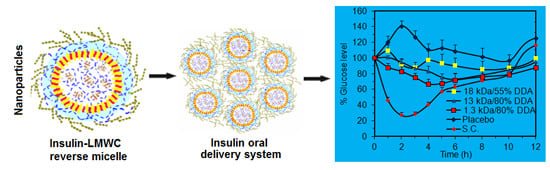
Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations
Abstract
:1. Introduction
2. Results and Discussion
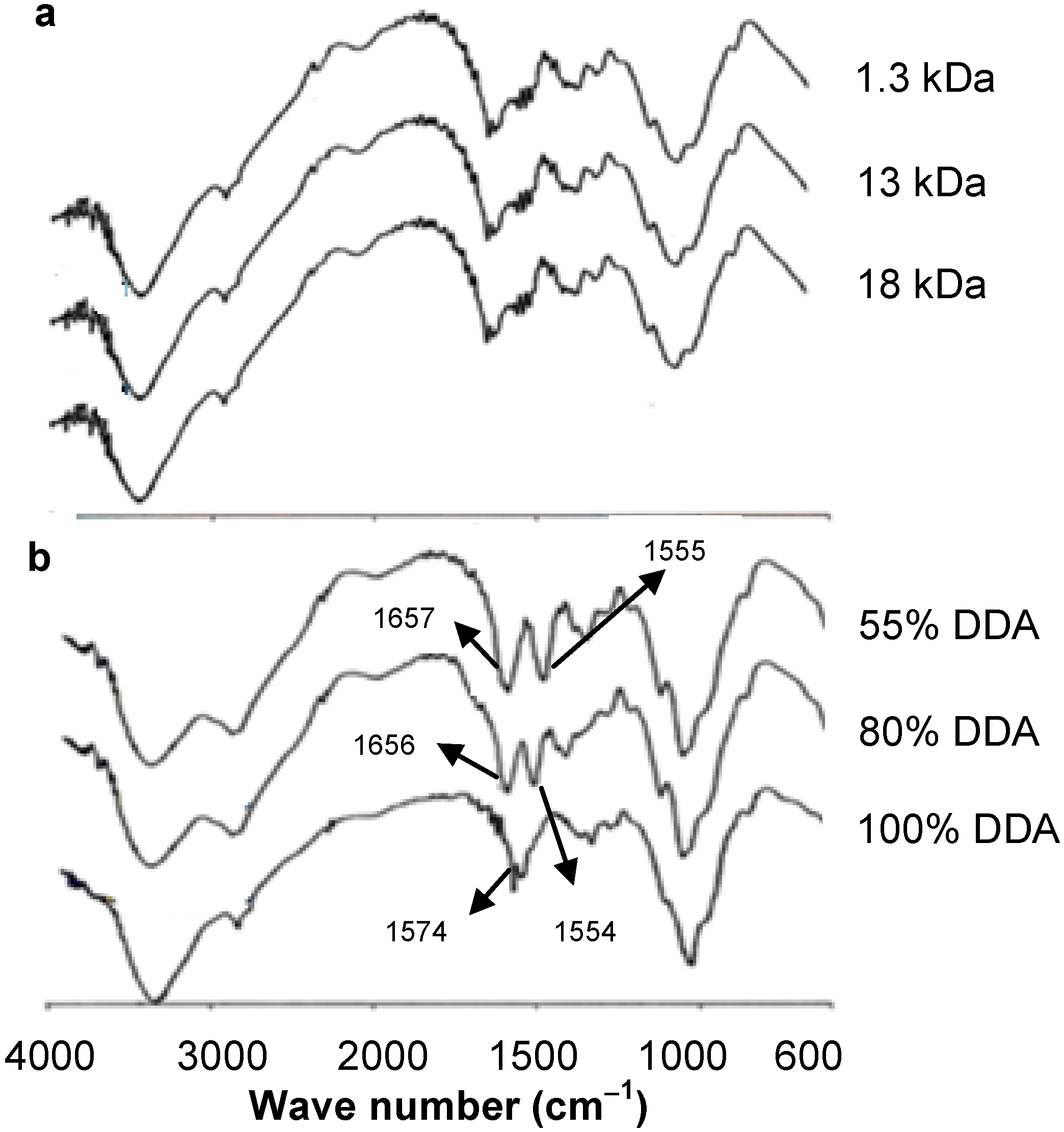
2.1. Preparation and Characterization of LMWCs with Different DDA
| Molecular Weight | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LMWC MW (kDa) | 1.3 | 13 | 18 | ||||||||
| Depolymerization time (h) | 24 | 3.2 | 2.0 | ||||||||
| Experimental MW (kDa) | 1.25 | 12.75 | 18.20 | ||||||||
| Degree of Deacetylation (DDA) | |||||||||||
| DDA % | 55 | 80 | 100 | ||||||||
| Molar ratio of chitosan:Ac2O | 1:0.60 | 1:0.15 | 1:0 | ||||||||
| LMWC MW (kDa) | 1.3 | 13 | 18 | 1.3 | 13 | 18 | 1.3 | 13 | 18 | ||
| Experimental DDA% | |||||||||||
| By UV/visible | 54.9 | 54.8 | 55.1 | 79.8 | 80.3 | 80.2 | 99.6 | 99.4 | 100.3 | ||
| By 1H-NMR | 55.0 | 55.4 | 56.6 | 79.6 | 79.1 | 80.6 | 100.0 | 100.0 | 100.0 | ||

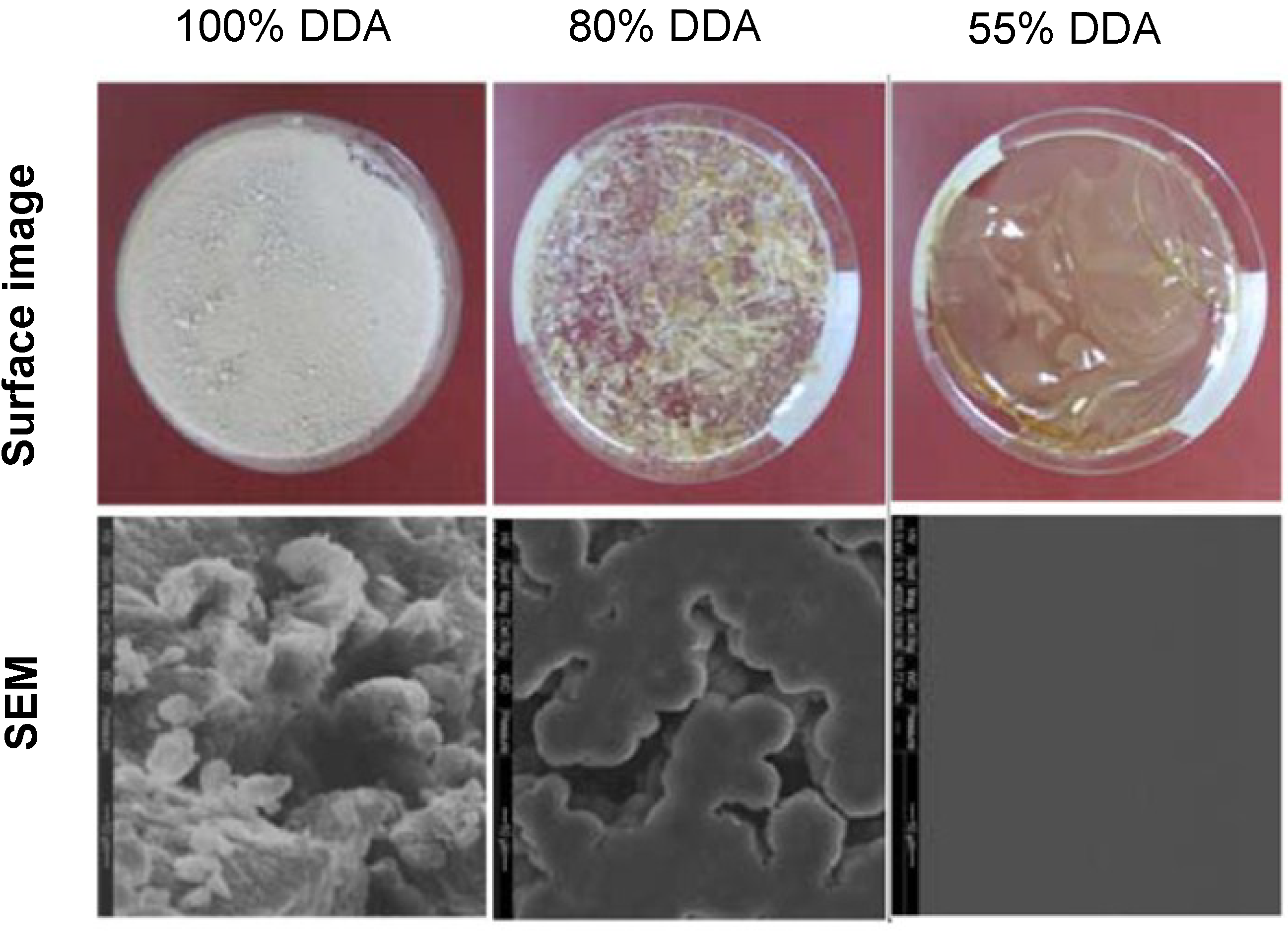
2.2. Surface Morphology
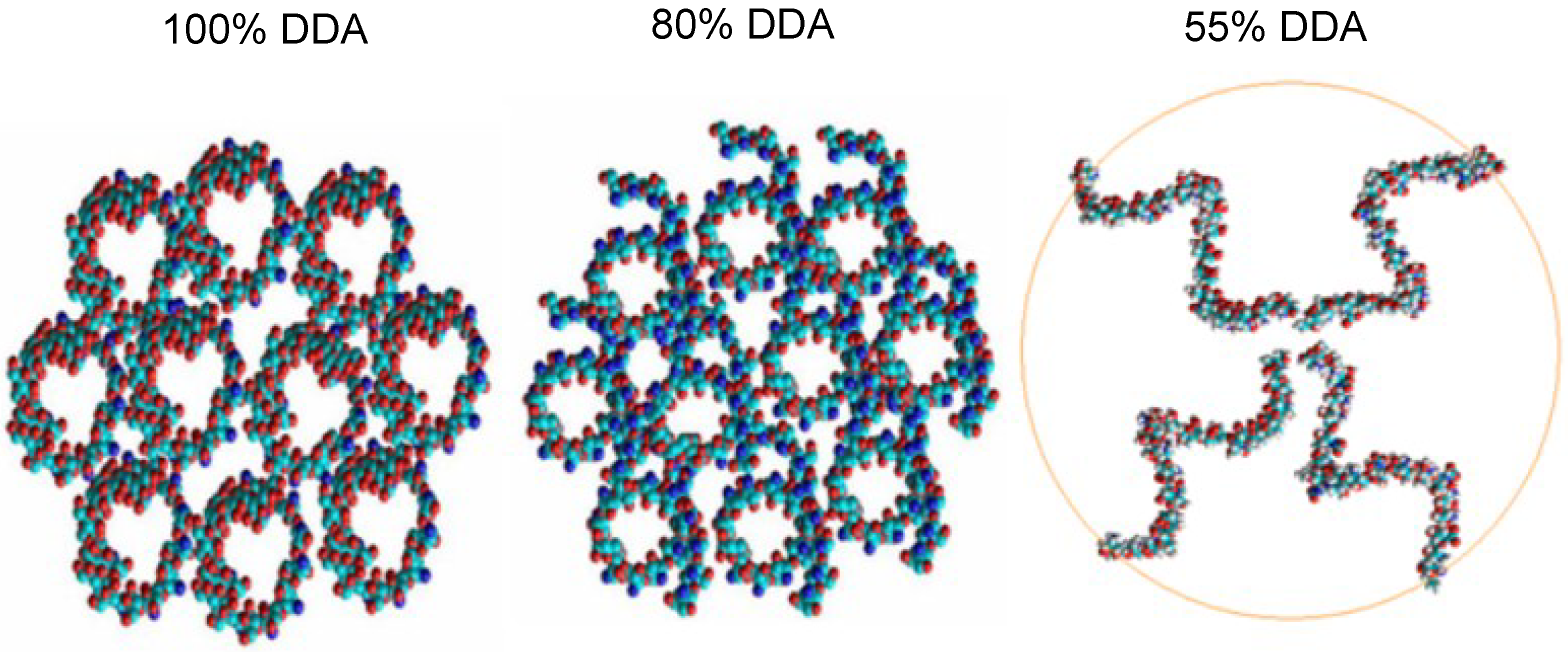
2.3. In Silico Characterization
2.4. Oral Insulin Formulation and Aggregation Size of the Reverse Micelle
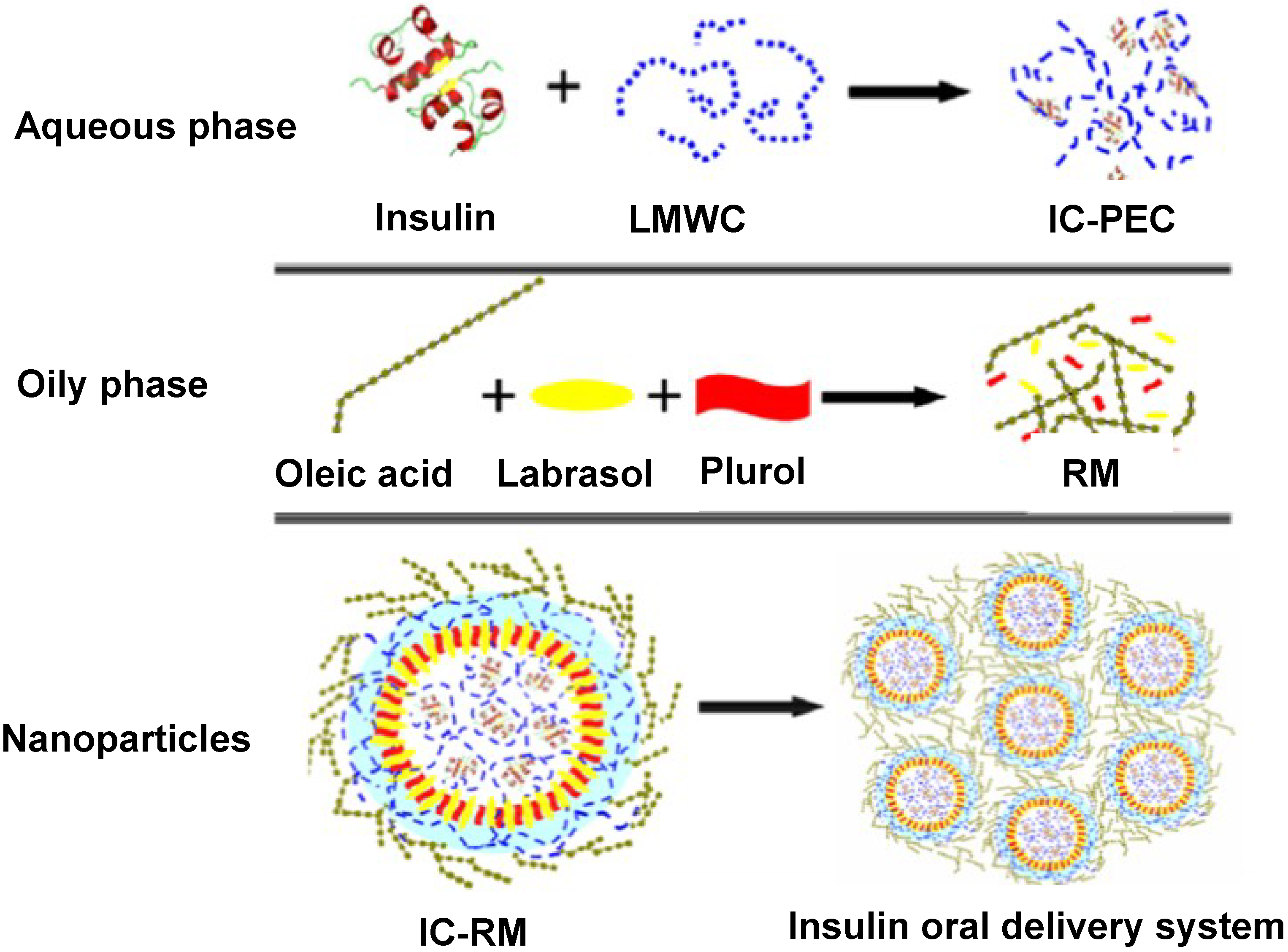
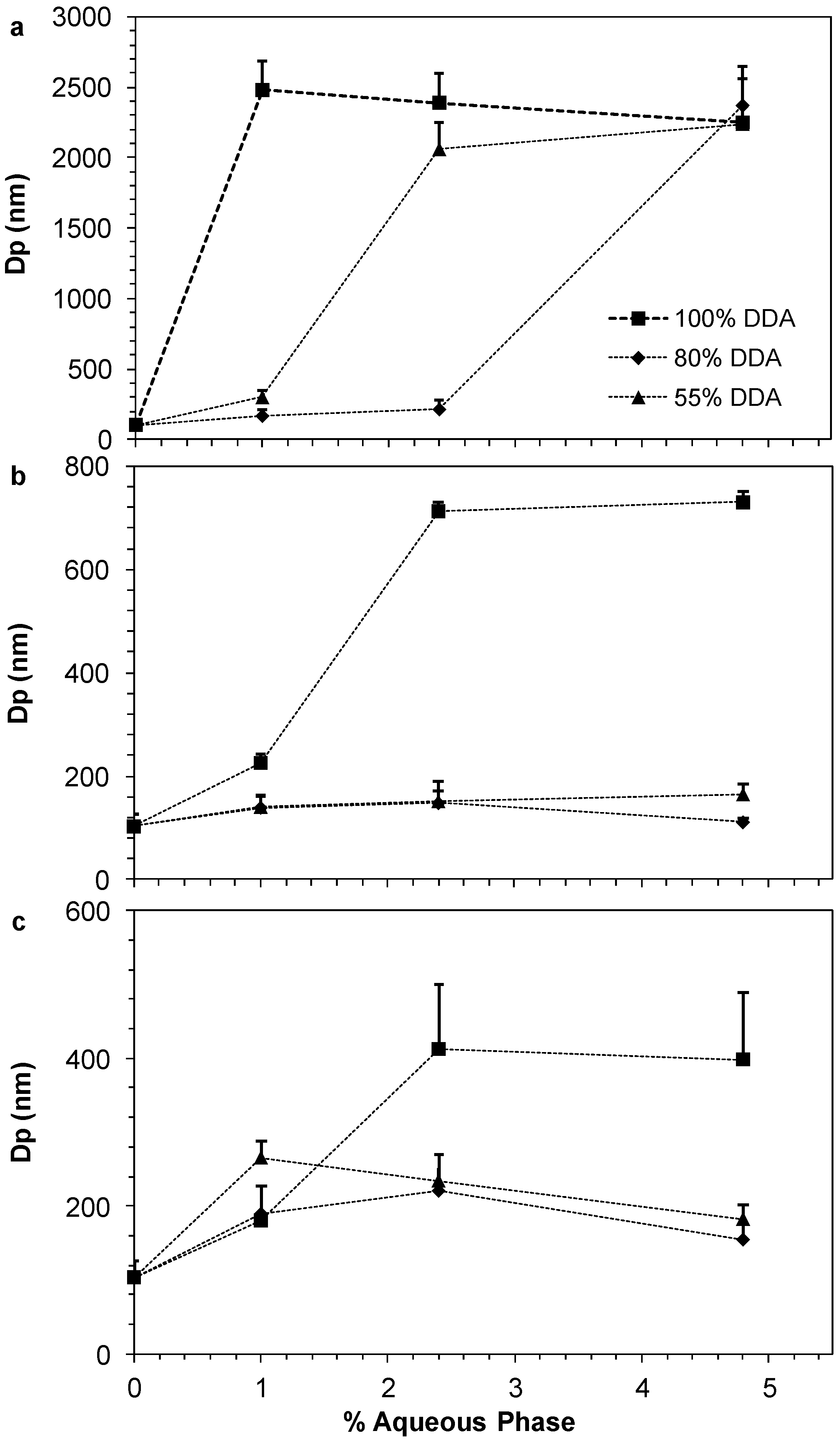
2.5. In Vivo Pharmacological Activity of the Nano Dispersion System


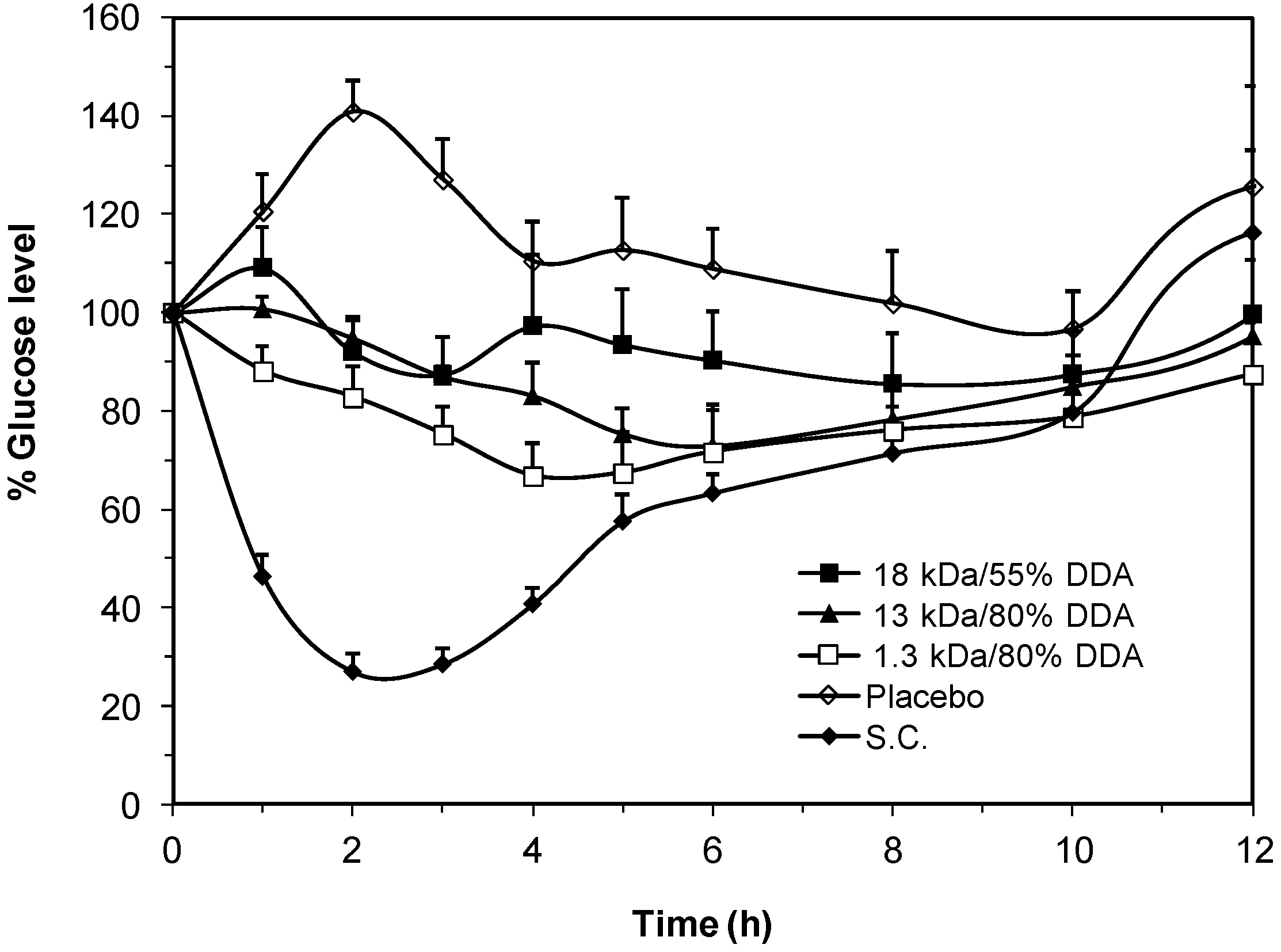
3. Experimental Section
3.1. Materials
3.2. Preparation of Fully Deacetylated LMWCs
3.3. Preparation of LMWCs with Different DDAs
3.4. Determination of the Molecular Weight of LMWCs
3.5. Determination of the DDA
3.6. FT-IR Spectroscopy
3.7. Surface Morphology
3.8. In Silico Molecular Mechanic Modeling
3.9. Formulation of the Oral Insulin Nanoparticle Dispersion System
3.10. Aggregation Size Determinations of the Nanoparticle Dispersion System
3.11. In vivo Studies on Streptozotocine (STZ) Diabetic Rats
3.11.1. Animal Handling
3.11.2. Induction of Diabetes using Streptozotocin
3.11.3. Pharmacological Activity Evaluations of the Insulin-Loaded Dispersions
3.12. Statistical Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, S.; Jain, A.; Chakraborty, M.; Sahni, J.K.; Ali, J.; Dang, S. Oral delivery of therapeutic proteins and peptides: A review on recent developments. Drug Deliv. 2013, 20, 237–246. [Google Scholar]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar]
- Assaf, S.; Al-Jbour, N.; Eftaiha, A.; Elsayed, A.; Al-Remawi, M.; Qinna, N.; Badwan, A. Factors involved in formulation of oily delivery system for proteins based on peg-8 caprylic/capric glycerides and polyglyceryl-6 dioleate in a mixture of oleic acid with chitosan. J. Dispers. Sci. Technol. 2011, 32, 623–633. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 2010, 8, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Qinna, N.A.; Akayleh, F.T.; Al Remawi, M.M.; Kamona, B.S.; Taha, H.; Badwan, A.A. Evaluation of a functional food preparation based on chitosan as a meal replacement diet. J. Funct. Foods 2013, 5, 1125–1134. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Hayes, D.G.; Weiss, J. Efficient reduction of chitosan molecular weight by high-intensity ultrasound: Underlying mechanism and effect of process parameters. J. Agric. Food Chem. 2008, 56, 5112–5119. [Google Scholar] [CrossRef] [PubMed]
- Einbu, A.; Grasdalen, H.; Varum, K.M. Kinetics of hydrolysis of chitin/chitosan oligomers in concentrated hydrochloric acid. Carbohydr. Res. 2007, 342, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Sadeghi, A.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, S.; Bouchemal, K.; Marsaud, V.; Vauthier, C.; Sharma, C.P. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J. Control. Release 2010, 147, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Badwan, A.; Al-Remawi, M.; Qinna, N.; Elsayed, A. Nanocapsules for oral delivery of proteins. Available online: https://data.epo.org/gpi/EP2042166A1-Nanocapsules-for-oral-delivery-of-proteins (accessed on 20 January 2015).
- Elsayed, A.; Remawi, M.A.; Qinna, N.; Farouk, A.; Badwan, A. Formulation and characterization of an oily-based system for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2009, 73, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Badwan, A.; Remawi, M.; Qinna, N.; Elsayed, A.; Arafat, T.; Melhim, M.; Hijleh, O.A.; Idkaidek, N.M. Enhancement of oral bioavailability of insulin in humans. Neuro Endocrinol. Lett. 2009, 30, 74–78. [Google Scholar] [PubMed]
- Park, K.; Kwon, I.C.; Park, K. Oral protein delivery: Current status and future prospect. React. Funct. Polym. 2011, 71, 280–287. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.S.; Cabral-Albuquerque, E.; Ferreira, D.; Sarmento, B. Chitosan: An option for development of essential oil delivery systems for oral cavity care? Carbohydr. Polym. 2009, 76, 501–508. [Google Scholar] [CrossRef]
- Tommeraas, K.; Koping-Hoggard, M.; Varum, K.M.; Christensen, B.E.; Artursson, P.; Smidsrod, O. Preparation and characterisation of chitosans with oligosaccharide branches. Carbohydr. Res. 2002, 337, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Preparation and pharmacodynamics of low-molecular-weight chitosan nanoparticles containing insulin. Carbohydr. Polym. 2009, 76, 368–373. [Google Scholar] [CrossRef]
- Palpandi, C.; Shanmugam, V.; Shanmugam, A. Extraction of chitin and chitosan from shell and operculum of mangrove gastropod nerita (dostia) crepidularia lamarck. Int. J. Med. Med. Sci. 2009, 1, 198–205. [Google Scholar]
- Heux, L.; Brugnerotto, J.; Desbrieres, J.; Versali, M.F.; Rinaudo, M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, L.; Primorac, M.; Stupar, M.; Krajisnik, D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int. J. Pharm. 2004, 271, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Jack, K.S.; Whittaker, A.K.; Hook, S.M.; Rades, T. Protein delivery using nanoparticles based on microemulsions with different structure-types. Eur. J. Pharm. Sci. 2008, 33, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Morawetz, H.; Sage, H. The effect of poly(acrylic acid) on the tryptic digestion of hemoglobin. Arch. Biochem. Biophys. 1955, 56, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, R.; Al-Jbour, N.; Al-Sou’d, K.; Sweidan, K.; Al-Remawi, M.; Badwan, A. Some physico-chemical properties of low molecular weight chitosans and their relationship to conformation in aqueous solution. J. Solut. Chem. 2010, 39, 575–588. [Google Scholar] [CrossRef]
- Kubota, N.; Tatsumoto, N.; Sano, T.; Toya, K. A simple preparation of half N-acetylated chitosan highly soluble in water and aqueous organic solvents. Carbohydr. Res. 2000, 324, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Qandil, A.; Obaidat, A.; Ali, M.; Al-Taani, B.; Tashtoush, B.; Al-Jbour, N.; Al Remawi, M.; Al-Sou’od, K.; Badwan, A. Investigation of the interactions in complexes of low molecular weight chitosan with ibuprofen. J. Solut. Chem. 2009, 38, 695–712. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qinna, N.A.; Karwi, Q.G.; Al-Jbour, N.; Al-Remawi, M.A.; Alhussainy, T.M.; Al-So'ud, K.A.; Omari, M.M.H.A.; Badwan, A.A. Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs 2015, 13, 1710-1725. https://doi.org/10.3390/md13041710
Qinna NA, Karwi QG, Al-Jbour N, Al-Remawi MA, Alhussainy TM, Al-So'ud KA, Omari MMHA, Badwan AA. Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Marine Drugs. 2015; 13(4):1710-1725. https://doi.org/10.3390/md13041710
Chicago/Turabian StyleQinna, Nidal A., Qutuba G. Karwi, Nawzat Al-Jbour, Mayyas A. Al-Remawi, Tawfiq M. Alhussainy, Khaldoun A. Al-So'ud, Mahmoud M. H. Al Omari, and Adnan A. Badwan. 2015. "Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations" Marine Drugs 13, no. 4: 1710-1725. https://doi.org/10.3390/md13041710
APA StyleQinna, N. A., Karwi, Q. G., Al-Jbour, N., Al-Remawi, M. A., Alhussainy, T. M., Al-So'ud, K. A., Omari, M. M. H. A., & Badwan, A. A. (2015). Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Marine Drugs, 13(4), 1710-1725. https://doi.org/10.3390/md13041710