MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt’s Lymphoma Cells to Trigger Apoptosis through Multiple Pathways
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glycan-Binding and Cell Agglutination of Recombinant MytiLec
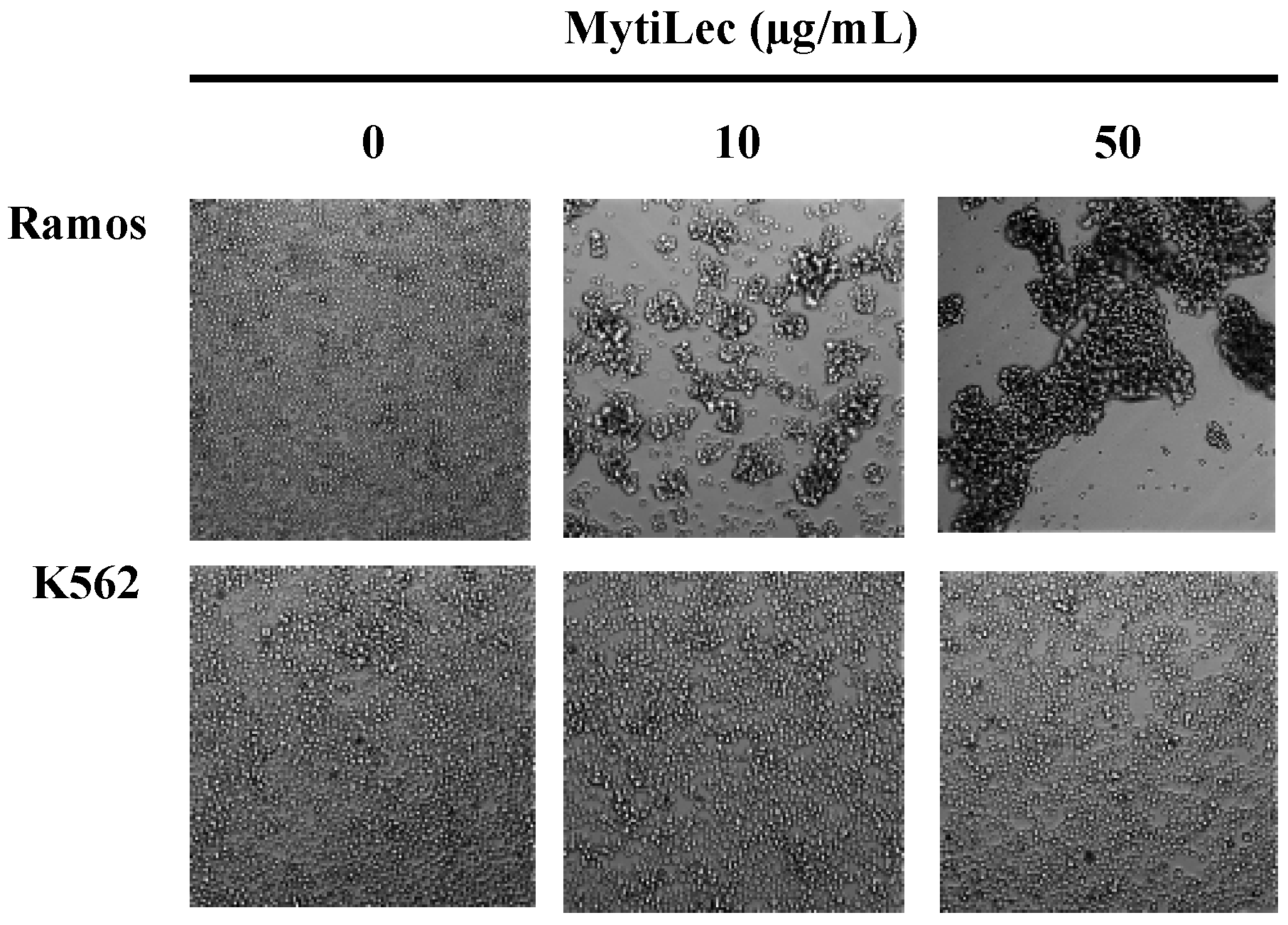
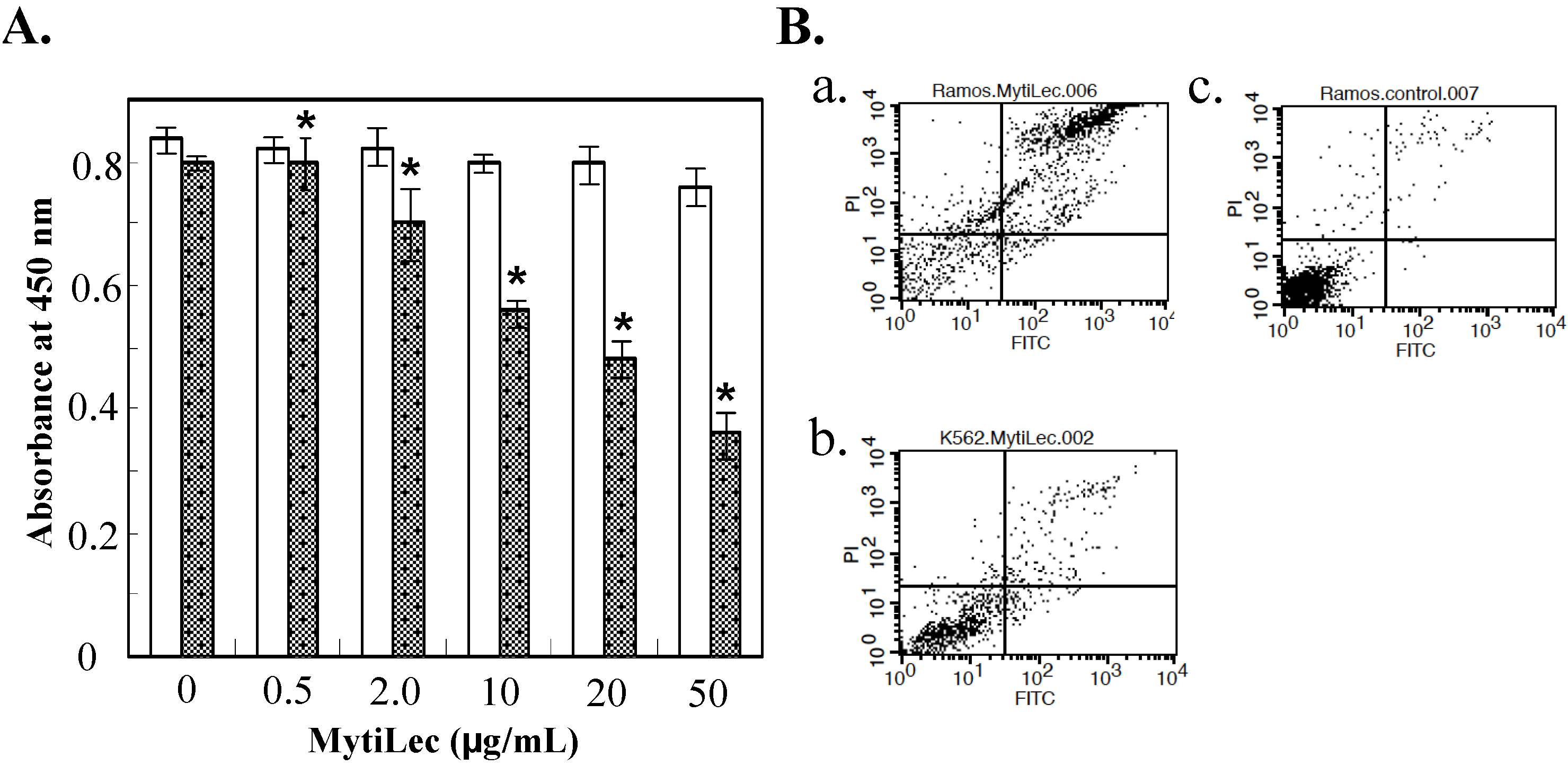
2.2. Cytotoxic Effects of MytiLec on Burkitt’s Lymphoma Cell Lines
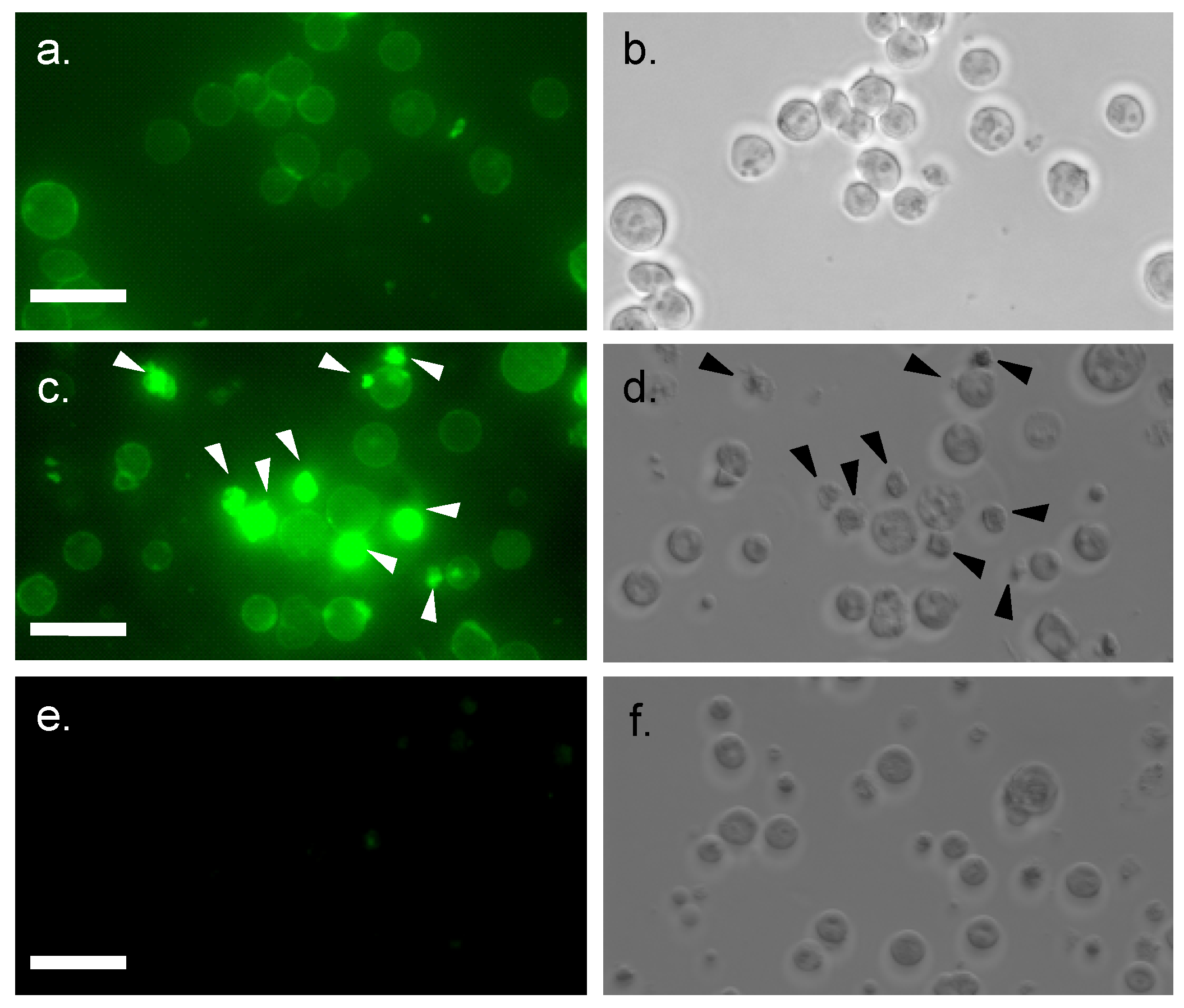
2.3. Internalization of MytiLec into Burkitt’s Lymphoma Cells
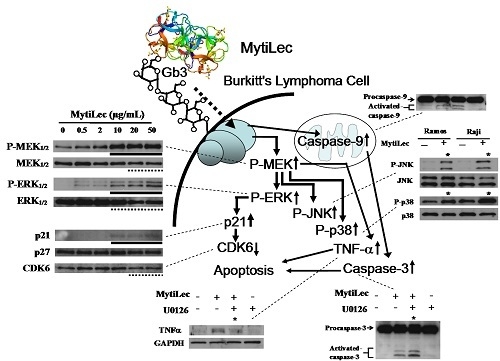
2.4. Activation of MAPK Pathways by MytiLec
2.4.1. Activation of MEK-ERK Pathway
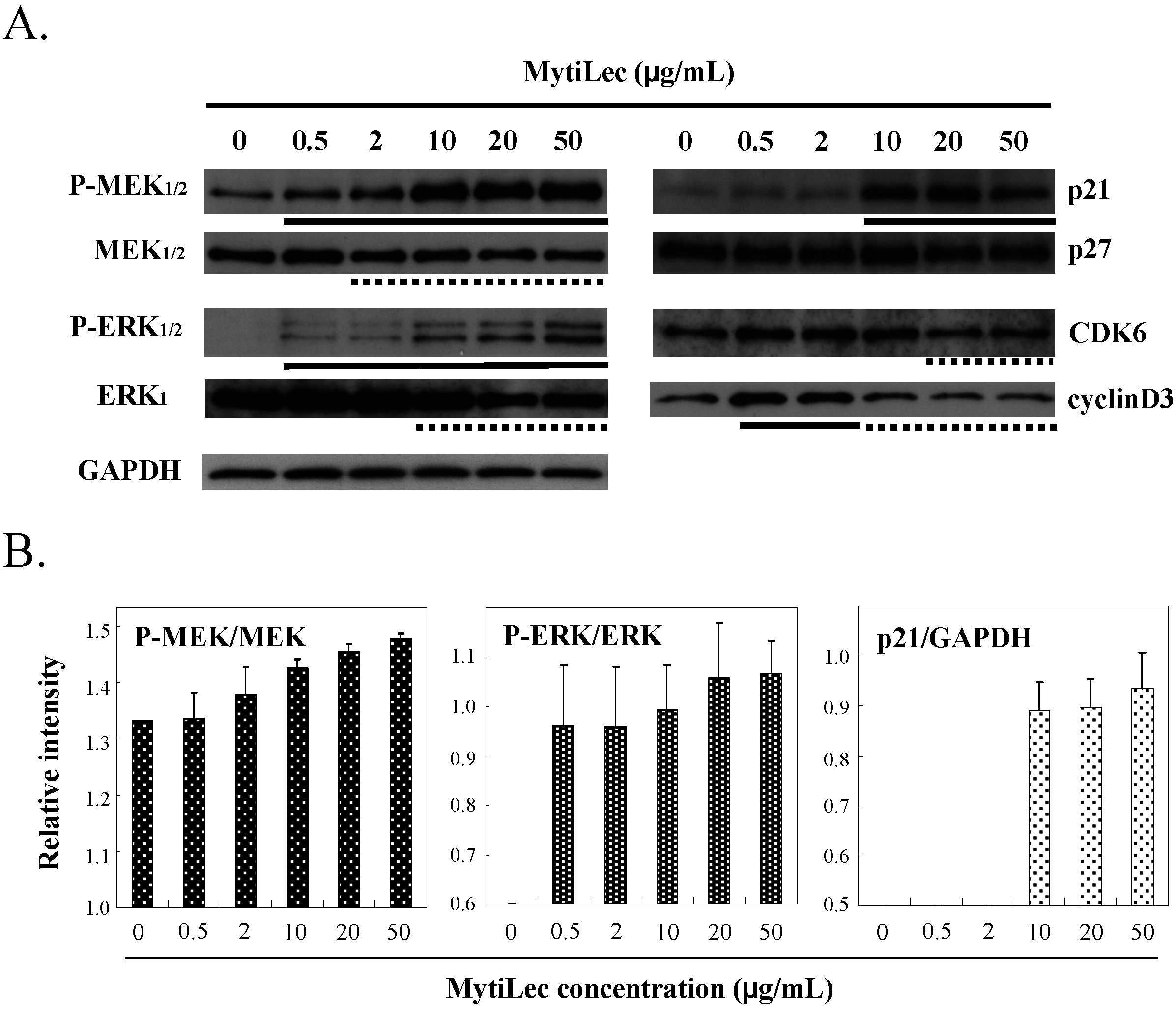
2.4.2. Phosphorylation of Stress-Activated MAPK Pathways (JNK, p38 Kinase)
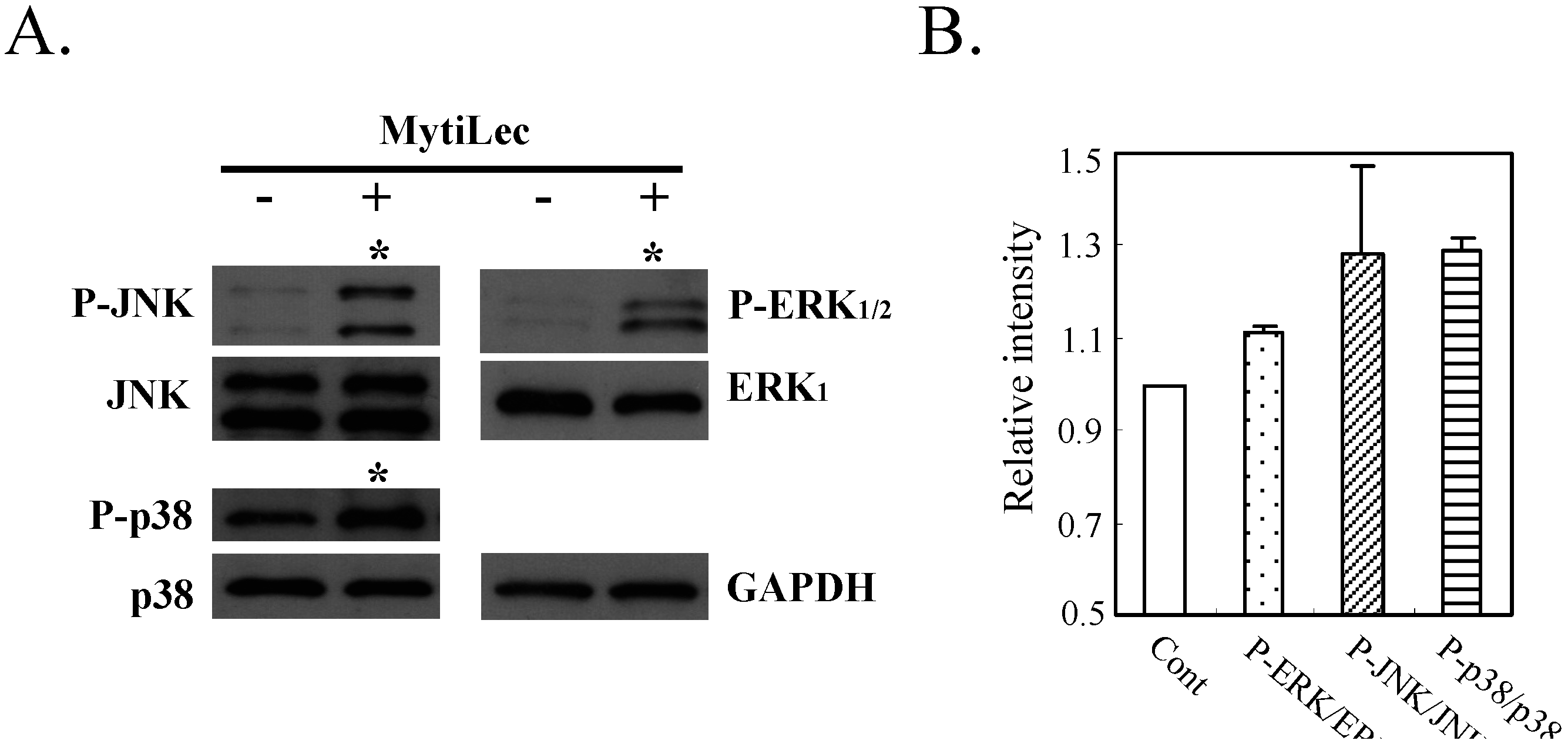
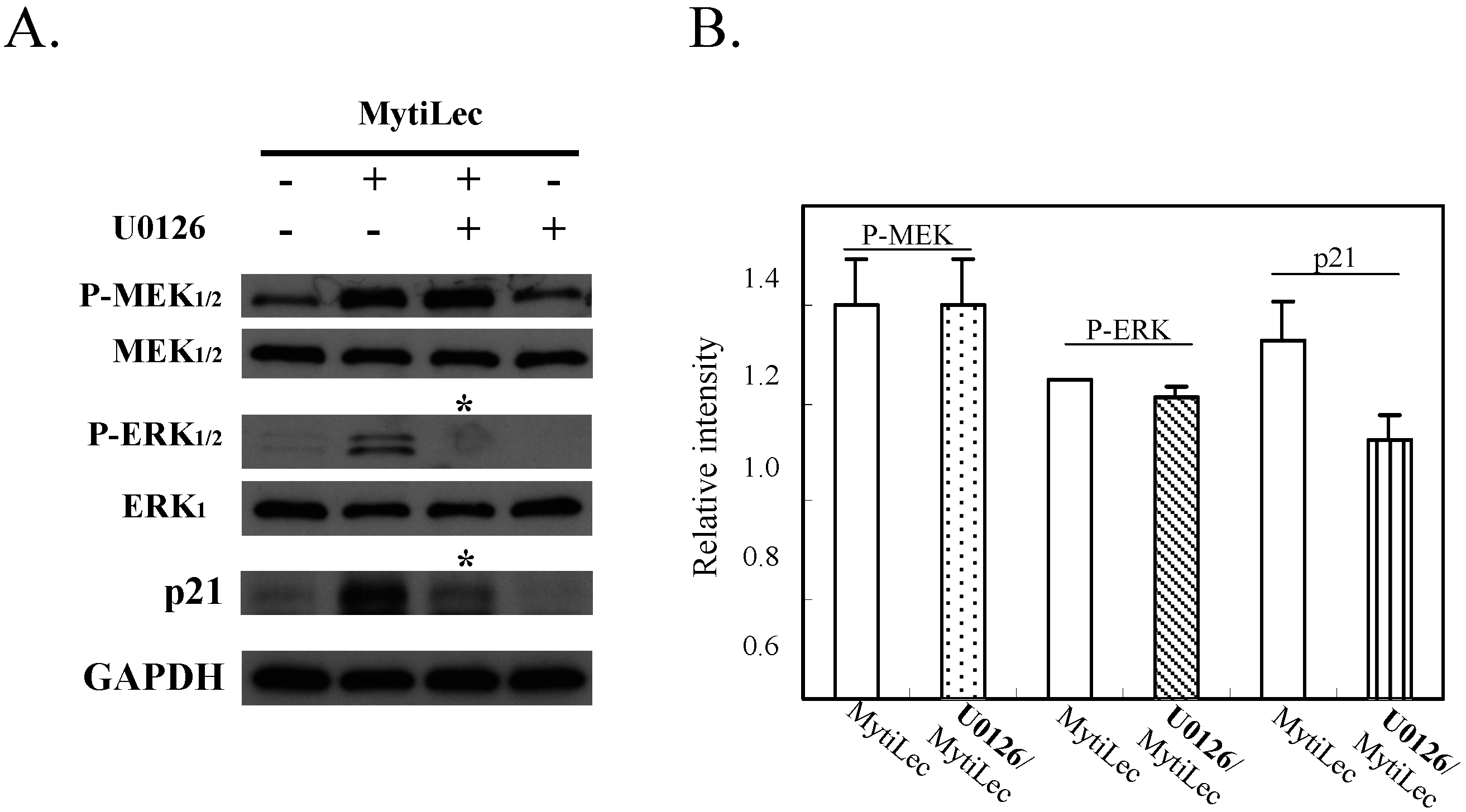
2.4.3. MytiLec-Induced Phosphorylation of MEK-ERK Pathway Causes Cell Cycle Arrest through p21 Up-Regulation
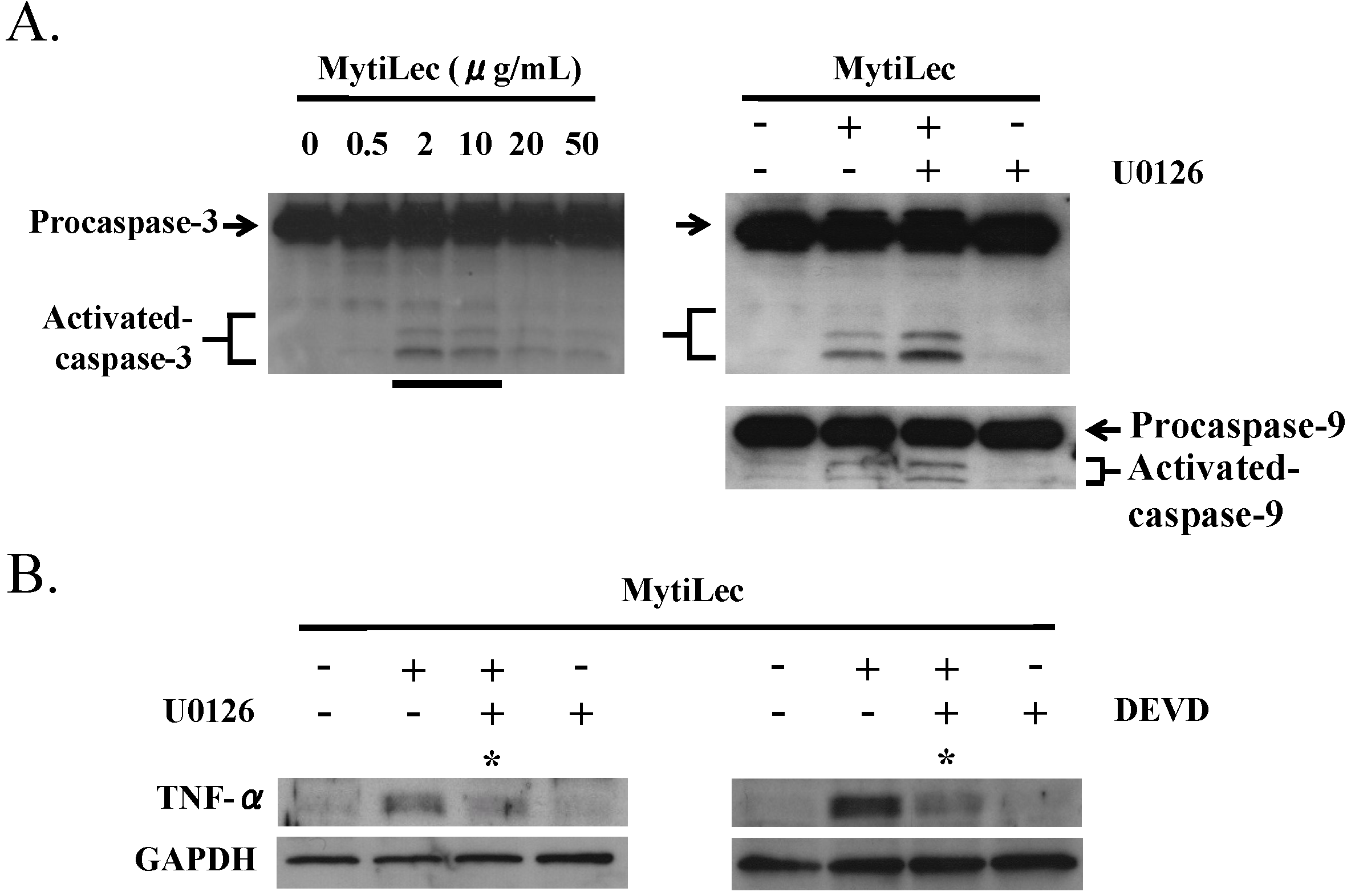
2.5. TNF-α Induction and Caspase Activation in Ramos Cells
3. Experimental Design
3.1. Preparation of MytiLec
3.2. Cell Lines and Culture
3.3. Cytotoxicity and Cell Viability Assays
3.4. Fluorescein Isothiocyanate (FITC)-Conjugated MytiLec
3.5. Protein Expression of Signal Transduction Molecules and Their Phosphorylated Forms
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suárez-Ulloa, V.; Fernández-Tajes, J.; Manfrin, C.; Gerdol, M.; Venier, P.; Eirín-López, J. Bivalve omics: State of the art and potential applications for the biomonitoring of harmful marine compounds. Mar. Drugs 2013, 11, 4370–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venier, P.; de Pittà, C.; Bernante, F.; Varotto, L.; de Nardi, B.; Bovo, G.; Roch, P.; Novoa, B.; Figueras, A.; Pallavicini, A.; et al. MytiBase: A knowledgebase of mussel (M. galloprovincialis) transcribed sequences. BMC Genomics 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Venier, P.; Varotto, L.; Rosani, U.; Millino, C.; Celegato, B.; Bernante, F.; Lanfranchi, G.; Novoa, B.; Roch, P.; Figueras, A.; Pallavicini, A. Insights into the innate immunity of the Mediterranean mussel Mytilus galloprovincialis. BMC Genomics 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Venier, P. An updated molecular basis for mussel immunity. Fish Shellfish Immunol. 2015, 46, 17–38. [Google Scholar] [Green Version]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Toubiana, M.; Gerdol, M.; Rosani, U.; Pallavicini, A.; Venier, P.; Roch, P. Toll-like receptors and MyD88 adaptors in Mytilus: Complete cds and gene expression levels. Dev. Comp. Immunol. 2013, 40, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, T.; Kondo, H.; Nii, H.; Kimura, K.; Egami, Y.; Oka, Y.; Yoshida, M.; Kida, E.; Ye, Y.; Akahoshi, S.; et al. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc. Natl. Acad. Sci. USA 2011, 108, 17533–17537. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Pittman, K.B.; Patterson, W.K.; Dickson, J.; Yeend, S.; Townsend, A.; Broadbridge, V.; Price, T.J. A phase I study to determine the safety, tolerability and maximum tolerated dose of green-lipped mussel (Perna canaliculus) lipid extract, in patients with advanced prostate and breast cancer. Ann. Oncol. 2010, 21, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.; Fedoseev, G.; Krasnoschekova, O.; Abulimity, A.; Trendeleva, T.; Bames, P.J. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur. Respir. J. 2002, 20, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Hasehira, K.; Tateno, H.; Onuma, Y.; Ito, Y.; Asashima, M.; Hirabayashi, J. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol. Cell Proteomics 2012, 11, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Kovbasnjuk, O.; Mourtazina, R.; Baibakov, B.; Wang, T.; Elowsky, C.; Choti, M.A.; Kane, A.; Donowitz, M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 19087–19092. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Dohmae, N.; Takio, K.; Kawsar, S.M.; Matsumoto, R.; Hasan, I.; Koide, Y.; Kanaly, R.A.; Yasumitsu, H.; Ogawa, Y.; et al. A lectin from the mussel Mytilus galloprovincialis has a highly novel primary structure and induces glycan-mediated cytotoxicity of globotriaosylceramide-expressing lymphoma cells. J. Biol. Chem. 2012, 287, 44772–44783. [Google Scholar] [CrossRef] [PubMed]
- UniProtKB-B3EWR1 (LEC_MYTGA). Available online: www.uniprot.org/uniprot/B3EWR1 (accessed on 11 December 2015).
- Mg_Nor01_51P18 Nor01 Mytilus galloprovincialis cDNA 3-, mRNA sequence. Available online: www.ncbi.nlm.nih.gov/nucest/223022238 (accessed on 11 December 2015).
- Kovalchuk, S.N.; Chikalovets, I.V.; Chernikov, O.V.; Molchanova, V.I.; Li, W.; Rasskazov, V.A.; Lukyanov, P.A. cDNA cloning and structural characterization of a lectin from the mussel Crenomytilus grayanus with a unique amino acid sequence and antibacterial activity. Fish Shellfish Immunol. 2013, 35, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Belogortseva, N.I.; Molchanova, V.I.; Kurika, A.V.; Skobun, A.S.; Glazkova, V.E. Isolation and characterization of new GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 119, 45–50. [Google Scholar] [CrossRef]
- PDBe > 3wmv. Available online: http://www.ebi.ac.uk/pdbe/entry/pdb/3WMV (accessed on 11 December 2015).
- Rutenber, E.; Robertus, J.D. Structure of ricin B-chain at 2.5 A resolution. Proteins 1991, 10, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Pohleven, J.; Renko, M.; Magister, Š.; Smith, D.F.; Künzler, M.; Štrukelj, B.; Turk, D.; Kos, J.; Sabotiè, J. Bivalent carbohydrate binding is required for biological activity of Clitocybe nebularis lectin (CNL), the N,Nʹ-diacetyllactosediamine (GalNAcâ1-4GlcNAc, LacdiNAc)-specific lectin from Basidiomycete C. nebularis. J. Biol. Chem. 2012, 287, 10602–10612. [Google Scholar] [CrossRef] [PubMed]
- Matsushima-Hibiya, Y.; Watanabe, M.; Hidari, K.I.; Miyamoto, D.; Suzuki, Y.; Kasama, T.; Kasama, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Identification of glycosphingolipid receptors for pierisin-1, a guanine-specific ADP-ribosylating toxin from the cabbage butterfly. J. Biol. Chem. 2003, 278, 9972–99728. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.W.; Buckley, J.T.; Postma, J.P.; Tucker, A.D.; Leonard, K.; Pattus, F.; Tsernoglou, D. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature 1994, 367, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Lingwood, C.A.; Taga, S.; Caillou, B.; Tursz, T.; Wiels, J. Apoptosis induced in Burkitt’s lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Res. 1993, 53, 5314–5319. [Google Scholar] [PubMed]
- Bovi, M.; Cenci, L.; Perduca, M.; Capaldi, S.; Carrizo, M.E.; Civiero, L.; Chiarelli, L.R.; Galliano, M.; Monaco, H.L. BEL β-trefoil: A novel lectin with antineoplastic properties in king bolete (Boletus edulis) mushrooms. Glycobiology 2013, 23, 578–592. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, P.P.; Danks, M.K.; Kriwacki, R.W.; Harris, L.C. p21Waf1/Cip1 dysfunction in neuroblastoma: A novel mechanism of attenuating G0-G1 cell cycle arrest. Cancer Res. 2003, 63, 3840–3844. [Google Scholar] [PubMed]
- Park, J.S.; Carter, S.; Reardon, D.B.; Schmidt-Ullrich, R.; Dent, P.; Fisher, P.B. Roles for basal and stimulated p21Cip-1/WAF1/MDA6 expression and mitogen-activated protein kinase signaling in radiation-induced cell cycle checkpoint control in carcinoma cells. Mol. Biol. Cell 1999, 10, 4231–4246. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Pellieux, C.; Briviba, K.; Pierlot, C.; Aubry, J.M.; Sies, H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur. J. Biochem. 1999, 260, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Bavaria, M.N.; Jin, S.; Ray, R.M.; Johnson, L.R. The mechanism by which MEK/ERK regulates JNK and p38 activity in polyamine depleted IEC-6 cells during apoptosis. Apoptosis 2014, 19, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, A.D.; Lei, Y.M.; Shan, G.Q.; Zhang, L.Y.; Lu, X.; Chen, Z.L. Mannose-binding lectin inhibits monocyte proliferation through transforming growth factor-â1 and p38 signaling pathways. PLoS ONE 2013, 8, e72505. [Google Scholar] [CrossRef] [PubMed]
- Tamma, S.M.; Kalyanaraman, V.S.; Pahwa, S.; Dominguez, P.; Modesto, R.R. The lectin jacalin induces phosphorylation of ERK and JNK in CD4+ T cells. J. Leukoc. Biol. 2003, 73, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Jain, K.; Basu, A. Regulation of autophagy by kinases. Cancers (Basel) 2011, 3, 2630–2654. [Google Scholar] [CrossRef] [PubMed]
- Favata, M.F.; Horiuchi, K.Y.; Manos, E.J.; Daulerio, A.J.; Stradley, D.A.; Feeser, W.S.; van Dyk, D.E.; Pitts, W.J.; Earl, R.A.; Hobbs, F.; et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998, 273, 18623–18632. [Google Scholar] [CrossRef] [PubMed]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yuan, H.; Liu, W.; Li, S.; Liu, Y.; Wan, J.; Li, X.; Zhang, R.; Chang, Y. Activation of RAW264.7 mouse macrophage cells in vitro through treatment with recombinant ricin toxin-binding subunit B: Involvement of protein tyrosine, NF-êB and JAK-STAT kinase signaling pathways. Int. J. Mol. Med. 2013, 32, 729–735. [Google Scholar] [PubMed]
- Betti, M.; Ciacci, C.; Lorusso, L.C.; Canonico, B.; Falcioni, T.; Gallo, G.; Canesi, L. Effects of tumour necrosis factor á (TNFá) on Mytilus haemocytes: Role of stress-activated mitogen-activated protein kinases (MAPKs). Biol. Cell 2006, 98, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Toubiana, M.; Rosani, U.; Giambelluca, S.; Cammarata, M.; Gerdol, M.; Pallavicini, A.; Venier, P.; Roch, P. Toll signal transduction pathway in bivalves: Complete cds of intermediate elements and related gene transcription levels in hemocytes of immune stimulated Mytilus galloprovincialis. Dev. Comp. Immunol. 2014, 45, 300–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Tateno, H.; Nakamura-Tsuruta, S.; Kominami, J.; Hirabayashi, J.; Nakamura, O.; Watanabe, T.; Kamiya, H.; Naganuma, T.; Ogawa, T.; et al. The function of rhamnose-binding lectin in innate immunity by restricted binding to Gb3. Dev. Comp. Immunol. 2009, 33, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, X.; Duan, X.; Cui, L.; Li, G. Exogenous expression of marine lectins DIFBL and SpRBL induces cancer cell apoptosis possibly through PRMT5-E2F-1 pathway. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.; Duan, X.; Cui, L.; Luo, J.; Li, G. Adenovirus carrying gene encoding Haliotis disus discus sialic acid binding lectin induces cancer cell apoptosis. Mar. Drugs 2014, 12, 3992–4004. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Russell, R.B. Identification of distant homologues of fibroblast growth factors suggests a common ancestor for all β-trefoil proteins. J. Mol. Biol. 2000, 302, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.E.; Cousens, L.S.; Weaver, L.H.; Matthews, B.W. Three-dimensional structure of human basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 1991, 88, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, B. Structure and function of interleukin-1, based on crystallographic and modeling studies. Biophys. J. 1992, 62, 112–115. [Google Scholar] [CrossRef]
- Fritz, T.A.; Hurley, J.H.; Trinh, L.B.; Shiloach, J.; Tabak, L.A. The beginnings of mucin biosynthesis: The crystal structure of UDP-GalNAc: Polypeptide á-N-acetylgalactosaminyltransferase-T1. Proc. Natl. Acad. Sci. USA 2004, 101, 15307–15312. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Sen, U.; Chakrabarti, C.; Dattagupta, J.K. Cryocrystallography of a Kunitz-type serine protease inhibitor: The 90 K structure of winged bean chymotrypsin inhibitor (WCI) at 2.13 Ǻ resolution. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Hosono, M.; Ogawa, Y.; Takayanagi, M.; Nitta, K. Catfish egg lectin causes rapid activation of multidrug resistance 1 P-glycoprotein as a lipid translocase. Biol. Pharm. Bull. 2005, 28, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Johansson, D.; Kosovac, E.; Moharer, J.; Ljuslinder, I.; Brännström, T.; Johansson, A.; Behnam-Motlagh, P. Expression of verotoxin-1 receptor Gb3 in breast cancer tissue and verotoxin-1 signal transduction to apoptosis. BMC Cancer 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Sugawara, S.; Hosono, M.; Tatsuta, T.; Nitta, K. Alteration of gene expression induced by Silurus asotus lectin in Burkitt's lymphoma cells. Biol. Pharm. Bull. 2008, 31, 998–1002. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.; Sugawara, S.; Fujii, Y.; Koide, Y.; Terada, D.; Iimura, N.; Fujiwara, T.; Takahashi, K.G.; Kojima, N.; Rajia, S.; et al. MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt’s Lymphoma Cells to Trigger Apoptosis through Multiple Pathways. Mar. Drugs 2015, 13, 7377-7389. https://doi.org/10.3390/md13127071
Hasan I, Sugawara S, Fujii Y, Koide Y, Terada D, Iimura N, Fujiwara T, Takahashi KG, Kojima N, Rajia S, et al. MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt’s Lymphoma Cells to Trigger Apoptosis through Multiple Pathways. Marine Drugs. 2015; 13(12):7377-7389. https://doi.org/10.3390/md13127071
Chicago/Turabian StyleHasan, Imtiaj, Shigeki Sugawara, Yuki Fujii, Yasuhiro Koide, Daiki Terada, Naoya Iimura, Toshiyuki Fujiwara, Keisuke G. Takahashi, Nobuhiko Kojima, Sultana Rajia, and et al. 2015. "MytiLec, a Mussel R-Type Lectin, Interacts with Surface Glycan Gb3 on Burkitt’s Lymphoma Cells to Trigger Apoptosis through Multiple Pathways" Marine Drugs 13, no. 12: 7377-7389. https://doi.org/10.3390/md13127071