Xyloketal B Attenuates Atherosclerotic Plaque Formation and Endothelial Dysfunction in Apolipoprotein E Deficient Mice
Abstract
:1. Introduction
2. Results
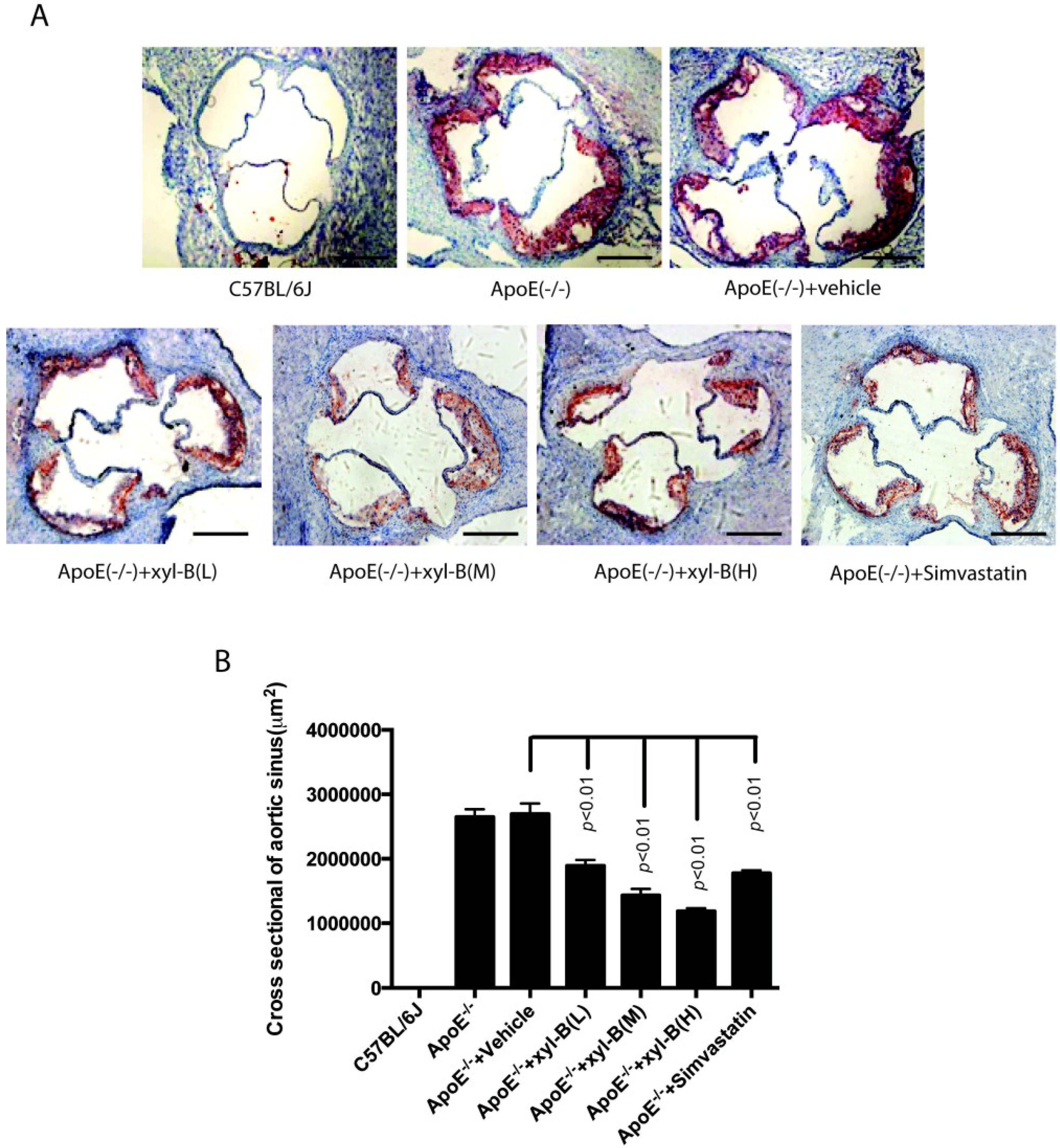
2.1. Effect of Xyloketal B on High-Fat Diet Induced Atherosclerotic Lesion Formation in ApoE−/− Mice
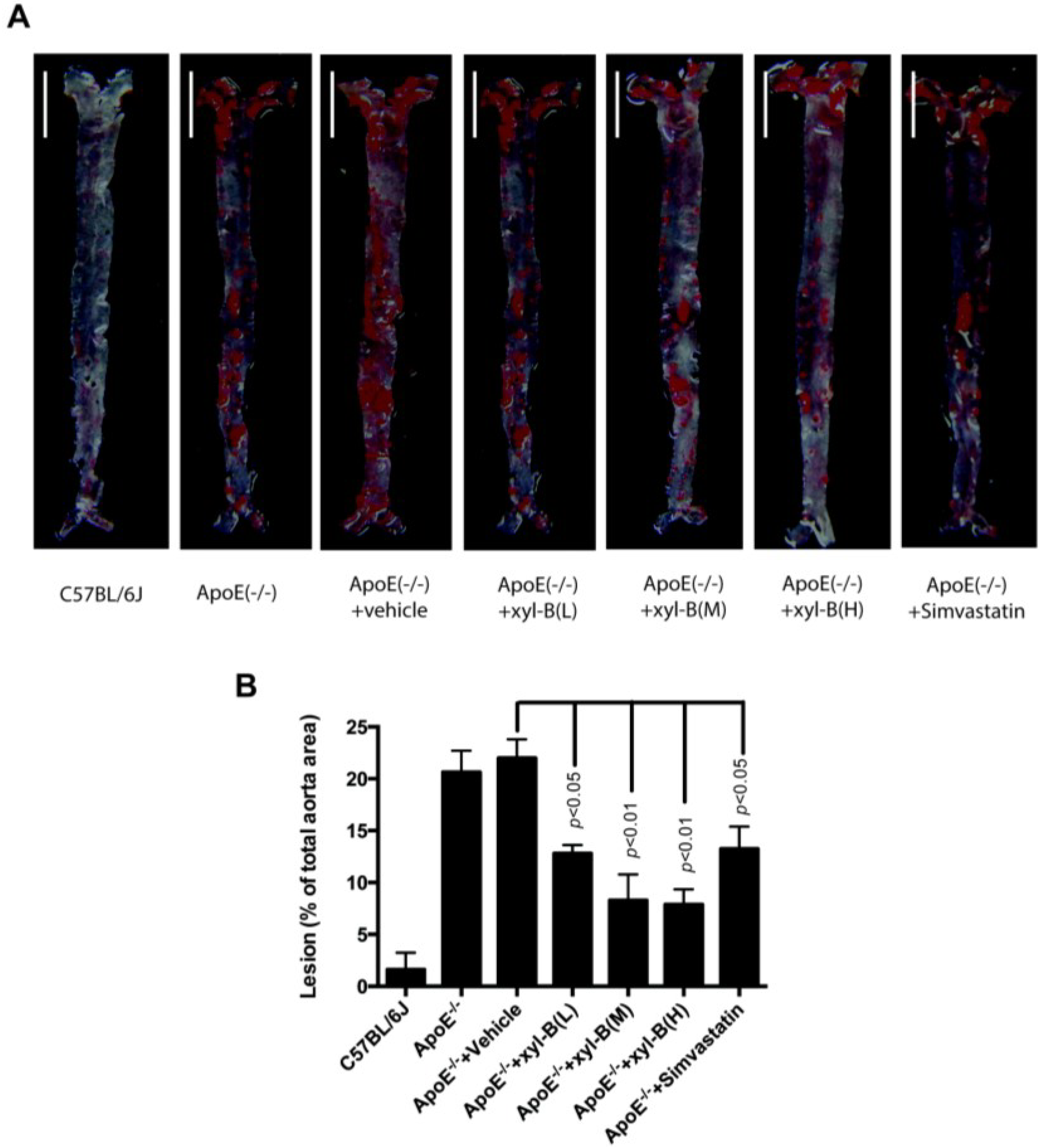
| C57BL/6J | ApoE−/− | ApoE−/− + Vehicle | ApoE−/− + xyl-B (M, 14 mg/kg/day) | ApoE−/− + Simvastatin (10mg/kg/day) | |
|---|---|---|---|---|---|
| 0 week | 18 ± 0.26 | 18 ± 0.32 | 19 ± 0.42 | 18 ± 0.36 | 18 ± 0.28 |
| 8 weeks | 24 ± 0.53 | 26 ± 0.52 | 25 ± 0.49 | 24 ± 0.61 | 24 ± 0.41 |
| 16 weeks | 29 ± 0.62 | 30 ± 0.77 | 31 ± 0.59 | 30 ± 0.69 | 30 ± 0.53 |
2.2. Effect of Xyloketal B on the Level of Serum Autoantibodies against oxLDL in Atherosclerotic Apoe−/− Mice

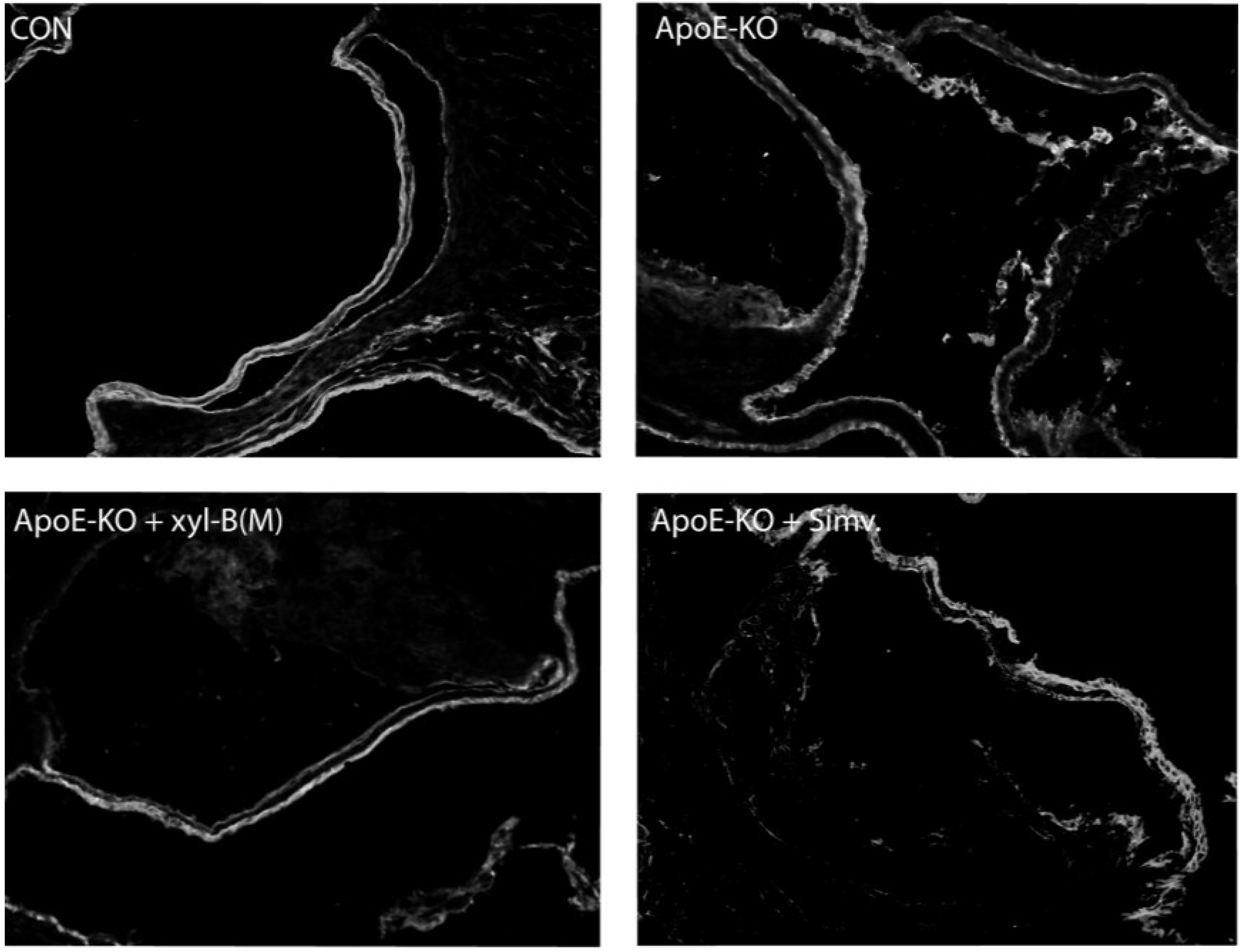
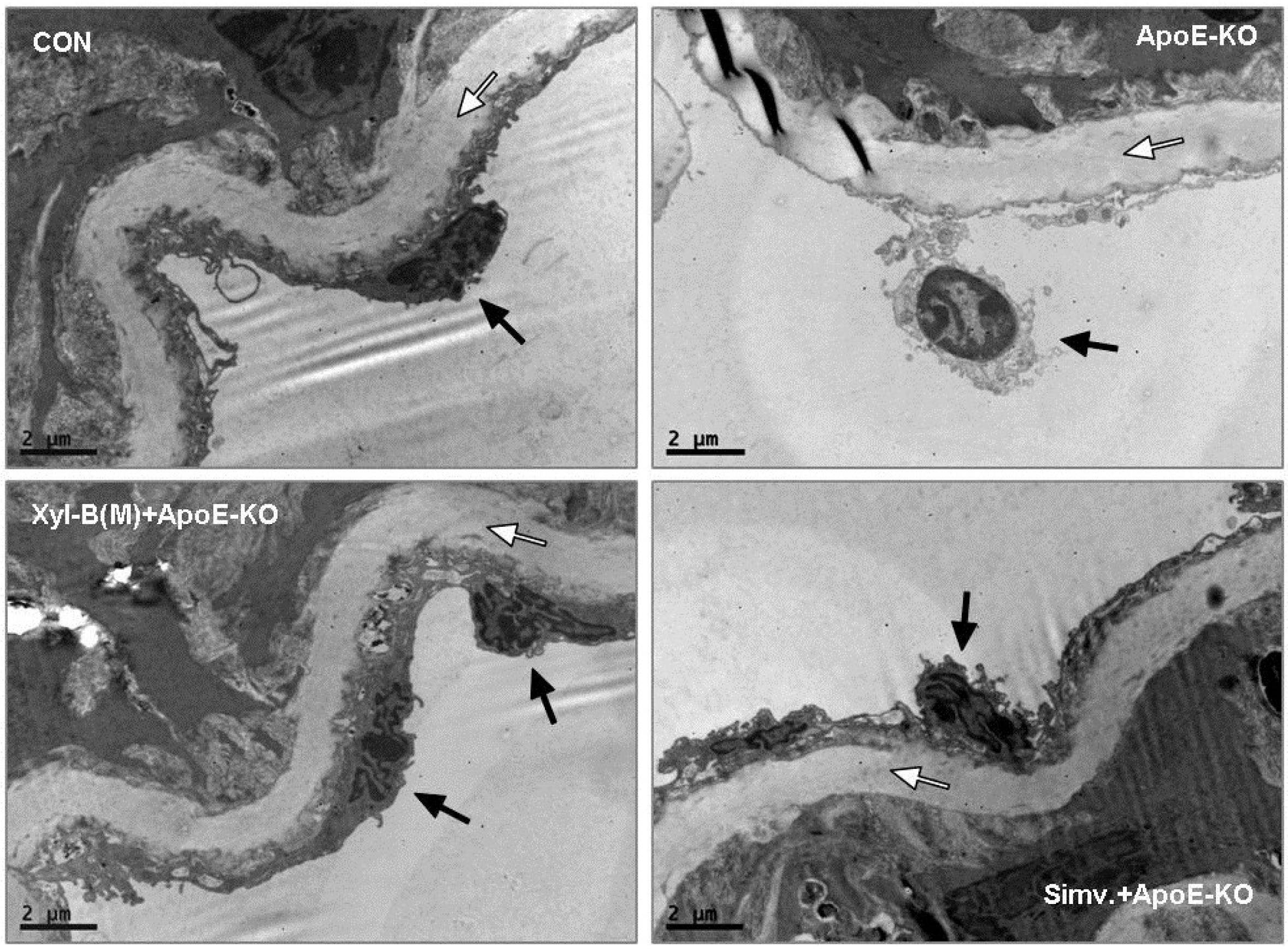
2.3. Protective Effects of Xyloketal B on Endothelial Integrity during Atherosclerosis
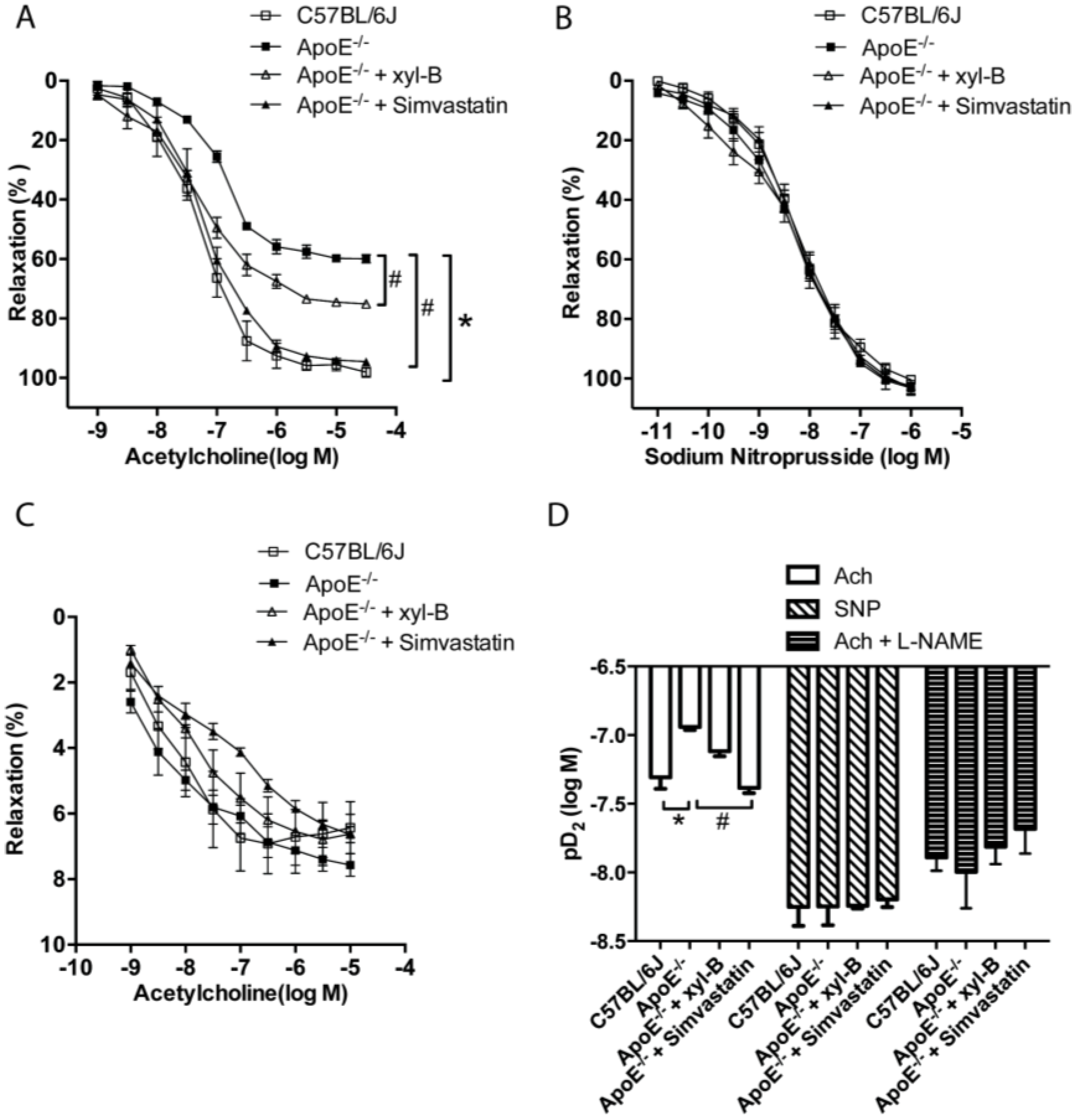
2.4. Xyloketal B Improved NO-Dependent Aortic Vasorelaxation in Atherosclerotic Apoe−/− Mice
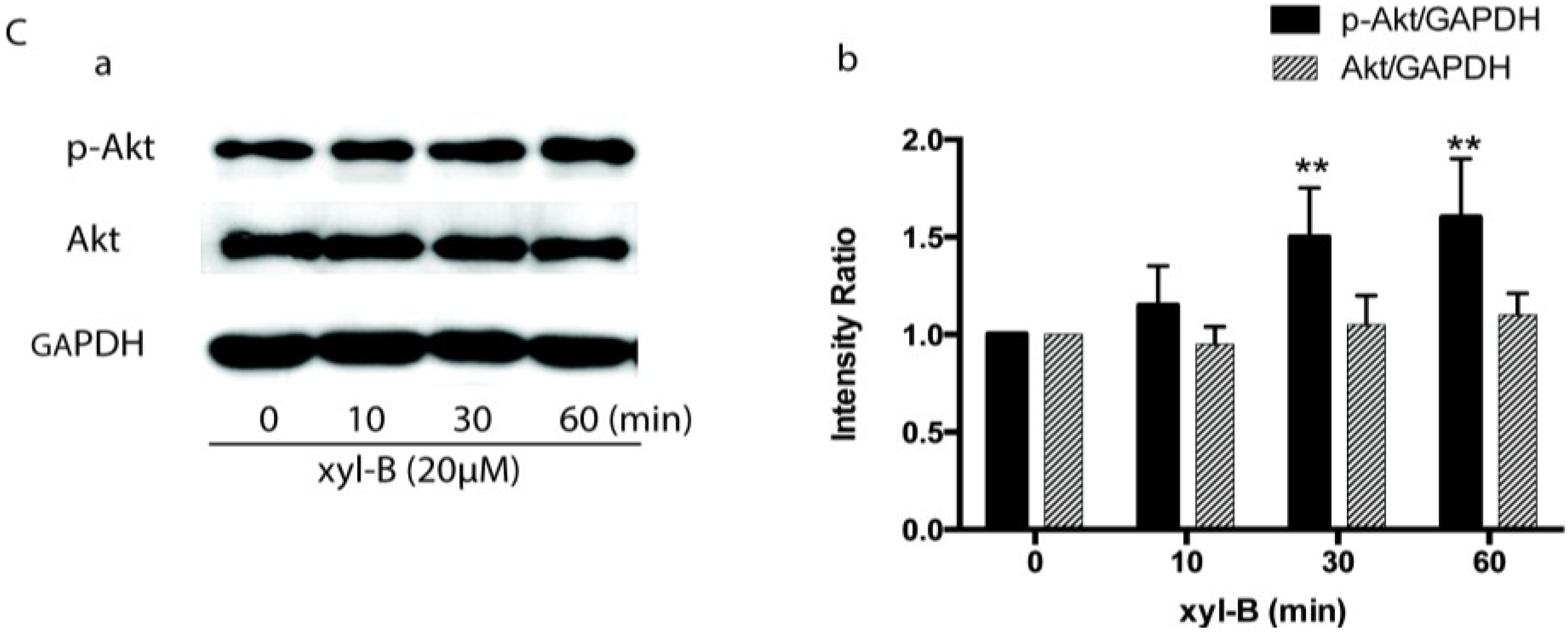
2.5. Effect of Xyloketal B on Akt/eNOS Signaling in HUVECs

3. Experimental Section
3.1. Animals
3.2. En Face Analysis of Aortic Lesion
3.3. Histologic Analysis of Aortic Root Plaque
3.4. Detection of Serum Anti-OxLDL Autoantibodies
3.5. Immunofluorescence Analysis of PECAM-1
3.6. Transmission Electron Microscopy of Aortic Vessels
3.7. Measurements of Vasorelaxation of the Aortic Rings
3.8. Isolation and Culture of Endothelial Cells
3.9. Western Blot Analysis
3.10. Statistical Methods
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc.Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Viecili, P.R.; Borges, D.O.; Kirsten, K.; Malheiros, J.; Viecili, E.; Melo, R.D.; Trevisan, G.; da Silva, M.A.; Bochi, G.V.; Moresco, R.N.; et al. Effects of campomanesia xanthocarpa on inflammatory processes, oxidative stress, endothelial dysfunction and lipid biomarkers in hypercholesterolemic individuals. Atherosclerosis 2014, 234, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Perticone, F.; Maio, R.; Perticone, M.; Sciacqua, A.; Shehaj, E.; Naccarato, P.; Sesti, G. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation 2010, 122, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Ghiadoni, L.; Cardinal, H.; Favilla, S.; Duranti, P.; Birindelli, R.; Magagna, A.; Bernini, G.; Salvetti, G.; Taddei, S.; et al. Mechanisms responsible for endothelial dysfunction induced by fasting hyperhomocystinemia in normotensive subjects and patients with essential hypertension. J. Am. Coll. Cardiol. 2001, 38, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Albiero, M.; Menegazzo, L.; de Kreutzenberg, S.; Fadini, G.P. Endothelial dysfunction in diabetes: The role of reparatory mechanisms. Diabetes Care 2011, 34 (Suppl. S2), S285–S290. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Balbarini, A. Exposure to passive smoking: A test to predict endothelial dysfunction and atherosclerotic lesions. Angiology 2008, 59, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.E. The unfolding tale of pecam-1. FEBS Lett. 2003, 540, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, N.; Ojaimi, C.; Kinugawa, S.; Wang, Z.; Xu, X.; Koller, A.; Recchia, F.A.; Hintze, T.H. Hyperhomocysteinemia alters cardiac substrate metabolism by impairing nitric oxide bioavailability through oxidative stress. Circulation 2007, 115, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide 2013, 35, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [PubMed]
- Michell, B.J.; Chen, Z.; Tiganis, T.; Stapleton, D.; Katsis, F.; Power, D.A.; Sim, A.T.; Kemp, B.E. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase c and the camp-dependent protein kinase. J. Biol. Chem. 2001, 276, 17625–17628. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, J.D.; Wilson, P.D. Synthesis of xyloketal a, b, c, d, and g analogues. J. Organ. Chem. 2006, 71, 1620–1625. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.; Jones, E.B.; Krohn, K.; Steingrover, K.; et al. Five unique compounds: Xyloketals from mangrove fungus xylaria sp. From the south China sea coast. J. Organ. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Y.; Wang, J.; Pang, J.; Huang, C.; Wu, X.; She, Z.; Vrijmoed, L.L.; Jones, E.B.; Lin, Y. Benzofuran derivatives from the mangrove endophytic fungus xylaria sp. (#2508). J. Nat. Prod. 2008, 71, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.L. A novel marine compound xyloketal b protects against oxidized ldl-induced cell injury in vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Y.; Xiang, Q.; Pei, Z.; Liu, X.; Lu, B.; Chen, L.; Wang, G.; Pang, J.; Lin, Y. Design and synthesis of novel xyloketal derivatives and their vasorelaxing activities in rat thoracic aorta and angiogenic activities in zebrafish angiogenesis screen. J. Med. Chem. 2010, 53, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.Y.; Lin, Y.C.; Liu, J.; Huang, R.; Wang, G.L.; Pei, Z.; et al. Marine compound xyloketal b protects pc12 cells against ogd-induced cell damage. Brain Res. 2009, 1302, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Chen, J.W.; Yuan, F.; Huang, Y.Y.; Zhao, L.Y.; Li, J.; Su, H.X.; Liu, J.; Pang, J.Y.; Lin, Y.C.; et al. Xyloketal b exhibits its antioxidant activity through induction of ho-1 in vascular endothelial cells and zebrafish. Mar. Drugs 2013, 11, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Yao, X.L.; Liu, Z.; Zhang, H.; Li, W.; Li, Z.; Wang, G.L.; Pang, J.; Lin, Y.; Xu, Z.; et al. Protective effects of xyloketal b against mpp+-induced neurotoxicity in caenorhabditis elegans and pc12 cells. Brain Res. 2010, 1332, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Pang, J.Y.; Lin, Y.C.; Liu, J.; Xie, Y.; Liu, C.Z. Application of Xyloketal B in preparing antiatherosclerotic medicaments. Patent CN102018699B, 26 March 2014. [Google Scholar]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Kou, R.; Lin, A.J.; Golan, D.E.; Michel, T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J. Biol. Chem. 2002, 277, 39554–39560. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. Apoe-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, C.P.; Burton, C.A.; Hernandez, M.; Mundt, S.; Hassing, H.; Patel, S.; Rosa, R.; Hermanowski-Vosatka, A.; Wang, P.R.; Zhang, D.; et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Ord, V.A.; Plump, A.S.; Breslow, J.L.; Steinberg, D.; Witztum, J.L. Apoe-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler. Thromb. 1994, 14, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Baglione, J.; Smith, J.D. Quantitative assay for mouse atherosclerosis in the aortic root. Methods Mol. Med. 2006, 129, 83–95. [Google Scholar] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Meir, K.S.; Leitersdorf, E. Atherosclerosis in the apolipoprotein-e-deficient mouse: A decade of progress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Zadelaar, S.; Kleemann, R.; Verschuren, L.; de Vries-Van der Weij, J.; van der Hoorn, J.; Princen, H.M.; Kooistra, T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Linton, M.F. Mouse models of hyperlipidemia and atherosclerosis. Front. Biosci. 2001, 6, D515–D525. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, T.E.; Dwyer, T.M.; Irwin, E.D.; Rossing, M.A.; Kieval, R.S. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension 2007, 49, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, T.E.; Iliescu, R.; Liu, B.; Henegar, J.R.; Maric-Bilkan, C.; Irwin, E.D. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012, 59, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Erdos, B.; Kirichenko, N.; Whidden, M.; Basgut, B.; Woods, M.; Cudykier, I.; Tawil, R.; Scarpace, P.J.; Tumer, N. Effect of age on high-fat diet-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H164–H172. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. High fat programming of beta cell compensation, exhaustion, death and dysfunction. Pediatr. Diabetes 2015, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Asahara, S.; Masuda, K.; Matsuda, T.; Kimura-Koyanagi, M.; Seino, S.; Ogawa, W.; Kido, Y. Compensatory hyperinsulinemia in high-fat diet-induced obese mice is associated with enhanced insulin translation in islets. Biochem. Biophys. Res. Commun. 2015, 458, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Dake, B.L.; Oltman, C.L. Cardiovascular, metabolic, and coronary dysfunction in high-fat-fed obesity-resistant/prone rats. Obesity 2015, 23, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, R.; Tudorancea, I.; Irwin, E.D.; Lohmeier, T.E. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1080–H1088. [Google Scholar] [CrossRef] [PubMed]
- McCully, B.H.; Brooks, V.L.; Andresen, M.C. Diet-induced obesity severely impairs myelinated aortic baroreceptor reflex responses. Am. J. Physiol. Heart Circ. Phys. 2012, 302, H2083–H2091. [Google Scholar] [CrossRef]
- McCully, B.H.; Hasan, W.; Streiff, C.T.; Houle, J.C.; Woodward, W.R.; Giraud, G.D.; Brooks, V.L.; Habecker, B.A. Sympathetic cardiac hyperinnervation and atrial autonomic imbalance in diet-induced obesity promote cardiac arrhythmias. Am. J. Physiol. Heart Circ. Phys. 2013, 305, H1530–H1537. [Google Scholar] [CrossRef]
- Zhao, D.; McCully, B.H.; Brooks, V.L. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. J. Pharmacol. Exp. Ther. 2012, 343, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Rajman, I.; Kendall, M.; Cramb, R. The oxidation hypothesis of atherosclerosis. Lancet 1994, 344, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Hulthe, J. Antibodies to oxidized ldl in atherosclerosis development—Clinical and animal studies. Clin. Chim. Acta 2004, 348, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Bielory, L. Late-phase reaction in ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Sezer, E.D.; Sozmen, E.Y.; Nart, D.; Onat, T. Effect of atorvastatin therapy on oxidant-antioxidant status and atherosclerotic plaque formation. Vasc. Health Risk Manag. 2011, 7, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, S.; Czech, T.; van Eickels, M.; Fleming, I.; Bohm, M.; Nickenig, G. Inhibition of diet-induced atherosclerosis and endothelial dysfunction in apolipoprotein e/angiotensin ii type 1a receptor double-knockout mice. Circulation 2004, 110, 3062–3067. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Meng, N.; Wang, S.; Zhao, F.; Zhao, J.; Su, L.; Zhang, S.; Zhang, Y.; Zhao, B.; Miao, J. An activator of mtor inhibits oxldl-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein e(−)/(−) mice. Sci. Rep. 2014, 4, 5519. [Google Scholar] [PubMed]
- Yoshioka, K.; Yoshida, K.; Cui, H.; Wakayama, T.; Takuwa, N.; Okamoto, Y.; Du, W.; Qi, X.; Asanuma, K.; Sugihara, K.; et al. Endothelial pi3k-c2alpha, a class ii pi3k, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 2012, 18, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Ramkhelawon, B.; Vilar, J.; Rivas, D.; Mees, B.; de Crom, R.; Tedgui, A.; Lehoux, S. Shear stress regulates angiotensin type 1 receptor expression in endothelial cells. Circ. Res. 2009, 105, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S. The two faces of endothelial nitric oxide synthase in the pathophysiology of atherosclerosis. Endothelium 2004, 11, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, T.; Genazzani, A.R. Raloxifene acutely stimulates nitric oxide release from human endothelial cells via an activation of endothelial nitric oxide synthase. J. Clin. Endocrinol. Metab. 2000, 85, 2966–2969. [Google Scholar] [CrossRef] [PubMed]
- Domagala, T.B.; Szeffler, A.; Dobrucki, L.W.; Dropinski, J.; Polanski, S.; Leszczynska-Wiloch, M.; Kotula-Horowitz, K.; Wojciechowski, J.; Wojnowski, L.; Szczeklik, A.; et al. Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by n1-methylnicotinamide in human blood vessels. Hypertension 2012, 59, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Kramer, M.F.; Becker, R.E.; Burnett, A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (ser-1177) by o-glcnac in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. USA 2005, 102, 11870–11875. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Fisslthaler, B.; Dimmeler, S.; Kemp, B.E.; Busse, R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 2001, 88, E68–E75. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.-Y.; Li, J.; Yuan, F.; Li, M.; Zhang, Q.; Pang, J.-Y.; Zhang, B.; Sun, F.-Y.; Sun, H.-S.; Li, Q.; et al. Xyloketal B Attenuates Atherosclerotic Plaque Formation and Endothelial Dysfunction in Apolipoprotein E Deficient Mice. Mar. Drugs 2015, 13, 2306-2326. https://doi.org/10.3390/md13042306
Zhao L-Y, Li J, Yuan F, Li M, Zhang Q, Pang J-Y, Zhang B, Sun F-Y, Sun H-S, Li Q, et al. Xyloketal B Attenuates Atherosclerotic Plaque Formation and Endothelial Dysfunction in Apolipoprotein E Deficient Mice. Marine Drugs. 2015; 13(4):2306-2326. https://doi.org/10.3390/md13042306
Chicago/Turabian StyleZhao, Li-Yan, Jie Li, Feng Yuan, Mei Li, Quan Zhang, Ji-Yan Pang, Bin Zhang, Fang-Yun Sun, Hong-Shuo Sun, Qian Li, and et al. 2015. "Xyloketal B Attenuates Atherosclerotic Plaque Formation and Endothelial Dysfunction in Apolipoprotein E Deficient Mice" Marine Drugs 13, no. 4: 2306-2326. https://doi.org/10.3390/md13042306





