Influences of Fucoxanthin on Alveolar Bone Resorption in Induced Periodontitis in Rat Molars
Abstract
:1. Introduction
2. Results
2.1. Biochemical Results
2.1.1. Serum TNF-α, IL-1β, and IL-6 Levels
2.1.2. Serum TOS, TAS, and OSI Levels
2.1.3. Serum B-ALP Levels
2.2. Stereologic Results
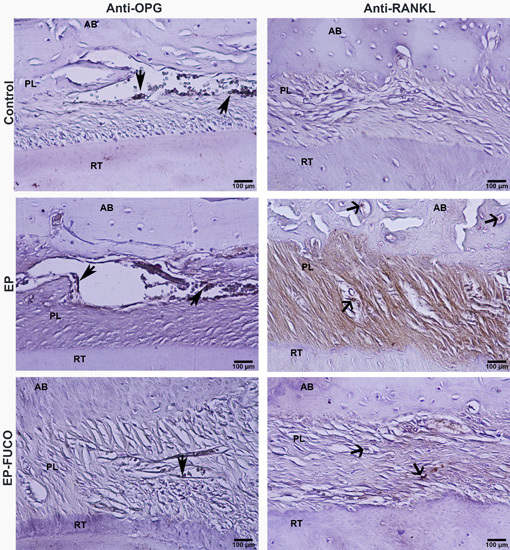
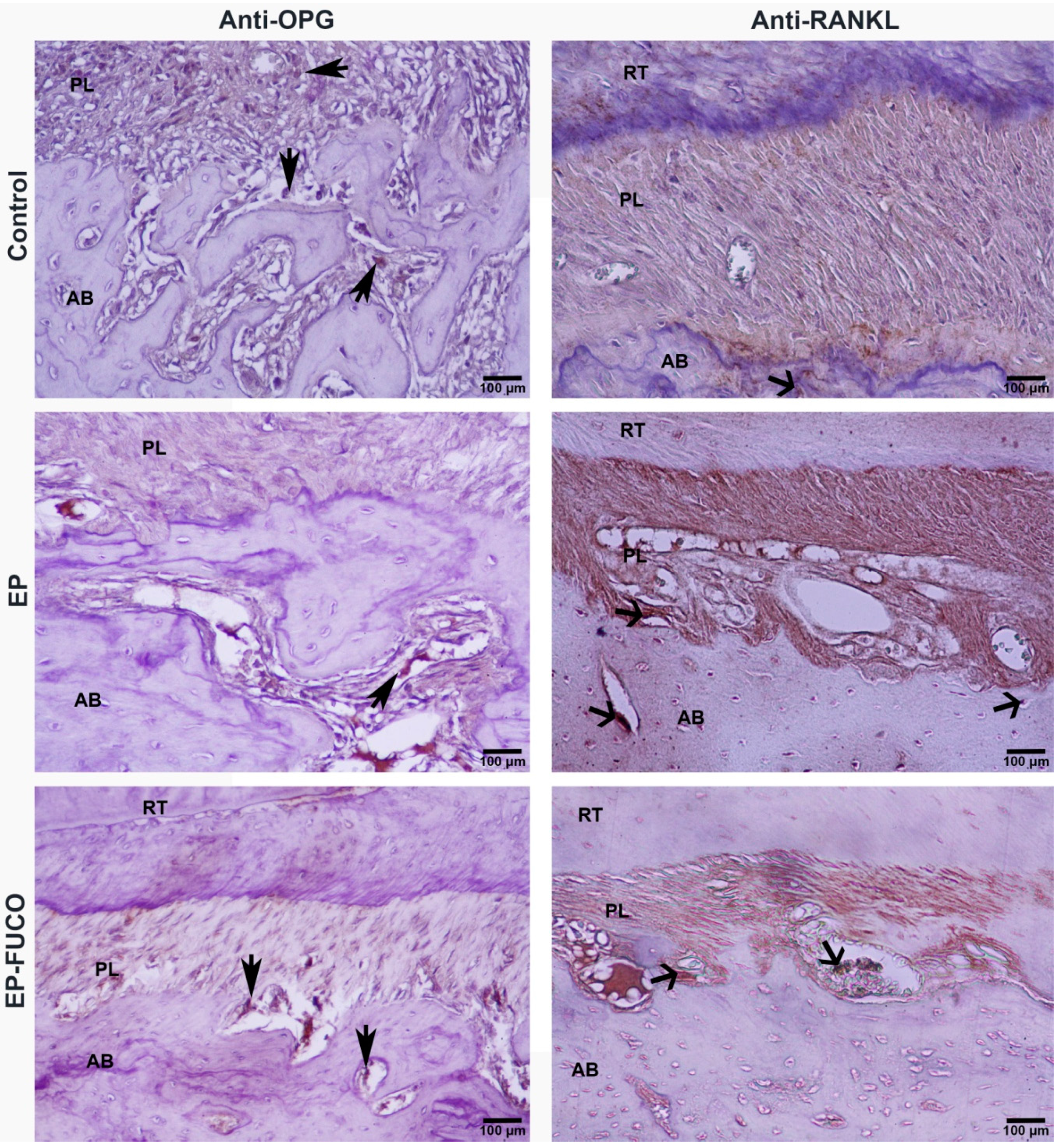
2.3. Histometric Results (Alveolar Bone Loss)
3. Discussion
4. Experimental Section
4.1. Extraction and Purification of FUCO
4.2. Induction of Periodontitis and FUCO Administration
4.3. Blood Sampling and Biochemical Assays
4.3.1. Serum TNF-α, IL-1β, and IL-6 Assays
4.3.2. Measurement of Serum TOS and TAS Levels and Calculation of OSI
4.3.3. Measurement of Serum Bone Alkaline Phosphatase (B-ALP) Activity
4.4. Histological and Immunohistochemical Analysis
4.4.1. Histological Imaging and Measurements
4.4.2. Immunohistochemical Analysis and Calculation of RANKL- and OPG-Positive Cells
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loos, B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005, 76, 2106–2215. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S. Mapping the pathogenesis of periodontitis: A new look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Kiji, M.; Yashiro, R.; Hormdee, D.; Lu, H.; Kunze, M.; Suda, T.; Koshy, G.; Kobayashi, H.; Oda, S.; et al. Roles of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin in periodontal health and disease. Periodontol. 2000 2007, 43, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.A.; Lopes de Souza, G.; Souza, T.O.; de Castro Brito, G.A.; Sabóia Aragão, K.; Xavier de Medeiros, C.A.; Lourenço, Y.; do Socorro Costa Feitosa Alves, M.; Fernandes de Araújo, R., Jr. Olmesartan decreases IL-1β and TNF-α levels; downregulates MMP-2, MMP-9, COX-2, and RANKL; and upregulates OPG in experimental periodontitis. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.A.; Souza, T.O.; Moura, L.M.; Brito, G.A.; Aragão, K.S.; Araújo, L.S.; Medeiros, C.A.; Alves, M.S.; Araújo, R.F., Jr. Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J. Clin. Periodontol. 2013, 40, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, R.; Usui, M.; Yamamoto, G.; Nishii, K.; Tsukamoto, Y.; Okamatsu, Y.; Sato, T.; Asou, Y.; Nakashima, K.; Yamamoto, M. Tumor necrosis factor-α enhances RANKL expression in gingival epithelial cells via protein kinase A signaling. J. Periodontal. Res. 2014, 49, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.C.; Martin, J.C.; King, P.J.; Powell, J.R.; Caves, J.; Cohen, M.E. Interleukin-1 beta, prostaglandin E2, and immunoglobulin G subclasses in gingival crevicular fluid in patients undergoing periodontal therapy. J. Periodontol. 1996, 67, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mautalen, C. Melatonin effects on bone: Experimental facts and clinical perspectives. J. Pineal. Res. 2003, 34, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Passeri, G.; Macaluso, G.M. FoxOs, Wnts and oxidative stress-induced bone loss: New players in the periodontitis arena? J. Periodontal. Res. 2011, 46, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Baltacıoğlu, E.; Yuva, P.; Aydın, G.; Alver, A.; Kahraman, C.; Karabulut, E.; Akalın, F.A. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: A new biomarker for periodontal disease? J. Periodontol. 2014, 85, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Yağan, A.; Kesim, S.; Liman, N. Effect of low-dose doxycycline on serum oxidative status, gingival antioxidant levels, and alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 2014, 85, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Longhini, R.; de Aparecida Oliveira, P.; Sasso-Cerri, E.; Cerri, P.S. Cimetidine reduces alveolar bone loss in induced periodontitis in rat molars. J. Periodontol. 2014, 85, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Arabacı, T.; Kermen, E.; Özkanlar, S.; Köse, O.; Kara, A.; Kızıldağ, A.; Duman, Ş.B.; Ibişoğlu, E. Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: A biochemical and immunohistochemical study. J. Periodontol. 2015, 86, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Akman, S.; Canakci, V.; Kara, A.; Tozoglu, U.; Arabaci, T.; Dagsuyu, I.M. Therapeutic effects of alpha lipoic acid and vitamin C on alveolar bone resorption after experimental periodontitis in rats: A biochemical, histochemical, and stereologic study. J. Periodontol. 2013, 84, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Wen-Jun, W.; Guang-Ce, W.; Ming, Z.; Tseng, C.K. Isolation of Fucoxanthin from the Rhizoid of Laminaria japonica Aresch. J. Integr. Plant. Biol. 2005, 47, 1009–1015. [Google Scholar]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative evaluation of the radical-scavenging activities of fucoxanthin and its stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.W.; Na, S.J.; Kim, W.K. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutr. Res. Pract. 2013, 7, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Hou, Y.H. First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation 2014, 37, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Chu, W.; Jiang, L.; Geng, C.; Li, J.; Ishikawa, N.; Kajima, K.; Zhong, L. Effect of fucoxanthin alone and in combination with d-glucosamine hydrochloride on carrageenan/kaolin-induced experimental arthritis in rats. Phytother. Res. 2014, 28, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, M.; Yamamoto, T.; Ichioka, H.; Honjo, K.; Yamamoto, K.; Oseko, F.; Kita, M.; Mazda, O.; Kanamura, N. β-Cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch. Oral Biol. 2013, 58, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Belludi, S.A.; Verma, S.; Banthia, R.; Bhusari, P.; Parwani, S.; Kedia, S.; Saiprasad, S.V. Effect of lycopene in the treatment of periodontal disease: A clinical study. J. Contemp. Dent. Pract. 2013, 14, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kadekaru, T.; Toyama, H.; Yasumoto, T. Safety evaluation of fucoxanthin purified from Undaria pinnatifida. JSNFS 2008, 55, 304–308. [Google Scholar] [CrossRef]
- Krishnan, B.; Lingaiah, H.B.; Shanmugam, V.; Peranantham, T.; Maruthaiveeran, P.B. Fucoxanthin, a marine carotenoid protects cadmium-induced oxidative renal dysfunction in rats. Biomed. Prev. Nutr. 2013, 3, 201–207. [Google Scholar]
- Thummuri, D.; Jeengar, M.K.; Shrivastava, S.; Nemani, H.; Ramavat, R.N.; Chaudhari, P.; Naidu, V.G. Thymoquinone prevents RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-κB and MAPK Signalling. Pharmacol. Res. 2015, 99, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tomofuji, T.; Ekuni, D.; Sanbe, T.; Irie, K.; Azuma, T.; Maruyama, T.; Tamaki, N.; Murakami, J.; Kokeguchi, S.; Yamamoto, T. Effects of vitamin C intake on gingival oxidative stress in rat periodontitis. Free Radic. Biol. Med. 2009, 46, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Ma, Y.; Hao, Z.; Chen, S.; Fu, T.; Chen, H.; Wang, H. Oral administration of 5-hydroxytryptophan aggravated periodontitis-induced alveolar bone loss in rats. Arch. Oral Biol. 2015, 60, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Akalin, F.A.; Baltacioğlu, E.; Alver, A.; Karabulut, E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J. Clin. Periodontol. 2007, 34, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 1997, 42, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ren, R.; Hashimoto, T.; Kanazawa, K. Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264.7 cells. J. Agric. Food. Chem. 2010, 58, 6090–6095. [Google Scholar] [CrossRef] [PubMed]
- Binder, T.A.; Goodson, J.M.; Socransky, S.S. Gingival fluid levels of acid and alkaline phosphatase. J. Periodontal Res. 1987, 22, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F.; Brixen, K.; Charles, P. New markers of bone metabolism: Clinical use in metabolic bone disease. Eur. J. Endocrinol. 1995, 132, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.N.; Reichert, C.; Jäger, A.; Deschner, J. Effect of overweight/obesity on response to periodontal treatment: Systematic review and a meta-analysis. J. Clin. Periodontol. 2015, 42, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Arabaci, T.; Kermen, E.; Kızıldag, A.; Yemenoglu, H.; Alkurt, M.; Ozkanlar, S. Effects of alpha-lipoic acid and its combined use with vitamin C on periodontal tissues and markers of oxidative stress in rats with experimental periodontitis. Oxid. Antioxid. Med. Sci. 2015, 4, 91–96. [Google Scholar] [CrossRef]
- Kose, O.; Arabaci, T.; Kara, A.; Yemenoglu, H.; Kermen, E.; Kizildag, A.; Gedikli, S.; Ozkanlar, S. Effects of Melatonin on Oxidative Stress Index and Alveolar Bone Loss in Diabetic Rats With Periodontitis. J. Periodontol. 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]

| Groups | TNF-α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) | TOS (μmol H2O2 Equiv/L) | TAS (mmol Trolox Equiv/L) | OSI (Ratio) |
|---|---|---|---|---|---|---|
| Control | 44.4 ± 8.6 a | 4.5 ± 2.1 a | 13.4 ± 4.6 a | 16.43 ± 5.43 a | 1.14 ± 0.33 a | 1.36 ± 0.41 a |
| EP | 61.1 ± 10.2 b | 7.9 ± 3.6 b | 22.2 ± 8.1 b | 23.87 ± 4.62 b | 0.61 ± 0.26 b | 3.11 ± 0.67 b |
| EP-FUCO | 58.8 ± 7.1 b | 7.5 ± 2.8 b | 19.8 ± 6.7 b | 18.50 ± 4.68 a | 0.86 ± 0.24 c | 1.66 ± 0.38 a |
| Groups | Serum B-ALP (U/L) | Anti-RANKL Positive Cells (n/µm2) | Anti-OPG Positive Cells (n/µm2) | CEJ-BC (µm) | |
|---|---|---|---|---|---|
| Control | 124.86 ± 12.40 a | 0.0000374 a | 0.0000877 a | L | 149.65 ± 11.36 a |
| B | 143.25 ± 13.68 x | ||||
| EP | 98.86 ± 9.40 b | 0.0000866 b | 0.0000397 b | L | 372.34 ± 32.47 b |
| B | 367.23 ± 43.58 y | ||||
| EP-FUCO | 105.10 ± 12.32 b | 0.0000402 a | 0.0000411 b | L | 338.37 ± 16.85 b |
| B | 336.71 ± 15.86 y | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, O.; Arabaci, T.; Yemenoglu, H.; Kara, A.; Ozkanlar, S.; Kayis, S.; Duymus, Z.Y. Influences of Fucoxanthin on Alveolar Bone Resorption in Induced Periodontitis in Rat Molars. Mar. Drugs 2016, 14, 70. https://doi.org/10.3390/md14040070
Kose O, Arabaci T, Yemenoglu H, Kara A, Ozkanlar S, Kayis S, Duymus ZY. Influences of Fucoxanthin on Alveolar Bone Resorption in Induced Periodontitis in Rat Molars. Marine Drugs. 2016; 14(4):70. https://doi.org/10.3390/md14040070
Chicago/Turabian StyleKose, Oguz, Taner Arabaci, Hatice Yemenoglu, Adem Kara, Seckin Ozkanlar, Sevki Kayis, and Zeynep Yesil Duymus. 2016. "Influences of Fucoxanthin on Alveolar Bone Resorption in Induced Periodontitis in Rat Molars" Marine Drugs 14, no. 4: 70. https://doi.org/10.3390/md14040070