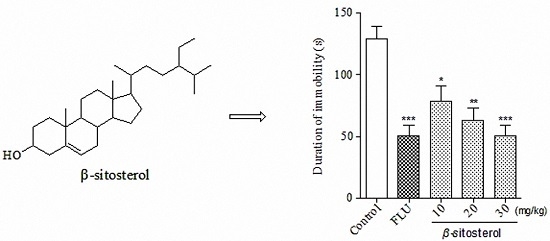
Structural Features and Potent Antidepressant Effects of Total Sterols and β-sitosterol Extracted from Sargassum horneri
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
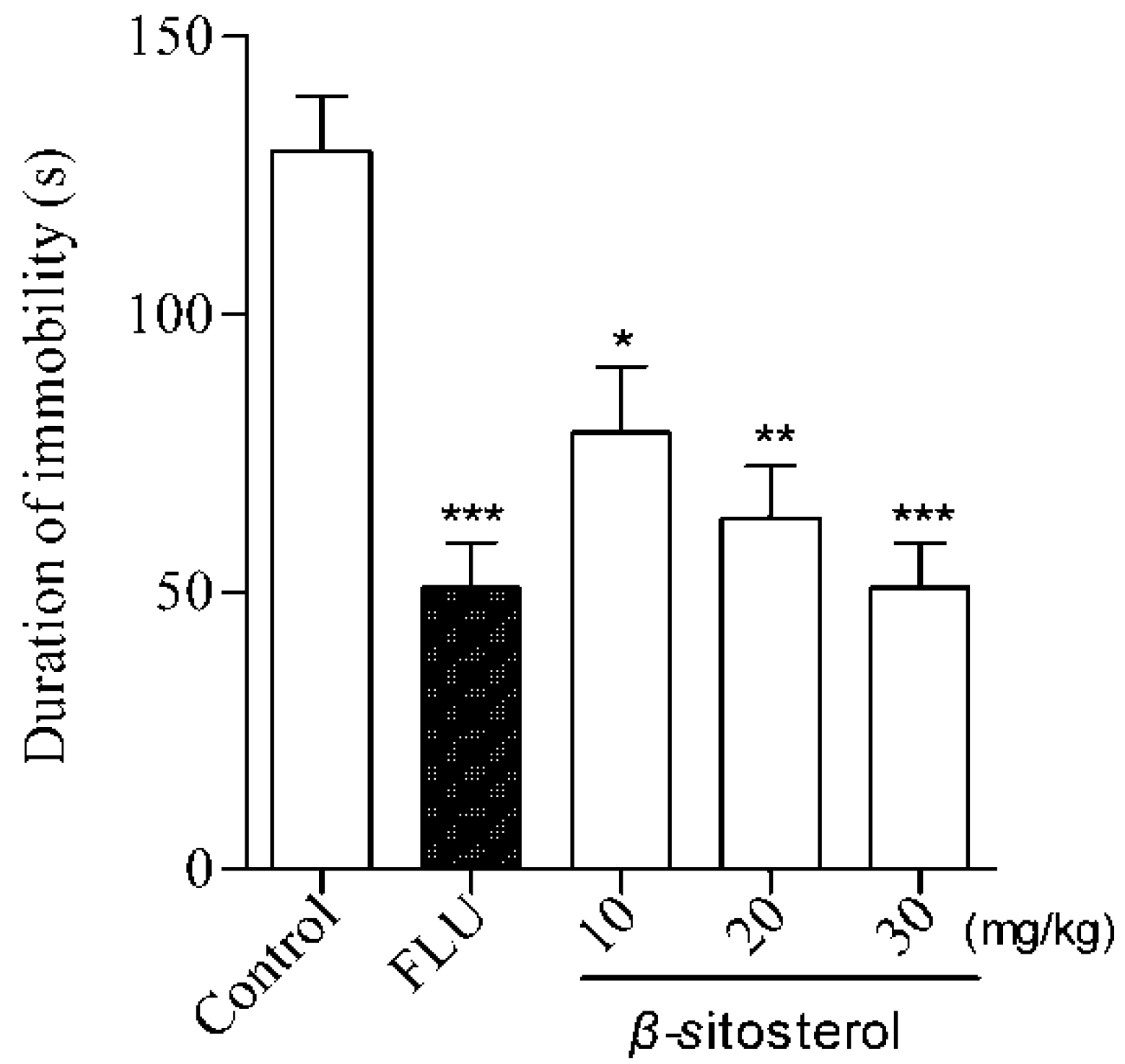
2.2. Effects of Total Sterol Extract and β-sitosterol on Immobility Time in the FST and TST
2.3. Effect on the Open-Field Test
2.4. Effects of Total Sterols and β-sitosterol on Monoamine Neurotransmitter Levels
3. Experimental Section
3.1. Materials and Agents
3.2. Animals
3.3. Isolation and Purification of Total Sterols and β-sitosterol
3.4. Drug Treatment
3.5. The Forced Swim Test
3.6. The Tail Suspension Test
3.7. The Open-Field Test
3.8. The Sample Preparation
3.9. HPLC Condition and Test
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaffrey, M.S.; Luby, J.L.; Barch, D.M. Towards the study of functional brain development in depression: An interactive specialization approach. Neurobiol. Dis. 2013, 52, 38–48. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: http://www.who.int/mediacentre/news/notes/2012/mental_health_day_20121009/en/ (accessed on 9 October 2012).
- Lopez, A.D.; Murray, C.C. The global burden of disease, 1990–2020. Nat. Med. 1998, 4, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, R.; Malki, K.; Maier, W.; Rietschel, M.; Mors, O.; Hauser, J.; Henigsberg, N.; Dernovsek, M.Z.; Souery, D.; Stahl, D.; et al. Combining clinical variables to optimize prediction of antidepressant treatment outcomes. J. Psychiatr. Res. 2016, 78, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bergner, C.L.; Smolinsky, A.N.; Hart, P.C.; Dufour, B.D.; Egan, R.J.; LaPorte, J.L.; Kalueff, A.V. Mouse models for studying depression-like states and antidepressant drugs. Methods Mol. Biol. 2016, 1438, 255–269. [Google Scholar] [PubMed]
- Alò, R.; Mele, M.; Fazzari, G.; Avolio, E.; Canonaco, M. Exposure to sub-chronic unpredictable stress accounts for antidepressant-like effects in hamsters treated with BDNF and CNQX. Brain Res. Bull. 2015, 118, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, X.L.; Mu, R.H.; Wu, Y.J.; Liu, B.B.; Geng, D.; Liu, Q.; Yi, L.T. Hippocampal BDNF signaling restored with chronic asiaticoside treatment in depression-like mice. Brain Res. Bull. 2015, 114, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Naert, G.; Ixart, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Brain-derived neurotrophic factor and hypothalamic pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol. Cell. Neurosci. 2011, 46, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Pilc, A.; Wieronska, J.M.; Skolnick, P. Glutamate-based antidepressants: Preclinical psychopharma-cology. Biol. Psychiatry 2013, 73, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Kessels, H.W. The developmental stages of synaptic plasticity. J. Physiol. 2014, 592, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Alò, R.; Mele, M.; Avolio, E.; Fazzari, G. Distint AMYAMPAergic/GABAergic-mechanisms promote anxiolitic-like effects in an unpredictable stress modelof the hamster. J. Mol. Neurosci. 2015, 55, 54. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Vishchuk, O.S.M.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Kicha, A.A. Four new sulfated polar steroids from the far eastern starfish Leptasterias ochotensis: Structures and activities. Mar. Drugs 2015, 13, 4418–4435. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C. Research progress of depression and the natural antidepressant drug. J. Pharm. Pract. 2005, 23, 3–5. [Google Scholar]
- Zhen, X.H.; Quan, Y.C.; Jiang, H.Y.; Wen, Z.S.; Qu, Y.L.; Guan, L.P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Liu, J.; Chen, X.; Fang, Z.; Sun, P. Structural features and antitumor activity of a purified polysaccharideextracted from Sargassum horneri. Int. J. Biol. Macromol. 2015, 73, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Xiang, X.W.; Jin, H.X.; Guo, X.Y.; Liu, L.J.; Huang, Y.N.; OuYang, X.K.; Qu, Y.L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.W.; Youn, H.Y.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. agardh on lipopolysa-ccharide-stimulated macrophage activation via NF-iB pathway regulation. Immunol. Investig. 2015, 44, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.C.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.C.; Guan, H.S.; Liu, H.B. Isolation of the molecular species of monogalactosyldiacylglycerols from brown edible seaweed Sargassum horneri and their inhibitory effects on triglyceride accumulation in 3T3-L1 adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.R.; Yang, R.; He, Y.Y.; Sun, Q.H. Genetic variation of Sargassum horneri populations detected by inter-simple sequence repeats. Genet. Mol. Res. 2015, 14, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; OuYang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Bansal, S. Antidepressant-like activity of plumbagin in unstressed and stressed mice. Pharmacol. Rep. 2015, 67, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Belzung, C. Treatment-resistant depression: are animal models of depressi-on fit for purpose? Psychopharmacology 2015, 232, 3473–3495. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Cryan, J.F. The ups and downs of modeling mood disorders in rodents. ILAS J. 2014, 55, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wang, J.X.; Hu, X.X.; Chen, L.; Qiu, Z.K.; Zhao, N.; Yu, Z.D.; Sun, S.Z.; Xu, Y.Y.; Guo, Y.; et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J. Ethnopharmacol. 2016, 79, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, R.; Tang, W.J.; Meng, G.; Hu, X.Y.; Wu, T.N. Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus-pituitary-adrenal axis. Eur. Neuropsychopharmacol. 2015, 25, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Hellemans, K.G.; Verma, P.; Gorzalka, B.B.; Weinberg, J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci. Biobehav. Rev. 2012, 36, 2085–2117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Fan, Y.; Shi, D.; Liu, C. Antidepressant-like effect of flavonoids extracted from Apocynum venetum leaves on brain monoamine levels and dopaminergic system. J. Ethnopharmacol. 2013, 147, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kordjazy, N.; Haj-Mirzaian, A.; Amiri, S.; Ostadhadi, S.; Kordjazy, M.; Sharifzadeh, M.; Dehpour, A.R. Elevated level of nitric oxide mediates the anti-depressant effect of rubidium chloride in mice. Eur. J. Pharmacol. 2015, 762, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Amato, D.; Pum, M.E.; Groos, D.; Lauber, A.C.; Huston, J.P.; Carey, R.J.; Silva, M.A.D.S.; Müller, C.P. Neuropharmacology of light-induced locomotor activation. Neuropharmacology 2015, 95, 243–251. [Google Scholar] [CrossRef] [PubMed]
- González-Cortazar, M.; Maldonado-Abarca, A.M.; Jiménez-Ferrer, E.; Marquina, S.; Ventura-Zapata, E.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Isosakuranetin-5-O-rutinoside: A new flavanone with antidepressant activity isolated from Salvia elegans Vahl. Molecules 2013, 18, 13260–13270. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, T.; Montgomery, T.R.; Hofmaier, T.; Kudlacek, O.; Yang, J.W.; Rickhag, M.; Jung, G.; Lubec, G.; Gether, U.; Freissmuth, M.; et al. Amphetamine action at the cocaine- and antidepressant-sensitive serotonin transporter is modulated by αCaMKII. J. Neurosci. 2015, 35, 8258–8271. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, H.; Zhou, C.; Jia, H.; Ma, Z.; Zou, Z. Identification of the chemical constituents in aqueous extract of Zhi-Qiao and evaluation of itsantidepressant effect. Molecules 2015, 20, 6925–6940. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Lin, S.H.; Chen, W.C.; Ho, C.T.; Lai, Y.S.; Panyod, S.; Sheen, L.Y. Antidepressant-like effects of water extract of Gastrodia elata Blume in rats exposed to unpredictable chronic mild stress via modulation of monoamine regulatory pathways. J. Ethnopharmacol. 2016, 187, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, H.; Zhao, L.; Li, X.; You, J.; Jiang, Q.; Li, S.; Jin, L.; Xu, Y. Protective effects of aqueous extract from Acanthopanax senticosus against corticosterone-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2013, 148, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.; Sandhir, R. Role of dopaminergic and serotonergic neurotransmitters in behavioral alterations observed in rodent model of hepatic encephalopathy. Behav. Brain Res. 2015, 286, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroye, J.O.; Galdino, P.M.; de Paula, J.A.; Rocha, F.F.; Akanmu, M.A.; Vanderlinde, F.A.; Zjawiony, J.K.; Costa, E.A. Anxiolytic and antidepressant like effects of natural food flavour (E)-methyl isoeugenol. Food Funct. 2014, 5, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.W.; Lai, W.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test. J. Funct. Foods 2013, 5, 370–379. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H.; Liu, Y.; He, H.; Li, G. Vanillin-induced amelioration of depression-like behaviors in rats by modulating monoamine neurotransmitters in the brain. Psychiatry Res. 2015, 225, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Cui, Y.; Feng, W.S.; Zhang, Y.Y.; Wang, G.F.; Wang, X.X.; Zhou, G. Involvement of the central monoaminergic system in the antidepressant-like effect of catalpol in mice. BioSci. Trends. 2014, 8, 248–252. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, W.; Zhang, J.J.; Qu, S.S.; Li, J.J.; Wang, L.Y. Determination of β-sitosterol and total sterols content and antioxidant activity of oil in acai (Euterpe oleracea). Zhongguo Zhong Yao Za Zhi 2014, 39, 4620–4624. [Google Scholar] [PubMed]
- Sriraman, S.; Ramanujam, G.M.; Ramasamy, M.; Dubey, G.P. Identification of beta-sitosterol and stigmasterol in Bambusa bambos (L.) Voss leaf extract using HPLC and its estrogenic effect in vitro. J. Pharm. Biomed. Anal. 2015, 115, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Haj-Mirzaian, A.; Kordjazy, N.; Amiri, S.; Haj-Mirzaian, A.; Amini-Khoei, H.; Ostadhadi, S.; Dehpour, A. Involvement of nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of tropisetron and ondansetron in mice forced swimming test and tail suspension test. Eur. J. Pharmacol. 2016, 780, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Rosca, A.E.; Zeca, V.; Zagrean, L.; Zagrean, A.M. Impact of maternal melatonin suppression on forced swim and tail suspension behavioral despair tests in adult offspring. J. Med. Life 2015, 8, 202–206. [Google Scholar] [PubMed]



| Compounds | Dose (mg/kg) | Antidepressant Activity 1 | |
|---|---|---|---|
| Duration of Immobility (s) | DID (%) 2 | ||
| FST | |||
| Total sterols | 50 | 91.6 ± 12.1 * | 30.44 |
| 100 | 75.2 ± 9.4 ** | 42.90 | |
| 200 | 60.3 ± 9.6 *** | 54.21 | |
| Fluoxetine | 20 | 62.5 ± 6.5 *** | 52.54 |
| Control | — | 131.7 ± 11.3 | — |
| β-sitosterol | 10 | 78.7 ± 11.9 * | 39.27 |
| 20 | 63.2 ± 9.5 ** | 51.23 | |
| 30 | 55.1 ± 10.1 *** | 57.48 | |
| Fluoxetine | 20 | 50.8 ± 8.1 *** | 60.80 |
| Control | — | 129.6 ± 9.8 | — |
| TST | |||
| Total sterols | 50 | 99.6 ± 10.1 | 28.45 |
| 100 | 70.2 ± 10.6 ** | 49.57 | |
| 200 | 66.3 ± 8.2 *** | 52.37 | |
| Fluoxetine | 20 | 65.5 ± 8.5 *** | 52.95 |
| Control | — | 139.2 ± 12.5 | — |
| β-sitosterol | 10 | 89.9 ± 12.7 * | 31.63 |
| 20 | 73.7 ± 10.5 ** | 43.95 | |
| 30 | 61.3 ± 11.3 *** | 53.38 | |
| Fluoxetine | 20 | 54.8 ± 9.3 *** | 58.33 |
| Control | — | 131.5 ± 13.3 | — |
| Groups | 5-HT | NE | DA | 5-HIAA |
|---|---|---|---|---|
| Normal vehicle | 316.4 ± 30.6 | 217.6 ± 27.3 | 401.3 ± 39.5 | 273.7 ± 28.3 |
| Stress vehicle | 202.1 ± 29.3 | 197.8 ± 22.4 | 346.3 ± 41.2 | 132.1 ± 26.4 |
| Total sterols | 348.2 ± 29.5 a,d | 358.5 ± 20.2 a,d | 299.8 ± 39.4 | 271.3 ± 27.2 b |
| β-sitosterol | 387.1 ± 38.5 b,d | 398.2 ± 31.5 c,e | 327.3 ± 38.6 | 312.1 ± 31.3 a |
| Fluoxetine | 373.6 ± 39.4 b,d | 389.5 ± 33.1 c,e | 302.4 ± 40.1 | 289.2 ± 33.4 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Zheng, L.; Qi, L.; Wang, S.; Guan, L.; Xia, Y.; Cai, J. Structural Features and Potent Antidepressant Effects of Total Sterols and β-sitosterol Extracted from Sargassum horneri. Mar. Drugs 2016, 14, 123. https://doi.org/10.3390/md14070123
Zhao D, Zheng L, Qi L, Wang S, Guan L, Xia Y, Cai J. Structural Features and Potent Antidepressant Effects of Total Sterols and β-sitosterol Extracted from Sargassum horneri. Marine Drugs. 2016; 14(7):123. https://doi.org/10.3390/md14070123
Chicago/Turabian StyleZhao, Donghai, Lianwen Zheng, Ling Qi, Shuran Wang, Liping Guan, Yanan Xia, and Jianhui Cai. 2016. "Structural Features and Potent Antidepressant Effects of Total Sterols and β-sitosterol Extracted from Sargassum horneri" Marine Drugs 14, no. 7: 123. https://doi.org/10.3390/md14070123
APA StyleZhao, D., Zheng, L., Qi, L., Wang, S., Guan, L., Xia, Y., & Cai, J. (2016). Structural Features and Potent Antidepressant Effects of Total Sterols and β-sitosterol Extracted from Sargassum horneri. Marine Drugs, 14(7), 123. https://doi.org/10.3390/md14070123