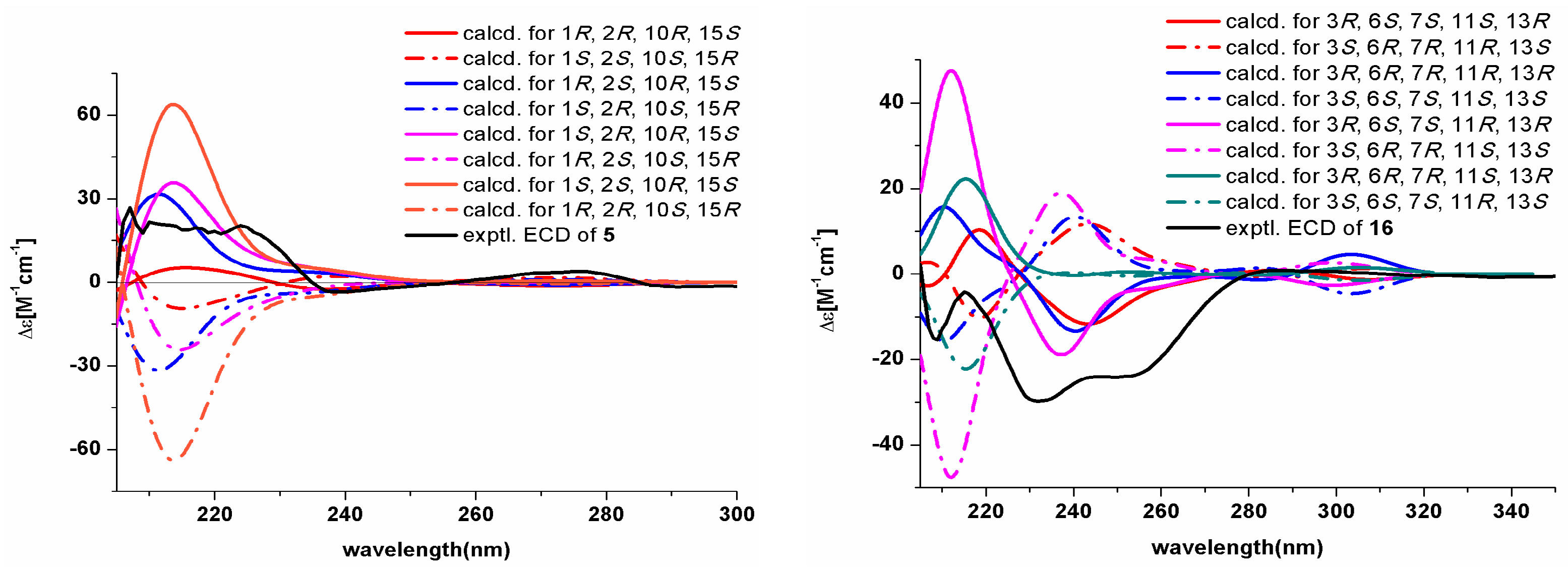
2.1. Structural Elucidation
2.2. Biological Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Culture, Extraction, and Isolation
3.4. Computational Methods
3.5. Cytotoxic Assay
3.6. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jesenska, Z.; Pieckova, E.; Bernat, D. Heat-resisitant fungi in the soil. Int. J. Food Microbiol. 1992, 16, 209–214. [Google Scholar] [CrossRef]
- Tranchida, M.C.; Centeno, N.D.; Cabello, M.N. Soil fungi: Their potential use as a forensic tool. J. Forensic Sci. 2014, 59, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Pieckova, E.; Jesenska, Z. Toxinogenicity of heat-resistant fungi detected by a bio-assay. Int. J. Food Microbiol. 1997, 36, 227–229. [Google Scholar] [CrossRef]
- Pieckova, E.; Roeijmans, H. Antibiotic secondary metabolites of Dichotomomyces cejpii. Mycopathologia 1999, 146, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.S.; Sahm, B.D.; Jimenez, P.C.; Pinto, F.C.; Mafezoli, J.; Mattos, M.C.; Rodrigues-Filho, E.; Pfenning, L.H.; Abreu, L.M.; Costa-Lotufo, L.V.; et al. Bioprospection of cytotoxic compounds in fungal strains recovered from sediments of the brazilian coast. Chem. Biodivers. 2015, 12, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Rempel, V.; Kehraus, S.; Kaiser, M.; Hufendiek, P.; Muller, C.E.; Konig, G.M. Indoloditerpenes from a marine-derived fungal strain of Dichotomomyces cejpii with antagonistic activity at GPR18 and cannabinoid receptors. J. Nat. Prod. 2014, 77, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Orlikova, B.; Ji, S.; Nesaei-Mosaferan, D.; Konig, G.M.; Diederich, M. Epipolythiodiketopiperazines from the marine derived fungus Dichotomomyces cejpii with NF-kB inhibitory potential. Mar. Drugs 2015, 13, 4949–4966. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Kehraus, S.; Nesaei-Mosaferan, D.; Hufendieck, P.; Meijer, L.; Konig, G.M. Abeta-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids 2015, 104, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Xu, M.Y.; Li, H.J.; Li, J.Q.; Chen, Y.X.; Ma, W.Z.; Li, Y.P.; Xu, J.; Yang, D.P.; Lan, W.J. Amino acid-directed strategy for inducing the marine-derived fungus Scedosporium apiospermum F41-1 to maximize alkaloid diversity. Org. Lett. 2017, 19, 4888–4891. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.J.; Wang, K.T.; Xu, M.Y.; Zhang, J.J.; Lam, C.K.; Zhong, G.H.; Xu, J.; Yang, D.P.; Li, H.J.; Wang, L.Y. Secondary metabolites with chemical diversity from the marine-derived fungus Pseudallescheria boydii F19-1 and their cytotoxic activity. RSC Adv. 2016, 6, 76206–76213. [Google Scholar] [CrossRef]
- Gallardo, G.L.; Butler, M.; Gallo, M.L.; Rodriguez, M.A.; Eberlin, M.N.; Cabrera, G.M. Antimicrobial metabolites produced by an intertidal Acremonium furcatum. Phytochemistry 2006, 67, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Le, X.; Li, H.J.; Yang, X.L.; Chen, J.X.; Xu, J.; Liu, H.L.; Wang, L.Y.; Wang, K.T.; Hu, K.C.; et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Chen, Y.X.; Yu, J.C.; Yuan, J.; Li, H.J.; Ma, W.Z.; Watanapokasin, R.; Hu, K.C.; Niaz, S.; Yang, D.P.; et al. Secondary metabolites from the marine-derived fungus Dichotomomyces sp. L-8 and their cytotoxic activity. Molecules 2017, 22, 444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Liu, P.; Wang, Y.; Li, J.; Hong, K.; Zhu, W. Cytotoxic polyphenols from the fungus penicillium expansum 091006 endogenous with the mangrove plant excoecaria agallocha. Plant. Med. 2012, 78, 1861–1866. [Google Scholar]
- Sargent, M.V.; Stransky, P.O. Naturally occurring dibenzofurans. Part 1. A synthesis of cannabifuran. J. Chem. Soc. 1982, 1605–1610. [Google Scholar] [CrossRef]
- Li, J.T.; Yin, B.L.; Liu, Y.; Wang, L.Q.; Chen, Y.G. Mono-aromatic constituents of Dendrobium longicornu. Chem. Natl. Compd. 2009, 45, 234–236. [Google Scholar] [CrossRef]
- Garba, S.; Salihu, L. Antibacterial activities of 2-o-butyl-1-o-(2′-ethylhexyl) benzene-1,8-dicarboxylate and 1-phenyl-1,4-pentanedione isolated from Vitellaria paradoxa root bark. Asian J. Sci. Res. 2011, 4, 149–157. [Google Scholar] [CrossRef]
- Wu, L.S.; Hu, C.L.; Han, T.; Zheng, C.J.; Ma, X.Q.; Rahman, K.; Qin, L.P. Cytotoxic metabolites from Perenniporia tephropora, an endophytic fungus from taxus chinensis var. Mairei. Appl. Microbiol. Biotechnol. 2013, 97, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.P.; Liang, X.R.; Liu, X.H.; Ji, N.Y. Aspewentins A–C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Ueda, J.Y.; Hoshi, M.; Hashimoto, J.; Nakashima, T.; Anzai, K.; Takagi, M.; Shinya, K. A novel indole-diterpenoid, JBIR-03 with anti-MRSA activity from Dichotomomyces cejpii var. cejpii NBRC 103559. J. Antibiot. 2007, 60, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Sun, Z.H.; Liu, Z.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Zhang, W.M. Dichotocejpins A–C: New diketopiperazines from a deep-sea-derived fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Mexia, N.; Gaitanis, G.; Velegraki, A.; Soshilov, A.; Denison, M.S.; Magiatis, P. Pityriazepin and other potent AhR ligands isolated from malassezia furfur yeast. Arch. Biochem. Biophys. 2015, 571, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.H.; Li, G.Y.; Qiao, L.; Gao, C.Y.; Wagner, H.; Lou, Z.C. Two new alkaloids from stellaria dichotoma var. Lanceolata. Natl. Prod. Res. 1995, 7, 59–64. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.J.; Jang, G.Y.; Kim, M.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Hwang, B.Y.; Song, J.; Lee, J.; et al. Isolation and identification of an antiproliferative compound from fructose-tryptophan maillard reaction products. J. Agric. Food Chem. 2016, 64, 3041–3047. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; Chandrapatya, A.; Manoch, L.; Silva, A.; Gales, L.; Bruyère, C.; Kiss, R.; Kijjoa, A. Sartorymensin, a new indole alkaloid, and new analogues of tryptoquivaline and fiscalins produced by Neosartorya siamensis (KUFC 6349). Tetrahedron 2012, 68, 3253–3262. [Google Scholar] [CrossRef]
- Nieman, J.; Coleman, J.; Wallace, D.; Piers, E.; Lim, L.; Roberge, M.; Andersen, R. Synthesis and antimitotic/cytotoxic activity of hemiasterlin analogues. J. Nat. Prod. 2003, 66, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.W.; Wang, D.C.; Cheng, H.; Zhang, M.Z.; Zhang, Y.M.; Wei, D.S.; Qin, J.C. Chemical constituent from endophytic fungus Chaetomium sp. RSQMK-9 isolated from Panax ginseng. Nat. Prod. Res. Dev. 2014, 26, 1202–1206. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision B.01; Gaussian: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Mazzeo, G.; Santoro, E.; Andolfi, A.; Cimmino, A.; Troselj, P.; Petrovic, A.G.; Superchi, S.; Evidente, A.; Berova, N. Absolute configurations of fungal and plant metabolites by chiroptical methods. Ord, ecd, and vcd studies on phyllostin, scytolide, and oxysporone. J. Nat. Prod. 2013, 76, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Pikulska, A.; Hopmann, K.H.; Bloino, J.; Pecul, M. Circular dichroism and optical rotation of lactamide and 2-aminopropanol in aqueous solution. J. Phys. Chem. B 2013, 117, 5136–5147. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, P.L.; Scalmani, G.; Hawkins, E.K.; Rizzo, C.; Jeirath, N.; Ibnusaud, I.; Habel, D.; Nair, D.S.; Haleema, S. Importance of solvation in understanding the chiroptical spectra of natural products in solution phase: Garcinia acid dimethyl ester. J. Nat. Prod. 2011, 74, 321–328. [Google Scholar] [CrossRef] [PubMed]
| No. | 1 | 2 | 3 | 4 | 5 | 15 | 16 |
|---|---|---|---|---|---|---|---|
| 1 | 170.4, C | 170.3, C | 169.1, C | 168.9, C | 75.8, CH | 156.7, C | 165.8, C |
| 2 | 127.3, C | 127.2, C | 127.3, C | 127.3, C | 31.0, CH | N | N |
| 3 | 134.6, CH | 134.6, CH | 134.5, CH | 134.1, CH | 36.3, CH2 | 66.8, CH | 71.7, C |
| 4 | 127.2, CH | 127.1, CH | 127.2, CH | 127.1, CH | 133.4, C | 161.8, C | 164.4, C |
| 5 | 136.7, CH | 136.7, CH | 136.6, CH | 136.2, CH | 130.5, CH | N | N |
| 6 | 18.9, CH3 | 19.0, CH3 | 18.9, CH3 | 18.9, CH3 | 134.6, C | 129.2, C | 70.9, CH |
| 7 | 13.0, CH3 | 13.0, CH3 | 12.9, CH3 | 12.6, CH3 | 134.4, C | 116.5, CH | 75.2, CH |
| 8 | 67.1, CH2 | 66.4, CH2 | 174.0, C | 66.6, CH2 | 130.6, CH | 128.3, CH | 190.9, C |
| 9 | 50.7, CH | 50.5, CH | 51.1, CH | 47.0, CH | 138.6, C | 125.7, CH | 127.2, CH |
| 10 | 40.5, CH2 | 38.3, CH2 | 42.1, CH2 | 40.8, CH2 | 37.8, CH | 122.8, CH | 148.4, CH |
| 11 | 25.3, CH | 31.5, CH | 25.1, CH | 24.9, CH | 17.2, CH3 | 134.9, C | 76.4, C |
| 12 | 23.2, CH3 | 29.1, CH2 | 22.9, CH3 | 23.0, CH3 | 18.2, CH3 | 115.1, CH | 51.1, CH2 |
| 13 | 22.4, CH3 | 11.2, CH3 | 22.3, CH3 | 22.4, CH3 | 19.3, CH3 | 127.9, C | 69.4, C |
| 14 | NH | 19.6, CH3 | 52.4, CH3 | 172.3, C | 43.0, CH2 | 32.2, CH3 | 15.3, CH3 |
| 15 | NH | NH | NH | 68.1, CH | 12.7, CH3 | 29.6, CH3 | |
| 16 | 31.5, CH2 | 23.2, CH3 | 64.0, CH2 | ||||
| 17 | 108.4, C | 13.7, CH3 | |||||
| 18 | 123.3, CH | 170.1, C | |||||
| 19 | NH | 20.7, CH3 | |||||
| 20 | 136.3, C | ||||||
| 21 | 111.5, CH | ||||||
| 22 | 122.3, CH | ||||||
| 23 | 119.8, CH | ||||||
| 24 | 118.8, CH | ||||||
| 25 | 127.4, C |
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 3 | 6.88 (d, 11.2) | 6.88 (d, 11.2) | 6.88 (d, 10.8) | 6.76 (d, 11.2) |
| 4 | 6.33 (ddq, 13.2, 11.2, 1.6) | 6.31 (ddq, 14.8, 11.2, 1.6) | 6.32 (ddq, 15.2, 10.8, 1.6) | 6.27 (ddq, 14.8, 11.2, 1.6) |
| 5 | 6.04 (dq, 13.2, 6.8) | 6.02 (dq, 14.8, 6.8) | 6.03 (dq, 15.2, 6.8) | 5.97 (dq, 14.8, 6.8) |
| 6 | 1.86 (d, 6.4) | 1.85 (d, 6.8) | 1.85 (d, 6.4) | 1.85 (d, 6.8) |
| 7 | 1.94 (s) | 1.93 (s) | 1.95 (s) | 1.72 (s) |
| 8 | 3.57 (dd, 10.8, 6.0); 3.72 (dd, 10.8, 3.2) | 3.55 (dd, 10.8, 6.0); 3.71 (dd, 10.8, 3.2) | 4.09 (dd, 11.2, 4.0); 4.20 (dd, 11.2, 5.2) | |
| 9 | 4.12 (m) | 4.11 (m) | 4.71 (td, 8.4, 5.2) | 4.33 (m) |
| 10 | 1.41 (dt, 8.4, 6.0) | 1.31 (dd, 13.2, 6.0); 1.54 (dt, 13.2, 6.4) | 1.58 (m); 1.70 (m) | 1.24 (m) |
| 11 | 1.66 (m) | 1.44 (m) | 1.67 (m) | 1.52 (m) |
| 12 | 0.95 (d, 6.8) | 1.14 (t, 6.8); 1.39 (m) | 0.95 (d, 6.4) | 0.85 (d, 6.4) |
| 13 | 0.94 (d, 6.8) | 0.86 (t, 6.8) | 0.95 (d, 6.4) | 0.85 (d, 6.4) |
| 14 | 5.77 (brd, 6.4) | 0.92 (d, 6.4) | 3.74 (s) | |
| 15 | 5.87 (brd, 7.2) | 6.10 (d, 8.0) | 5.54 (d, 8,8) | |
| 16 | 3.79 (s) | |||
| 18 | 7.11 (s) | |||
| 19 | 8.38 (brs) | |||
| 21 | 7.34 (d, 8.0) | |||
| 22 | 7.18 (dd, 8.0, 8.0) | |||
| 23 | 7.11 (dd, 8.0, 8.0) | |||
| 24 | 7.61 (d, 8.0) |
| No. | 5 | 15 | 16 |
|---|---|---|---|
| 1 | 3.71 (dd, 9.2, 4.8) | ||
| 2 | 2.07, m | ||
| 3 | 2.41 (dd, 16.8, 9.6); 2.92 (dd, 16.8, 6.4) | 5.04 (s) | |
| 5 | 6.87 (s) | ||
| 6 | 5.14 (d, 11.2) | ||
| 7 | 8.42 (d, 8.0) | 5.89 (d, 11.2) | |
| 8 | 6.94 (s) | 7.53 (t, 8.0) | |
| 9 | 7.40 (t, 8.0) | 6.10 (d, 10.4) | |
| 10 | 3.03 (m) | 7.70 (d, 8.0) | 6.92 (d, 10.4) |
| 11 | 1.25 (d, 7.2) | ||
| 12 | 1.11 (d, 6.4) | 7.46 (s) | 2.80 (d, 16.0); 3.42 (d, 16.0) |
| 13 | 2.26 (s) | ||
| 14 | 2.67 (dd, 13.6, 8.4); 2.76 (dd, 13.6, 4.4) | 3.24 (s) | 2.23 (s) |
| 15 | 4.00, m | 2.05 (s) | 3.10 (s) |
| 16 | 1.27 (d, 6.4) | 3.85 (d, 12.0); 4.31 (d, 12.0) | |
| 17 | 2.19 (s) | ||
| 19 | 2.17 (s) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-X.; Xu, M.-Y.; Li, H.-J.; Zeng, K.-J.; Ma, W.-Z.; Tian, G.-B.; Xu, J.; Yang, D.-P.; Lan, W.-J. Diverse Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces cejpii F31-1. Mar. Drugs 2017, 15, 339. https://doi.org/10.3390/md15110339
Chen Y-X, Xu M-Y, Li H-J, Zeng K-J, Ma W-Z, Tian G-B, Xu J, Yang D-P, Lan W-J. Diverse Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces cejpii F31-1. Marine Drugs. 2017; 15(11):339. https://doi.org/10.3390/md15110339
Chicago/Turabian StyleChen, Yan-Xiu, Meng-Yang Xu, Hou-Jin Li, Kun-Jiao Zeng, Wen-Zhe Ma, Guo-Bao Tian, Jun Xu, De-Po Yang, and Wen-Jian Lan. 2017. "Diverse Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces cejpii F31-1" Marine Drugs 15, no. 11: 339. https://doi.org/10.3390/md15110339




