Indole Alkaloids of the Stigonematales (Cyanophyta): Chemical Diversity, Biosynthesis and Biological Activity
Abstract
:1. Introduction
2. Biology of the Stigonematales
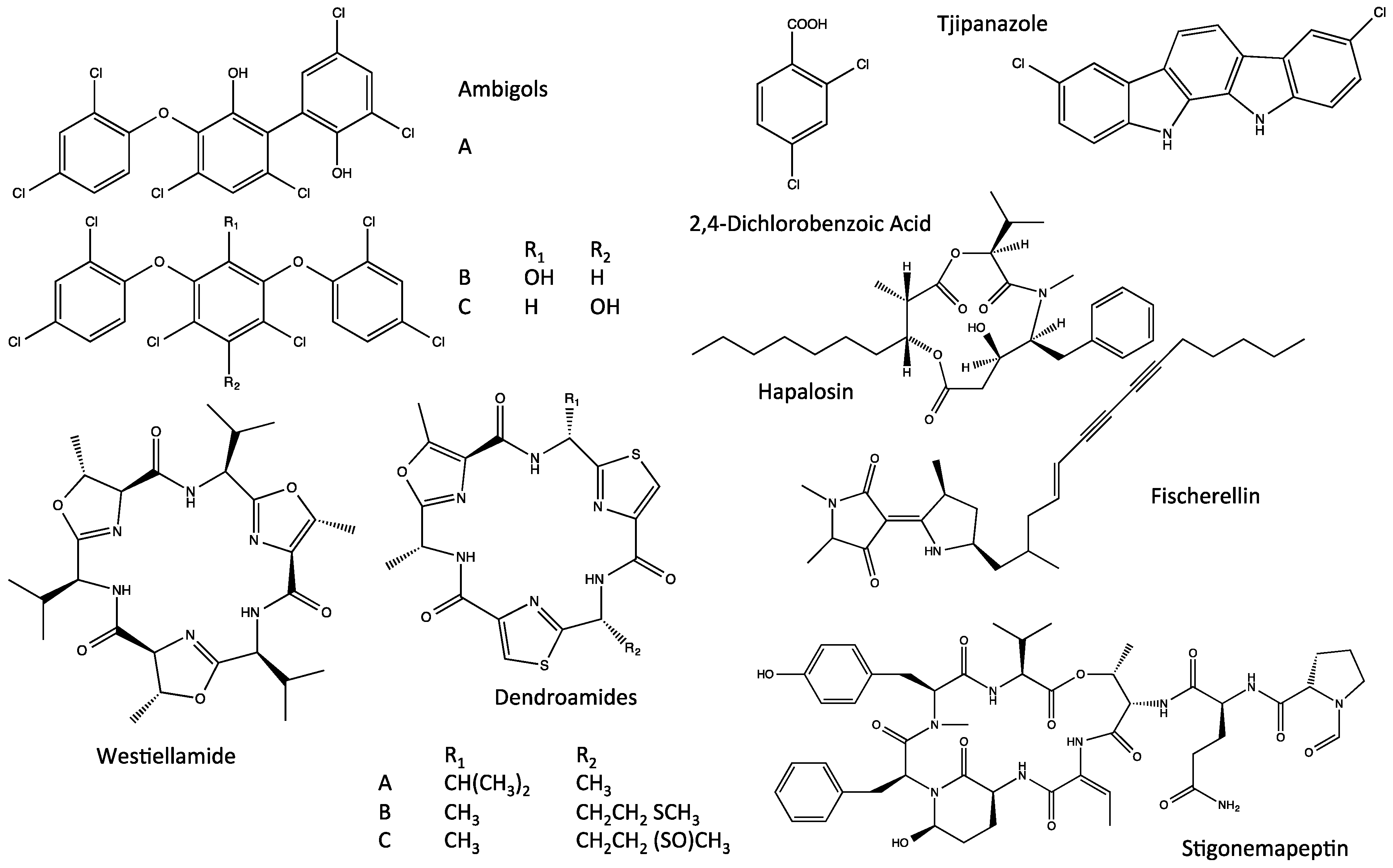
3. Stigonematales as a Source of Bioactive Metabolites
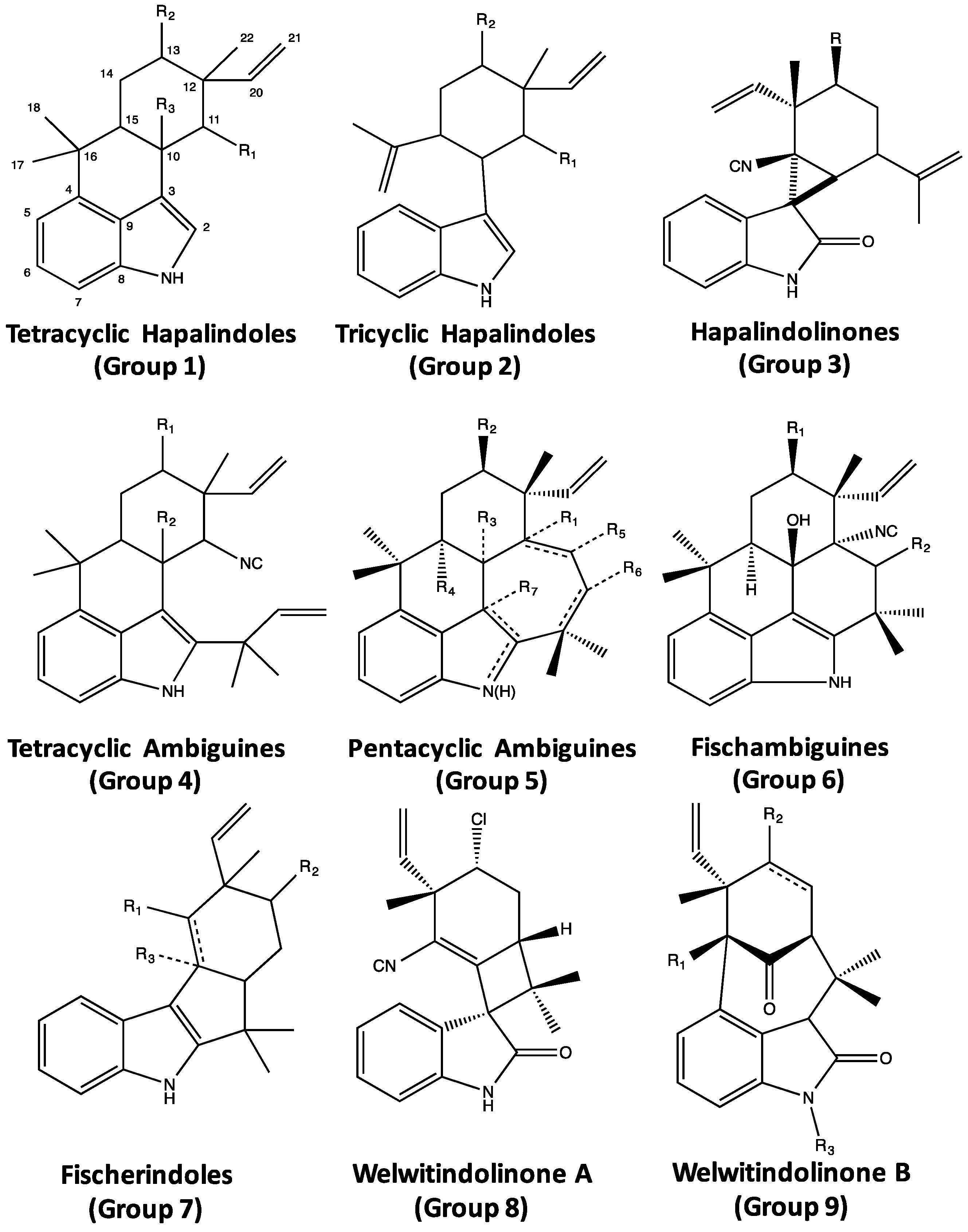
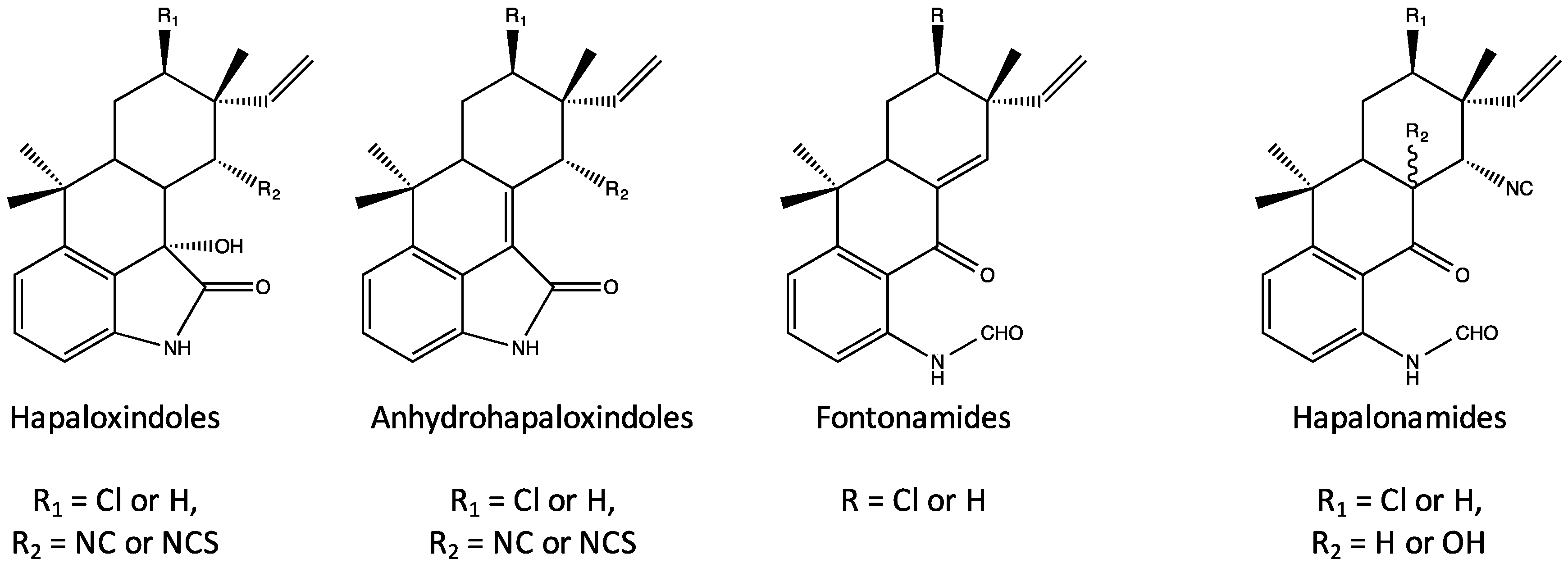
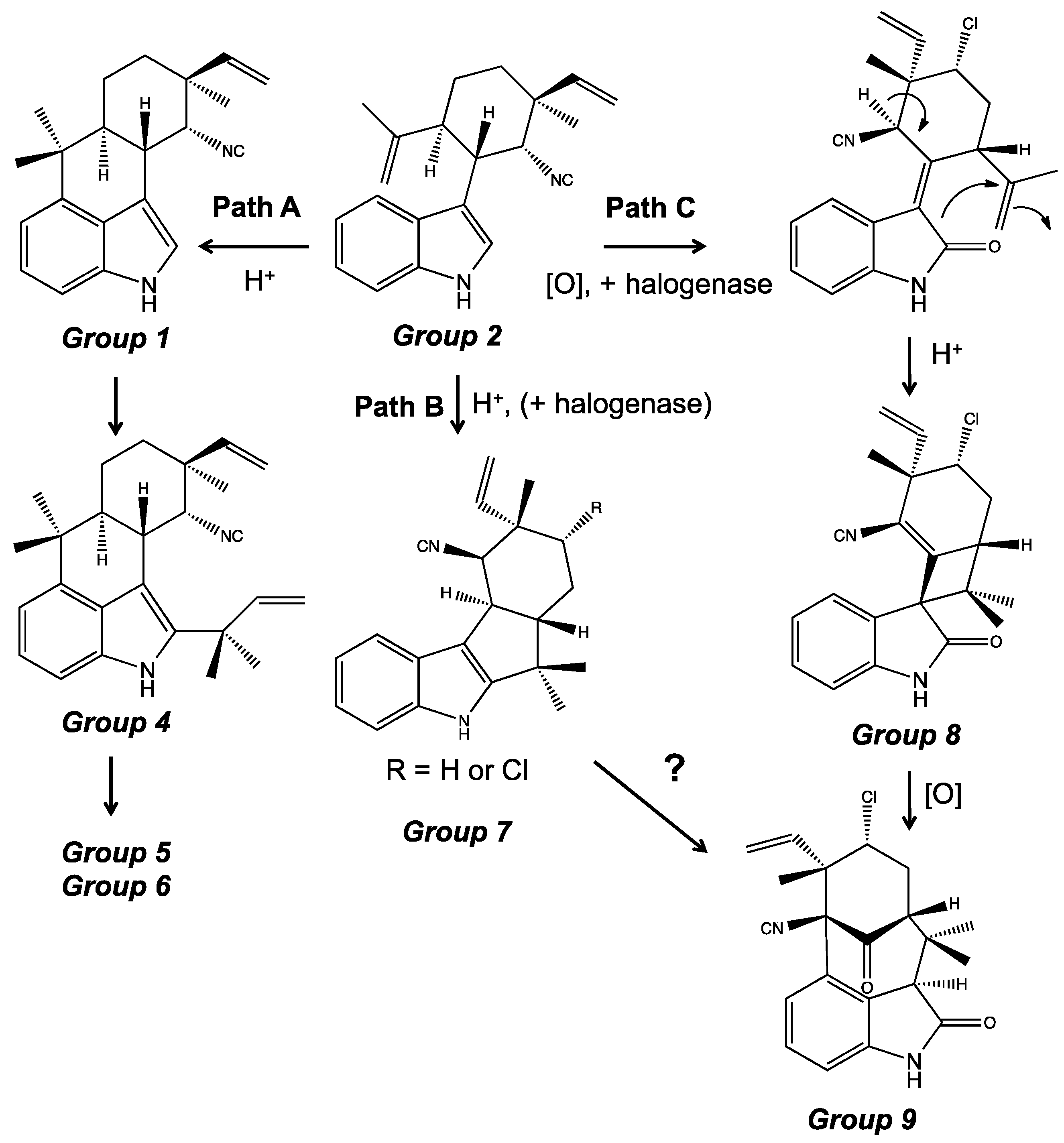
4. Indole Alkaloids from the Stigonematales: Chemical Diversity
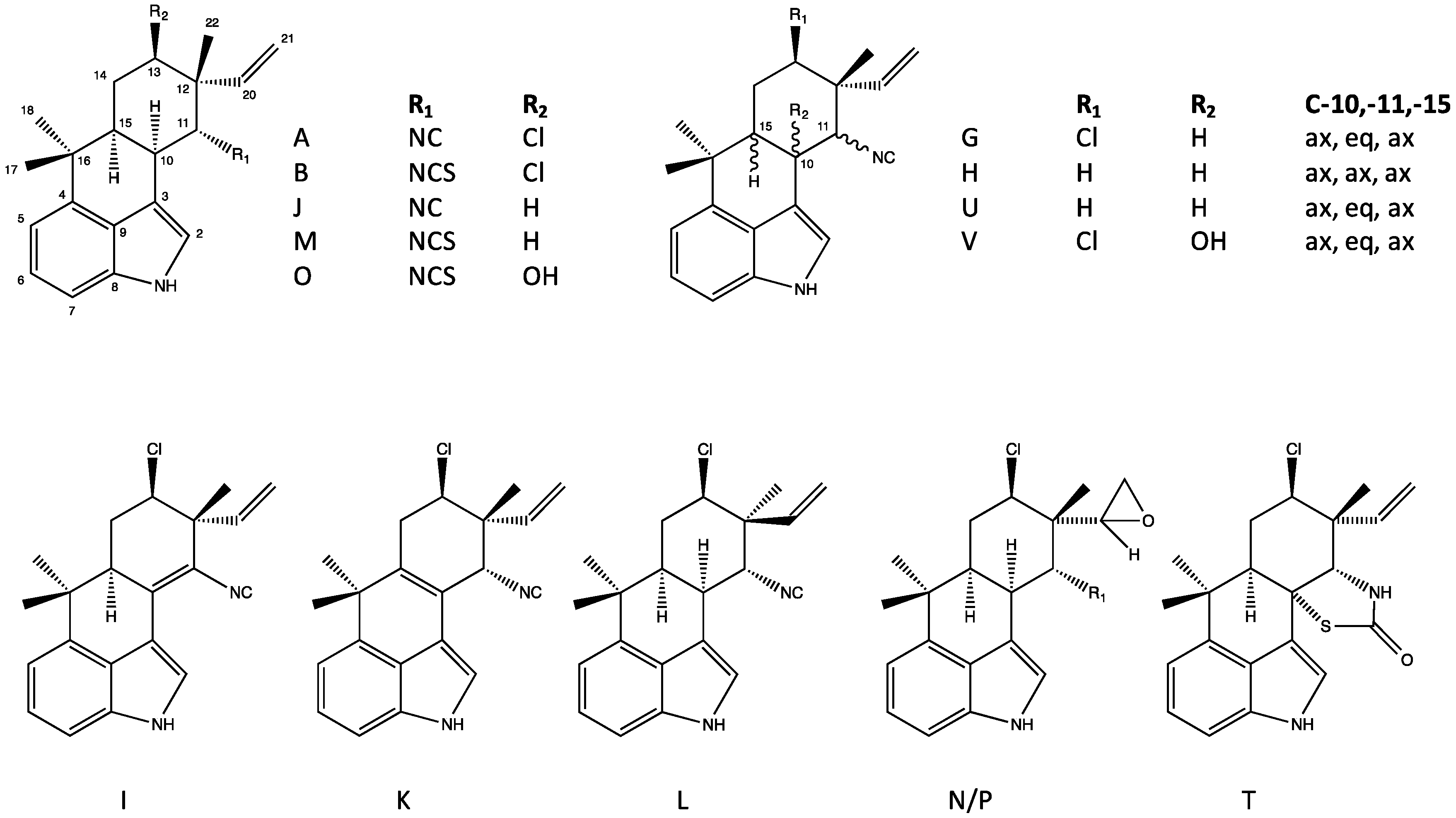
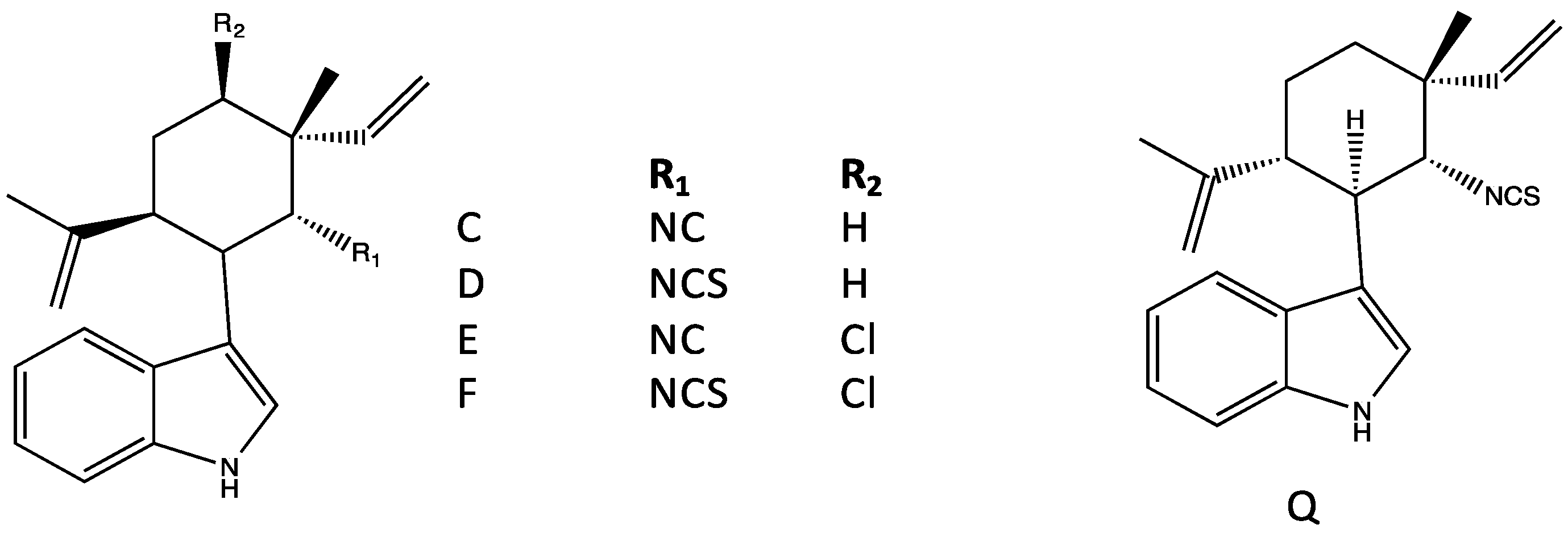
4.1. Hapalindoles
4.2. Hapalindolinones
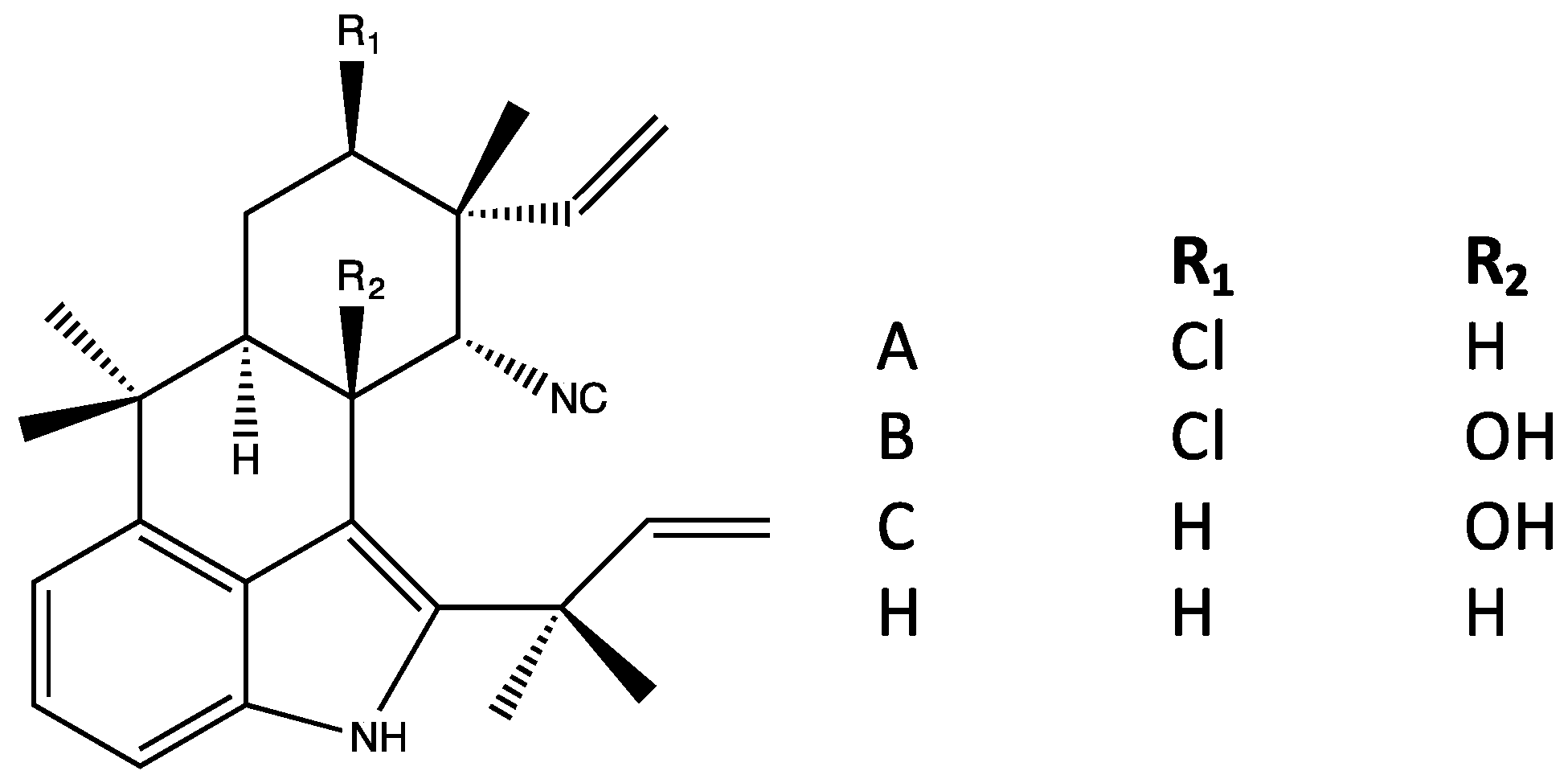
4.3. Ambiguines
4.4. Fischambiguines
4.5. Fischerindoles
4.6. Welwitindolinones
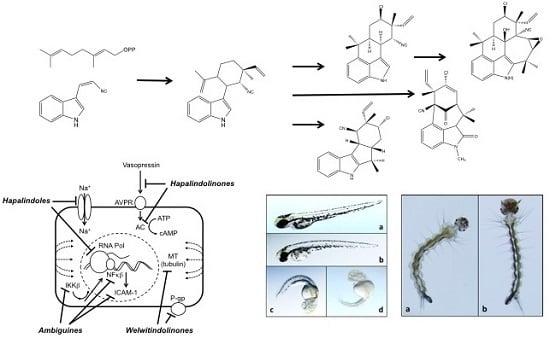
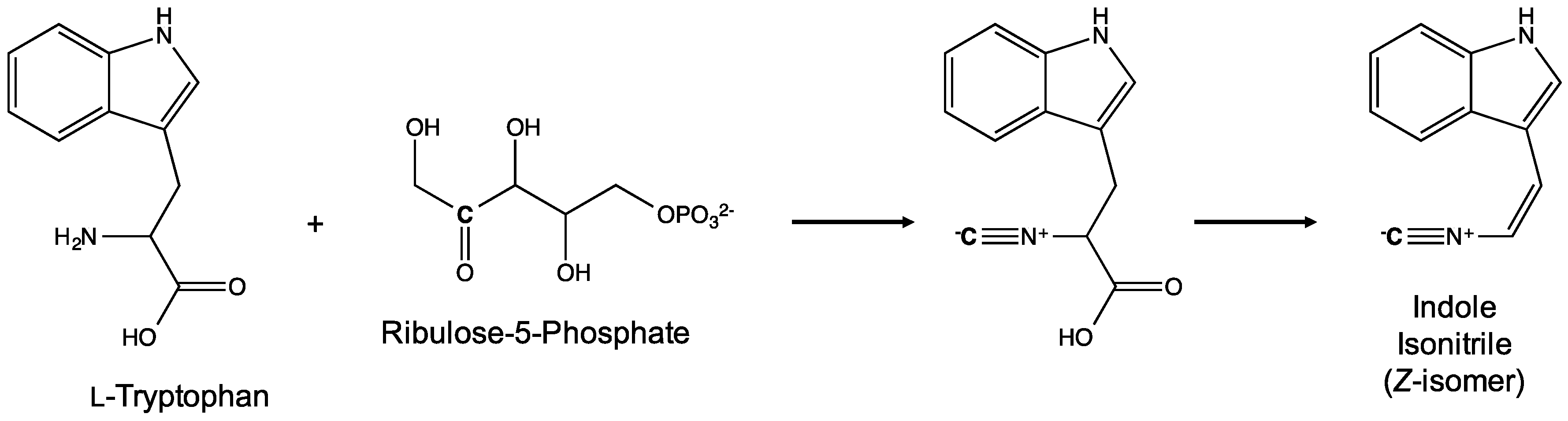
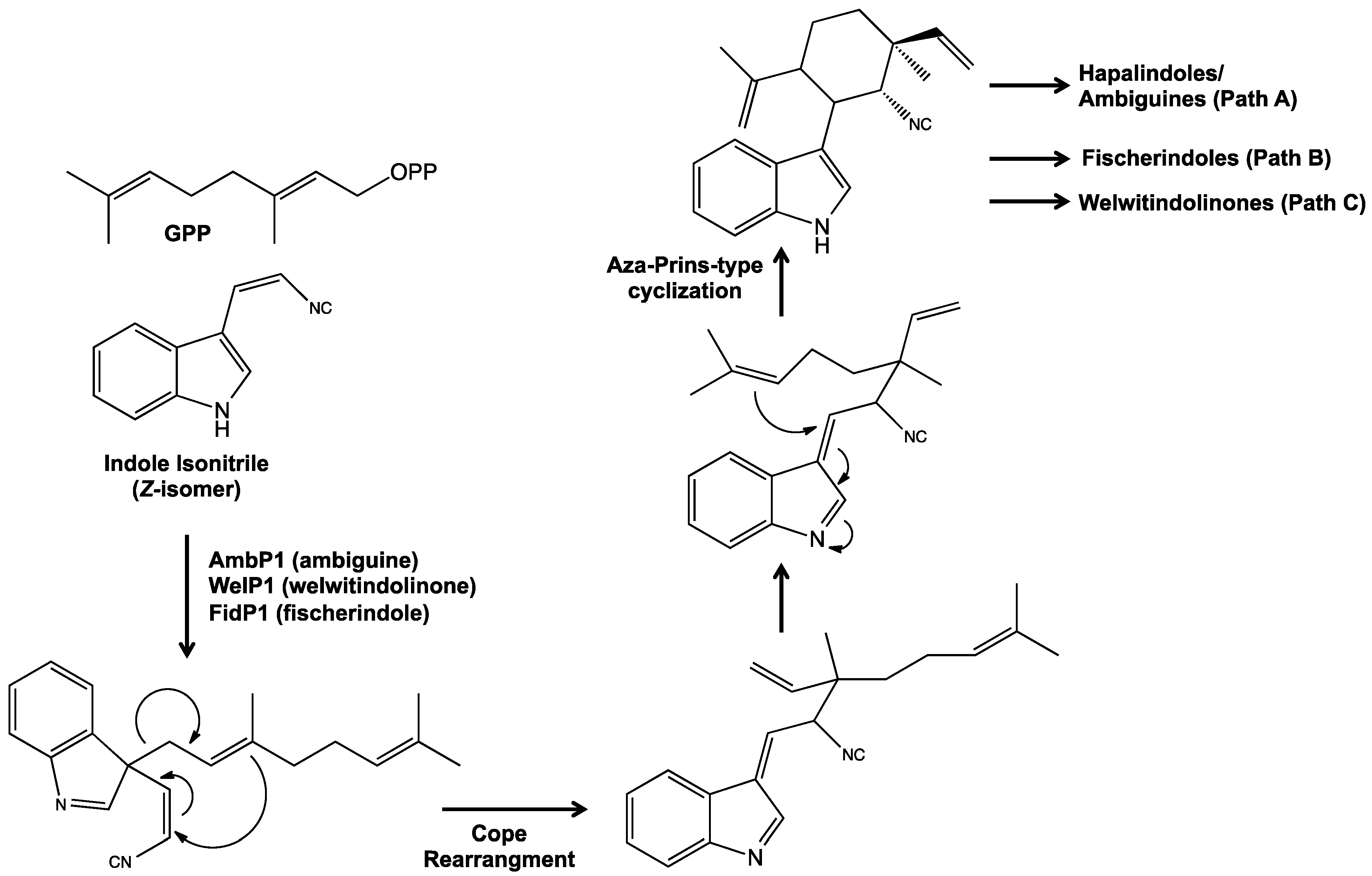
5. Biosynthesis of Indole Alkaloids from the Stigonematales
6. Biological Activity of the Hapalindole-Type Alkaloids
6.1. Antimicrobial Activity
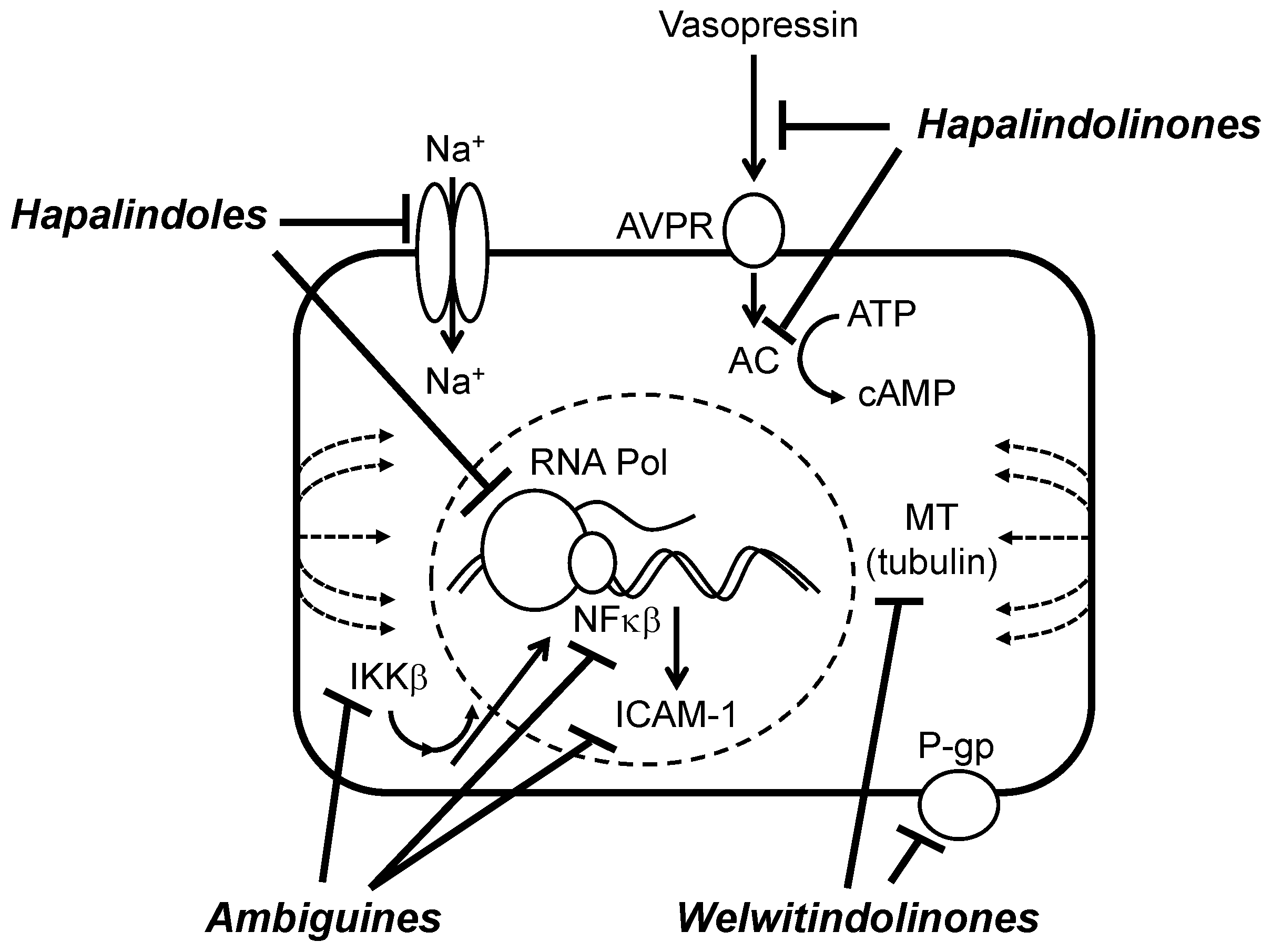
6.2. Biochemical, Molecular and Cellular Bioactivity
6.3. Toxicity to Plants and Animals
7. Possible Ecological Functions
8. Relevance as Harmful Algae
9. Potential Source of Drug Leads
10. Conclusions
Author Contributions
Conflicts of Interest
References
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011, 64, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.B.; Suseela, M.R. Cyanobacteria: Potential candidates for drug discovery. Antonie Van Leeuwenhoek 2013, 103, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Ibelings, B.W.; Backer, L.C.; Kardinaal, W.E.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2015, 49, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wilmotte, A.; Herdman, M. Phylogenetic relationships among the cyanobacteria based on 16s rRNA sequences. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, R.W., Eds.; Springer: New York, NY, USA, 2001; Volume 1, pp. 487–493. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of Cyanophytes 5-Stigonematales. Arch. Hydrobiol. Suppl. 1990, 86, 1–73. [Google Scholar]
- Gugger, M.F.; Hoffmann, L. Polyphyly of true branching cyanobacteria (Stigonematales). Int. J. Sytemat. Evol. Microbiol. 2004, 54, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Dagan, T.; Roettger, M.; Stucken, K.; Landan, G.; Koch, R.; Major, P.; Gould, S.B.; Goremykin, V.V.; Rippka, R.; Tandeau de Marsac, N.; et al. Genomes of Stigonematalean cyanobacteria (Subsection V) and the evolution of oxygenic photosynthesis from prokaryote to plastids. Genome Biol. Evol. 2013, 5, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.B.; Johansen, J.R.; Wilde, H.D.; Jiang, P.; Bartelme, B.A.; Haynie, R.S. Aetokthenos hydrillicola gen. et sp. nov.: Epiphytic cyanobacteria on invasive aquatic plants implicated in Avian Vacuolar Myelinopathy. Phytotaxa 2014, 181, 243–260. [Google Scholar] [CrossRef]
- Fiore, M.F.; Genuária, D.B.; da Silva, C.S.; Shishido, T.K.; Moraes, L.A.; Cantúsio, N.R.; Silva-Stenico, M.E. Microcystin production by a freshwater spring cyanobacterium of the genus Fischerella. Toxicon 2009, 53, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Prinsep, M.R.; Caplan, F.R.; Moore, R.E.; Patterson, G.M.L.; Honkanen, R.E.; Boynton, A.L. Microcystin-LA from a blue-green algae belonging to the Stigonematales. Phytochemistry 1992, 31, 1247–1248. [Google Scholar] [CrossRef]
- Cirés, R.; Alvarez-Roa, C.; Wood, S.A.; Puddick, J.; Loza, V.; Heiman, K. First report of microcystins producing Fischerella sp. (Stigonematales, Cyanobacteria) in tropical Australia. Toxicon 2014, 88, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Bidigare, R.R.; Christensen, S.J.; Wilde, S.B.; Banack, S.A. Cyanobacteria and BMAA: Possible linkage with avian vacuolar myelinopathy (AVM) in the southeastern United States. Amyotroph. Lateral Scler. 2009, 10, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Tuji, A.; Tsujimura, S. Umezakia natans does not belong to the Stigonemataceae to Nostocaceae. Fottea 2011, 11, 163–169. [Google Scholar] [CrossRef]
- Falch, B.S.; König, G.M.; Wright, A.D.; Sticher, O.; Angerhofer, C.K.; Pezzuto, J.M.; Bachmann, H. Biological activities of cyanobacteria: Evaluation of extracts and pure compounds. Planta Med. 1995, 61, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Papendorf, O.; König, G.M. Ambigol C and 2,4-dichlorobenzoic acid, natural products produced by the terrestrial cyanobacterium Fischerella ambigua. J. Nat. Prod. 2005, 68, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Hilliwig, M.L.; Liu, X. A new family of iron-dependent halogenases acts on freestanding substrates. Nat. Chem. Biol. 2014, 10, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, V.; Morvan, H.; Hoffmann, L.; Catherine, O.S.; Kraemer, M.; Krausz, P. Bioactive capsular polysaccharide from the thermophilic cyanophyte/cyanobacterium Mastigocladus laminosus—Cytotoxic properties. Planta Med. 2007, 73, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Prinsep, M.R.; Moore, R.E.; Levine, I.A.; Patterson, G.M. Westiellamide, a bistratamide-related cyclic peptide from the blue-green alga Westelliopsis prolifica. J. Nat. Prod. 1992, 55, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, K.; Burgoyne, D.L.; Moore, R.E.; Patterson, G.M.L.; Smith, C.D. Hapalosin, a cyanobacterial cyclic depsipeptide with multidrug-resistance reversing activity. J. Org. Chem. 1994, 59, 7219–7226. [Google Scholar] [CrossRef]
- Kang, H.S.; Krunic, A.; Orjala, J. Stigonemapeptin, an Ahp-containing depsipeptide with elastase inhibitory activity from the bloom-forming freshwater cyanobacterium Stigonema sp. J. Nat. Prod. 2012, 75, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Ogino, J.; Moore, R.E.; Patterson, G.M.; Smith, C.D. Dendroamides, new cyclic hexapeptides from a blue-green alga. Multidrug resistance reversing activity of dendroamide A. J. Nat. Prod. 1995, 59, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.P.; Concepcion, G.P.; Caraan, G.B.; Ireland, C.M. Bistratamides C and D. Two new oxazole-containing cyclic hexapeptides isolated from a Philippine Lissoclinum bistratum ascidian. J. Org. Chem. 1992, 57, 6671–6675. [Google Scholar] [CrossRef]
- Todorova, A.K.; Jüttner, F. Nostocyclamide: A new macrocyclic, thiazole-containing allelochemical from Nostoc sp. 31. J. Org. Chem. 1995, 60, 7891–7895. [Google Scholar] [CrossRef]
- Jüttner, F.; Todorova, A.K.; Walch, N.; von Philipsborn, W. Nostocyclamide M: A cyanobacterial cyclic peptide with allelopathic activity from Nostoc 31. Phytochemistry 2001, 57, 613–619. [Google Scholar] [CrossRef]
- Hagmann, L.; Jüttner, F. Fischerellin A, a novel photosystem-II inhibiting allelochemical of the cyanobacterium, Fischerella muscicola, with antifungal and herbicidal activity. Tetrahedron Lett. 1996, 37, 6539–6542. [Google Scholar] [CrossRef]
- Papke, U.; Gross, E.M.; Francke, W. Isolation, identification and determination of the absolute configuration of fischerellin B. A new algicide from the freshwater cyanobacterium Fischerella muscicola. Tetrahedron Lett. 1997, 38, 379–382. [Google Scholar] [CrossRef]
- Moore, R.E.; Cheuk, C.; Patterson, G.M.L. Hapalindoles: New alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 1984, 106, 6456–6457. [Google Scholar] [CrossRef]
- Moore, R.E.; Cheuk, C.; Yang, X.-Q.G.; Patterson, G.M.L.; Bonjouklian, R.; Smitka, T.; Mynderse, J.S.; Foster, R.S.; Jones, N.D.; Swartzendruber, J.K.; et al. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 1036–1043. [Google Scholar] [CrossRef]
- Asthana, R.K.; Srivastava, A.; Singh, A.P.; Deepali; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Identification of an antimicrobial entity from the cyanobacterium Fischerella sp. isolated from the bark of Azadirachta indica (Neem) tree. J. Appl. Phycol. 2006, 18, 33–39. [Google Scholar] [CrossRef]
- Becher, P.G.; Keller, S.; Jung, G.; Süssmuth, R.D.; Jüttner, F. Insecticidal activity of 12-epi-hapalindole J isonitrile. Phytochemistry 2007, 68, 2493–2497. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Yang, X.-Q.G.; Patterson, G.M.L.; Bonjouklian, R.; Smitka, T.A. Hapalonamides and other oxidized hapalindoles from Hapalosiphon fontinalis. Phytochemistry 1989, 28, 1565–1567. [Google Scholar] [CrossRef]
- Kim, H.; Lantvit, D.; Hwang, C.H.; Kroll, D.J.; Swanson, S.M.; Franzblau, S.G.; Orjala, J. Indole alkaloids from two cultured cyanobacteria, Westelliopsis sp. and Fischerella muscicola. Bioorg. Med. Chem. 2012, 20, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Hirsch, C.F.; Spring, J.P.; Pettibone, J.; Zink, D.L. Unusual cyclopropane-containing hapalindolinones from a cultured cyanobacterium. J. Org. Chem. 1987, 52, 3704–3706. [Google Scholar] [CrossRef]
- Smitka, T.A.; Bonjouklian, R.; Doolin, L.; Jones, N.D.; Deeter, J.B.; Yoshida, W.Y.; Prinsep, M.R.; Moore, R.E.; Patterson, G.M.L. Ambiguine isonitriles, fungicidal hapalindole-type alkaloids from three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem. 1992, 57, 857–861. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Chlipala, G.; Orjala, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009, 72, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Raveh, A.; Carmeli, S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp. collected in Israel. J. Nat. Prod. 2007, 70, 196–201. [Google Scholar] [CrossRef] [PubMed]
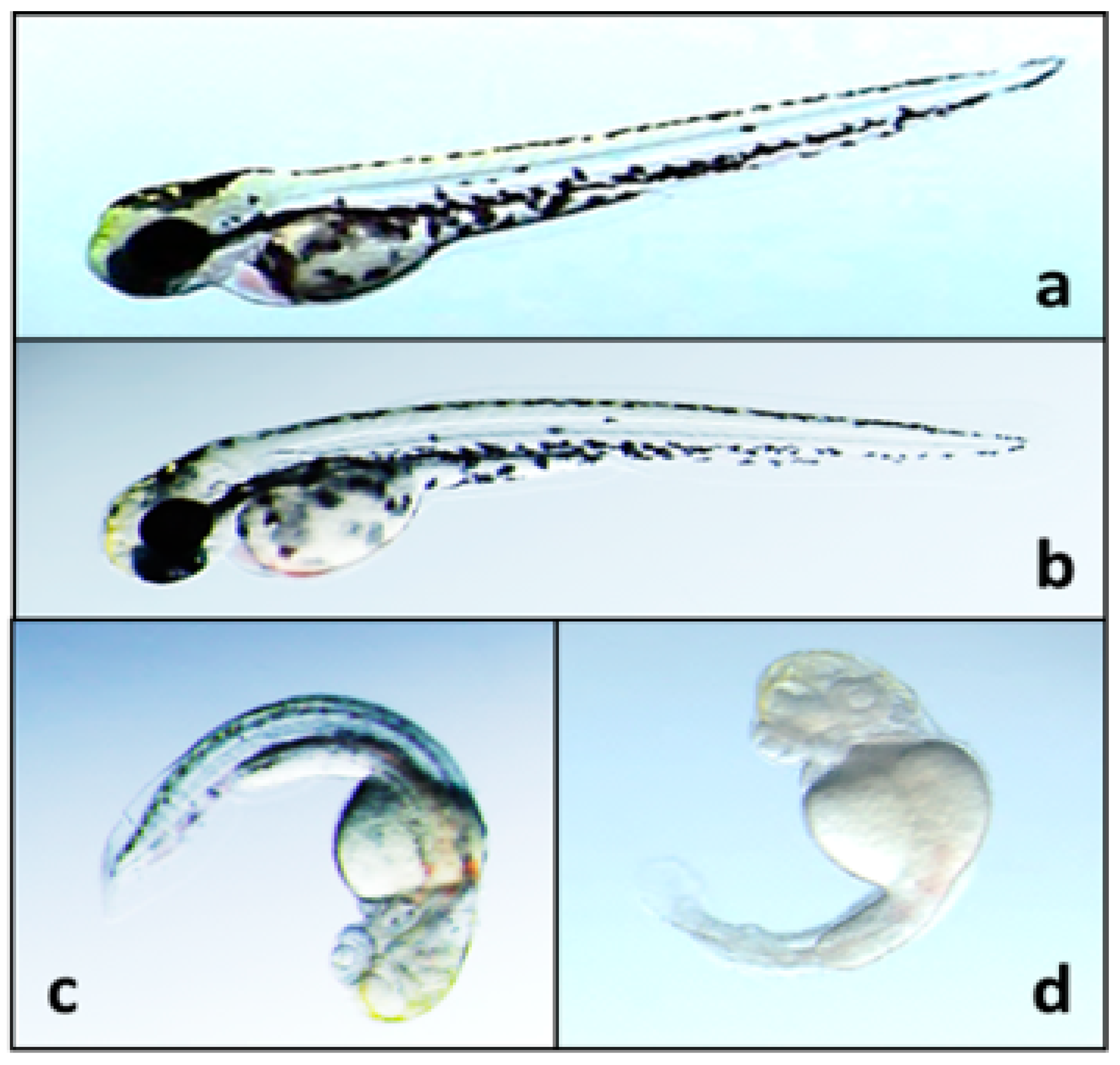
- Walton, K.; Gantar, M.; Gibbs, P.D.; Schmale, M.C.; Berry, J.P. Indole alkaloids from Fischerella inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Toxins 2014, 6, 3568–3581. [Google Scholar] [CrossRef] [PubMed]
- Huber, U.; Moore, R.E.; Patterson, G.M.L. Isolation of a nitrile-containing indole alkaloid from the terrestrial blue-green alga Hapalosiphon delicatulus. J. Nat. Prod. 1998, 61, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.; Berry, J.P. Isolation, chemical characterization and comparative toxicology of structurally diverse indole alkaloids from Fischerella as teratogens. Mar. Drugs. forthcoming.
- Mo, S.; Krunic, A.; Santarsiero, B.D.; Franzblau, S.G.; Orjala, J. Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Moore, R.E.; Patterson, G.M.L. Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett. 1992, 33, 3257–3260. [Google Scholar] [CrossRef]
- Kim, H.; Krunic, A.; Lantvit, D.; Shen, Q.; Kroll, D.J.; Swanson, S.M.; Orjala, J. Nitrile-containing fischerindoles from the cultured cyanobacterium Fischerella sp. Tetrahedron 2012, 68, 3205–3209. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, K.; Moore, R.E.; Bonjouklian, R.; Deeter, J.B.; Patterson, G.M.L.; Shaffer, S.; Smith, C.D.; Smitka, T.A. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricate: Relationship to the fischerindoles and hapalindoles. J. Am. Chem. Soc. 1994, 116, 9935–9942. [Google Scholar] [CrossRef]
- Wood, J.L. Total synthesis: Welwitindolinone is well worth it. Nat. Chem. 2012, 4, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Dave, A.; MacKay, J.A.; Rawal, V.H. The chemistry of hapalindoles, fischerindoles, ambiguines and welwitindolinones. Alkaloids Chem. Biol. 2014, 73, 65–160. [Google Scholar] [PubMed]
- Jimenez, J.I.; Huber, U.; Moore, R.E.; Patterson, G.M. Oxidized welwitindolinones from terrestrial Fischerella sp. J. Nat. Prod. 1999, 62, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Hilliwig, M.L.; Fuhrman, H.A.; Ittiiamornkul, K.; Sevco, T.J.; Kwak, D.H.; Liu, X. Identification and characterization of a welwitindolinone alkaloid biosynthetic gene cluster in the Stigonematalean cyanobacterium Hapalosiphon welwitschii. ChemBioChem 2014, 15, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Knowles, C.J. Microorganisms and cyanide. Bacteriol. Rev. 1976, 40, 662–680. [Google Scholar]
- Pistorius, E.K.; Voss, H. Some properties of a basic l-amino acid oxidase from Anacystis nidulans. Biochim. Biophys. Acta 1980, 611, 227–240. [Google Scholar] [CrossRef]
- Bornemann, V.; Patterson, G.M.L.; Moore, R.E. Isonitrile biosynthesis in the cyanophyte Hapalosiphon fontinalis. J. Am. Chem. Soc. 1988, 110, 2339–2340. [Google Scholar] [CrossRef]
- Garson, M.J.; Simpson, J.S. Marine isocyanides and related natural products—Structure, biosynthesis and ecology. Nat. Prod. Rep. 2004, 21, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Hilliwig, M.L.; Zhu, Q.; Liu, X. Biosynthesis of ambiguine indole alkaloids in cyanobacterium Fischerella ambigua. ACS Chem. Biol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hilliwig, M.L.; Koharudin, L.M.; Gronenborn, A.M. Unified biogenesis of ambiguine, fischerindole, hapalindole and welwitindolinone: Identification of a monogeranylated indolenine as a cryptic common biosynthetic intermediate by an unusual magnesium-dependent aromatic prenyltransferase. Chem. Commun. 2016, 52, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.M.; Ishihara, Y.; Masuda, T.; Whitefield, B.W.; Llamas, T.; Pohjakallio, A.; Baran, P.S. Enantiospecific total synthesis of the hapalindoles, fischerindoles and welwitindolinones via a redox economic approach. J. Am. Chem. Soc. 2008, 130, 17938–17954. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.L.; Sharma, D.; Bunn, B.M.; Gerwick, L.; Viswanathan, R.; Moffitt, M.C. Comparative analysis of hapalindole, ambiguine and welwitindolinone gene clusters and reconstitution of indole-isonitrile biosysynthesis from cyanobacteria. BMC Microbiol. 2014, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lowell, A.N.; Yu, F.; Raveh, A.; Newmister, S.A.; Bair, N.; Schaub, J.M.; Williams, R.M.; Sherman, D.H. Hapalindole/ambiguine biogenesis is mediated by a Cope Rearrangement, C-C bond-forming cascade. J. Am. Chem. Soc. 2015, 137, 15366–15369. [Google Scholar] [CrossRef] [PubMed]
- Cagide, E.; Becher, P.G.; Louzao, M.C.; Espiña, B.; Vieytes, M.R.; Jüttner, F.; Botana, L.M. Hapalindoles from the cyanobacterium Fischerella: Potential sodium channel modulators. Chem. Res. Toxicol. 2014, 27, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Jüttner, F. Insecticidal compounds of the biofilm-forming cyanobacterium Fischerella sp. (ATCC 43239). Environ. Toxicol. 2005, 20, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.E. Identification, Isolation and Characterization of Developmental Toxins from the Cyanobacterium Fischerella 52–1 Using the Zebrafish (Danio rerio) Embryo Model. M.S. Thesis, Florida International University, Miami, FL, USA, 30 March 2012. [Google Scholar]
- Doan, N.T.; Rickards, R.W.; Rotschild, J.M.; Smith, G.D. Allelopathic actions of the alkaloids 12-epi-hapalindole E isonitrile and calothrixin A from cyanobacteria of the genera Fischerella and Calothrix. J. Appl. Phycol. 2000, 12, 409–416. [Google Scholar] [CrossRef]
- Etchagaray, A.; Rabello, E.; Dieckmann, R.; Moon, D.H.; Fiore, M.F.; von Döhren, H.; Tsai, S.M.; Neilan, B.A. Algicide production by the filamentous cyanobacterium Fischerella sp. CENA 19. J. Appl. Phycol. 2004, 16, 237–243. [Google Scholar] [CrossRef]
- Doan, N.T.; Stewart, P.R.; Smith, G.D. Inhibition of bacterial RNA polymerase by the cyanobacterial metabolite 12-epi-hapalindole E isonitrile and calothrixin A. FEMS Microbiol. Lett. 2001, 196, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Acuña, U.M.; Zi, J.; Orjala, J.; Carcache de Blanco, E.J. Ambiguine I isonitrile from Fischerella ambigua induces caspase-independent cell death in MCF-7 hormone dependent breast cancer cells. Int. J. Cancer Res. 2015, 49, 1655–1662. [Google Scholar]
- Koodkaew, I.; Sunohara, Y.; Matsuyama, S.; Matsumoto, H. Isolation of ambiguine D isonitrile from Hapalosiphon sp. and characterization of its phytotoxic activity. Plant Growth Regul. 2012, 68, 141–150. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, C.D. Microtubule effects of welwistatin, a cyanobacterial indolinone that circumvents multiple drug resistance. Mol. Pharmacol. 1996, 49, 288–294. [Google Scholar] [PubMed]
- Smith, C.D.; Zilfou, J.T.; Stratmann, K.; Patterson, G.M.; Moore, R.E. Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol. 1995, 47, 241–247. [Google Scholar] [PubMed]
- Klein, D.; Daloze, D.; Braekman, J.C.; Hoffmann, L.; Demoulin, V. New hapalindoles from the cyanophyte Hapalosiphon laingii. J. Nat. Prod. 1995, 58, 1781–1785. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Gibbs, P.D.L.; Schmale, M.C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Wiley, F.E.; Wilde, S.B.; Birrenkott, A.H.; Williams, S.K.; Murphy, T.M.; Hope, C.P.; Bowerman, W.W.; Fischer, J.R. Investigation of the link between avian vacuolar myelinopathy and a novel species of cyanbacteria through laboratory feeding trails. J. Wildl. Dis. 2007, 43, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Berry, J.P.; Thomas, S.; Wang, M.; Perez, R.; Rein, K.S. Allelopathic activity among cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol. Ecol. 2008, 64, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.M.; Wolk, C.P.; Jüttner, F. Fischerellin, a new allelochemical from the freshwater cyanobacterium Fischerella muscicola. J. Phycol. 1991, 27, 686–692. [Google Scholar] [CrossRef]
- Srivastava, A.; Jüttner, F.; Strasser, R.J. Action of the allelochemical, fischerellin A, on photosystem II. Biochim. Biophys. Acta Bioenerg. 1998, 1364, 326–336. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F. Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Birrenkott, A.H.; Wilde, S.B.; Hains, J.J.; Fischer, J.R.; Murphy, T.M.; Hope, C.P.; Parnell, P.G.; Bowermann, W.W. Establishing a food-chain link between aquatic plant material and avian vacuolar myelinopathy in mallards (Anas platyrhynchos). J. Wildlif. Dis. 2004, 40, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.B.; Murphy, T.M.; Hope, C.P.; Habrun, S.K.; Kempton, J.; Birrenkott, A.; Wiley, F.; Bowermann, W.W.; Lewitus, A.J. Avian vacuolar myelinopathy linked to exotic aquatic plants and novel cyanobacterial species. Environ. Toxicol. 2005, 20, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Wiley, F.E.; Twiner, M.J.; Leighfield, T.A.; Wilde, S.B.; Van Dolah, F.M.; Fischer, J.R.; Bowerman, W.W. An extract of Hydrilla verticillata and associated epiphytes induces avian vacuolar myelinopathy in laboratory mallards. Environ. Toxicol. 2009, 24, 362–368. [Google Scholar] [CrossRef] [PubMed]
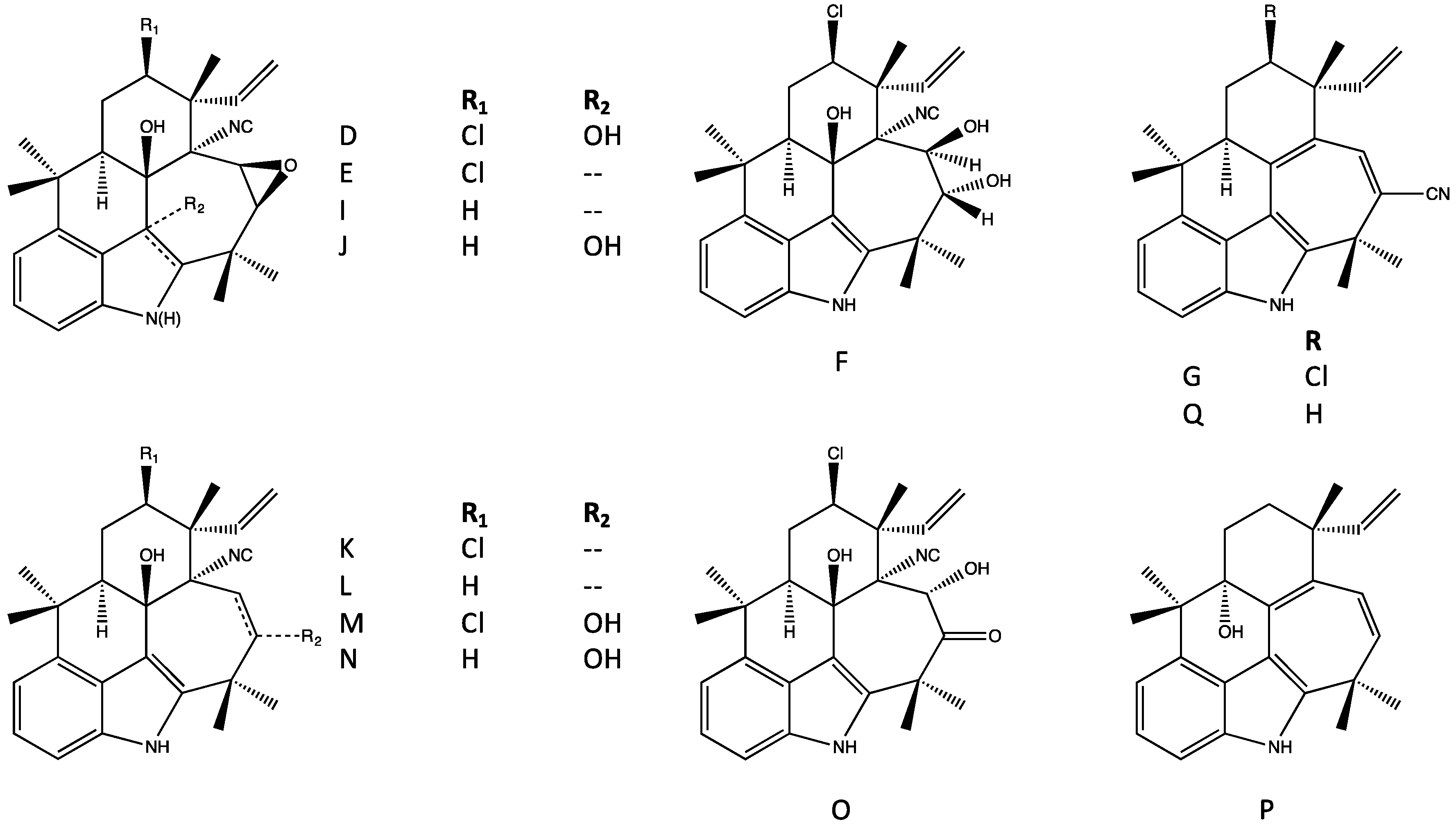

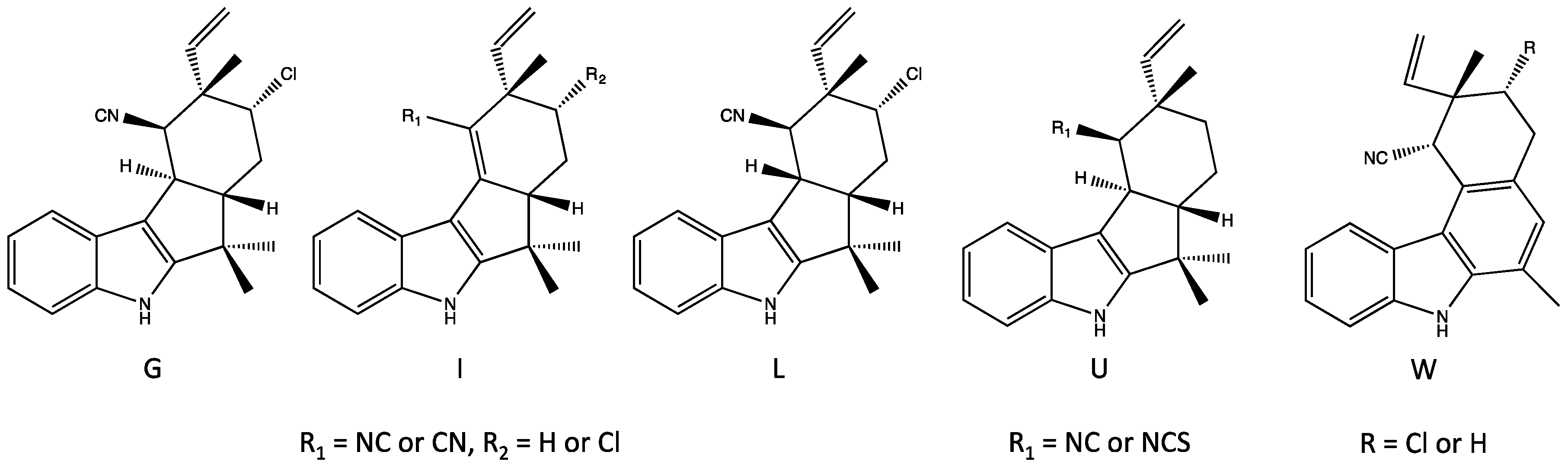
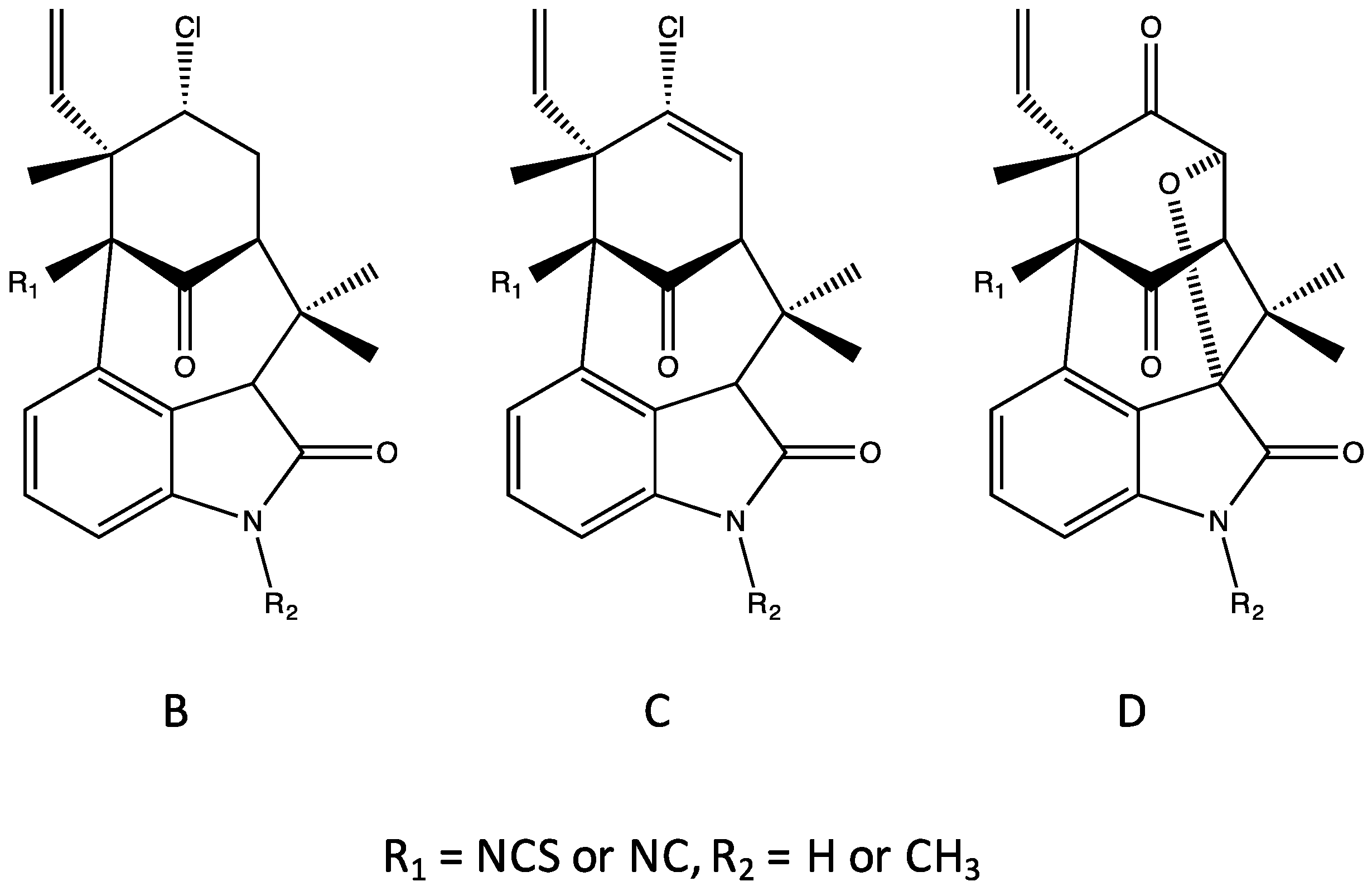
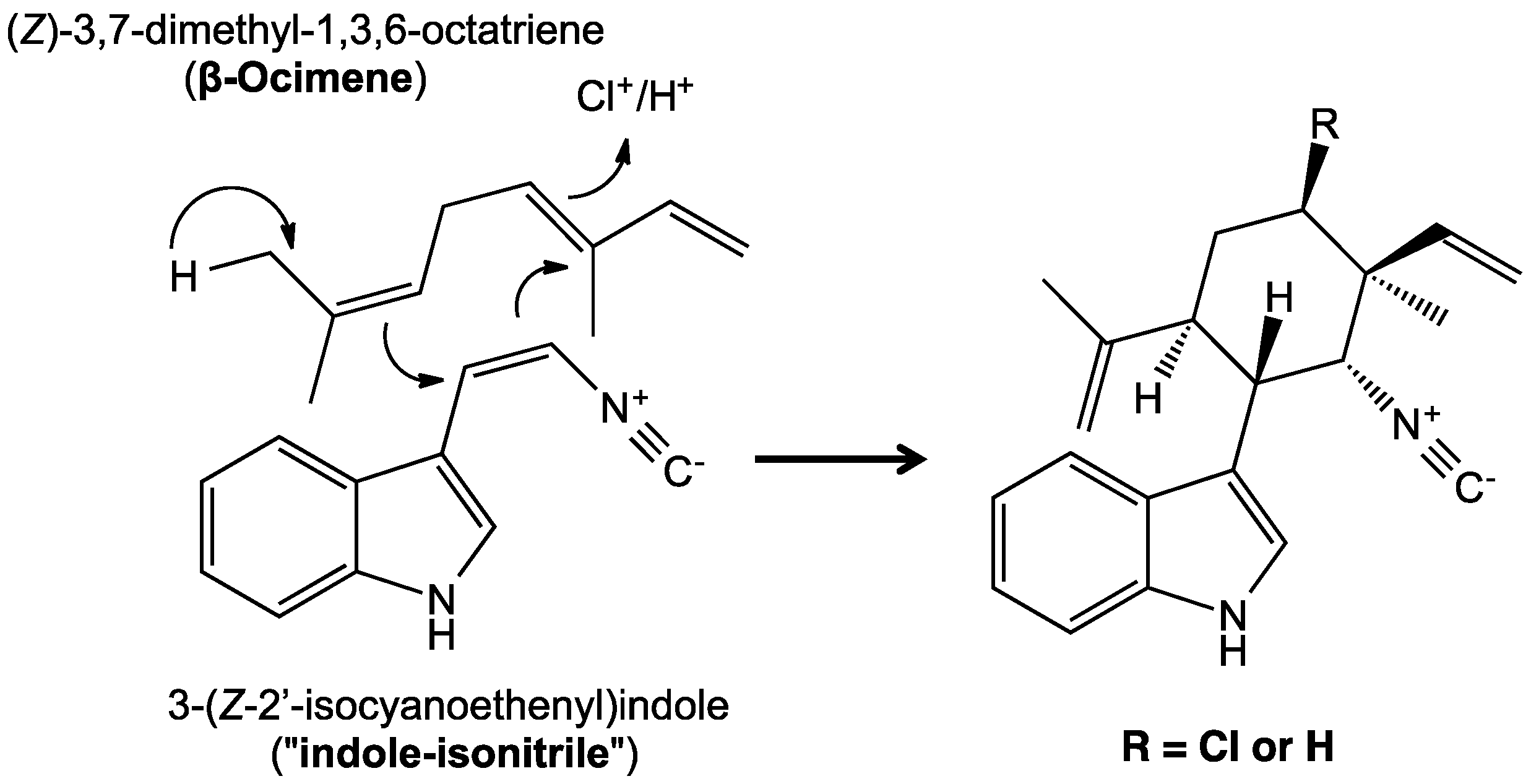

| Antimicrobial | Cytotoxicity | Biochemical, Molecular or Cellular | Plant/Animal Toxicity | |||
|---|---|---|---|---|---|---|
| Bacteria | Fungi | Algae | ||||
| Hapalindoles | ||||||
| Tetracyclic (Group 1) | A, G–H, I–J, T, X [29,31,34,42] | G [42] | A, J, X [34] | A, H–J, U, X [34] | Modulate Na+ channels in neuroblastoma [59] | Insecticidal [32,60,61] Teratogenicity/vertebrate toxicity [39,41] |
| Tricyclic (Group 2) | E [62] | E [62] | E–F [62,63] | C, E [34,62] | Modulate Na+ channels in neuroblastoma [59] RNA polymerase inhibition [62,64] | Insecticidal [32,60] |
| Hapalindolinones (Group 3) | Inhibits arginine vassopressin binding, and adenylate cyclase [35] | |||||
| Ambiguines | ||||||
| Tetracyclic (Group 4) | A–C, H [38,42] | A–C, H [38,42] | A–C [42] | Teratogenicity/vertebrate toxicity [39,41] | ||
| Pentacyclic (Group 5) | E–F, G nitrile, I, K–O [37,38,42] | E–F, I, K–O, P [37,38,42] | E–F, G nitrile, K–O [37,42] | Apoptosis, inhibition of NF-kb, IKKb and ICAM-1 [65] Inhibits mitosis (plant cells) [66] Elevates ROS [65,66] | Phytotoxic [66] | |
| Fischambiguines (Group 6) | A, B [42] | A [42] | ||||
| Fischerindoles (Group 7) | L [34] | L [34] | descholor I nitrile, L [34,44] | Teratogenicity/vertebrate toxicity [41] | ||
| Welwitindolinones | ||||||
| Welwitindolinone A (Group 8) | A [45] | |||||
| Welwitindolinone B (Group 9) | N-methyl C isothiocyanate [67] | Inhibits microtubules and mitosis [67] Inhibit P-glycoprotein MDR [67,68] | Insecticidal [45] | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walton, K.; Berry, J.P. Indole Alkaloids of the Stigonematales (Cyanophyta): Chemical Diversity, Biosynthesis and Biological Activity. Mar. Drugs 2016, 14, 73. https://doi.org/10.3390/md14040073
Walton K, Berry JP. Indole Alkaloids of the Stigonematales (Cyanophyta): Chemical Diversity, Biosynthesis and Biological Activity. Marine Drugs. 2016; 14(4):73. https://doi.org/10.3390/md14040073
Chicago/Turabian StyleWalton, Katherine, and John P. Berry. 2016. "Indole Alkaloids of the Stigonematales (Cyanophyta): Chemical Diversity, Biosynthesis and Biological Activity" Marine Drugs 14, no. 4: 73. https://doi.org/10.3390/md14040073
APA StyleWalton, K., & Berry, J. P. (2016). Indole Alkaloids of the Stigonematales (Cyanophyta): Chemical Diversity, Biosynthesis and Biological Activity. Marine Drugs, 14(4), 73. https://doi.org/10.3390/md14040073