Marine Carotenoids against Oxidative Stress: Effects on Human Health
Abstract
:1. Introduction
2. Oxidative Stress: The Role of Antioxidants

3. Bioactivity and the Protective Effects of Natural Carotenoids: New Perspectives from the Sea
3.1. Astaxanthin

3.2. Fucoxanthin
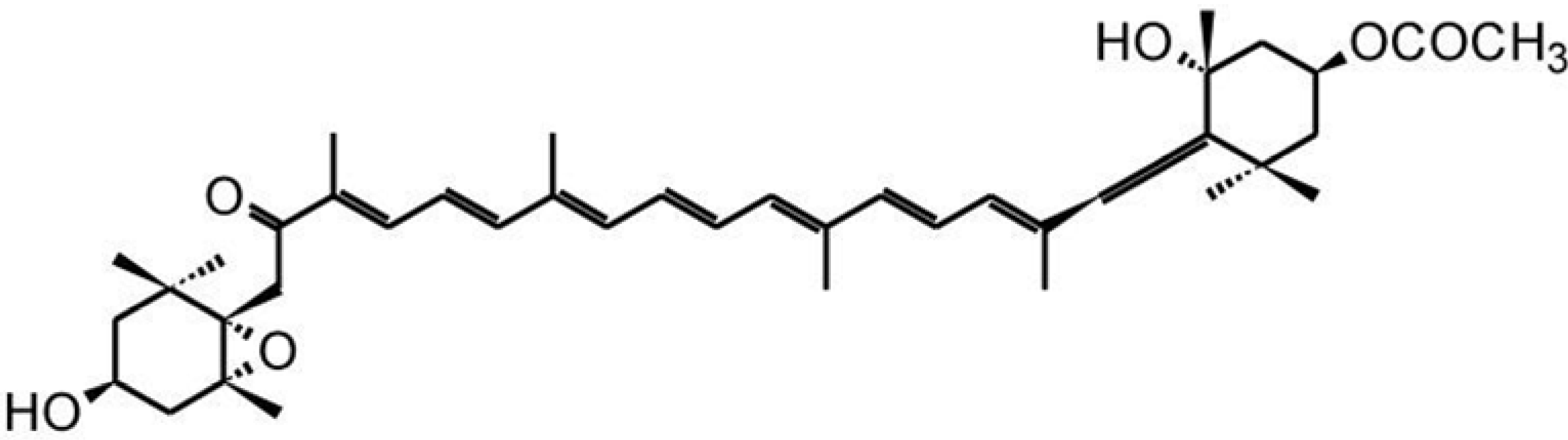
3.3. Zeaxanthin

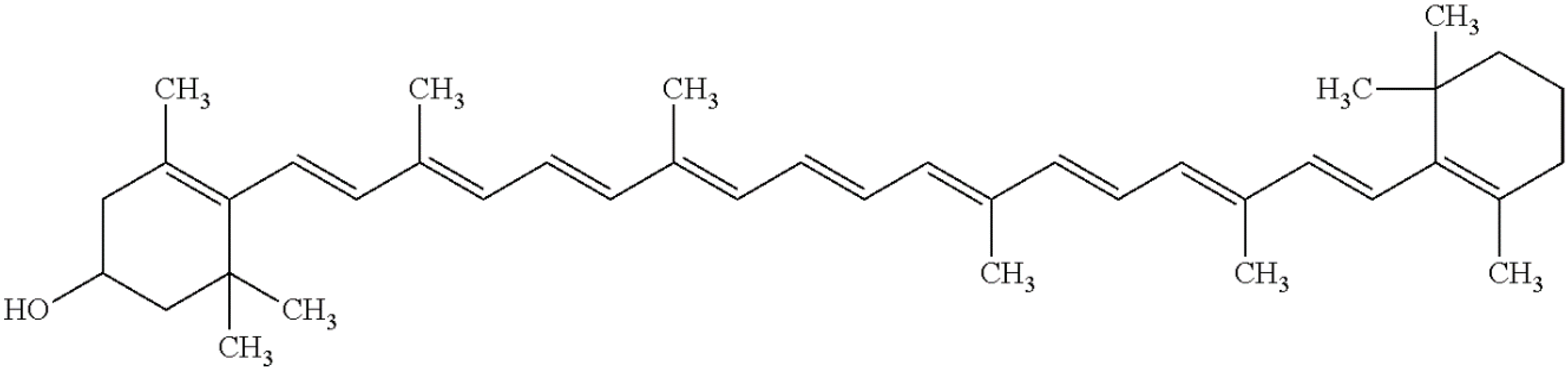
3.4. β-Cryptoxanthin
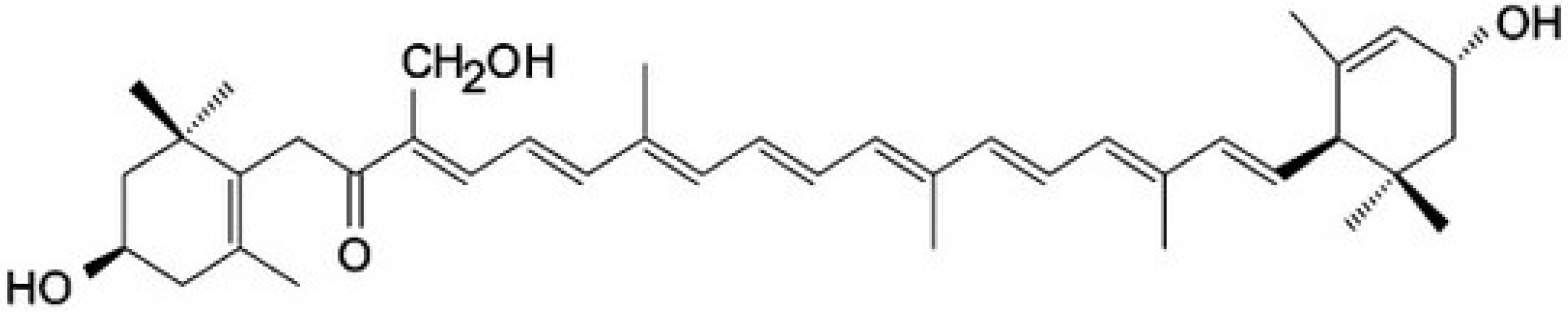
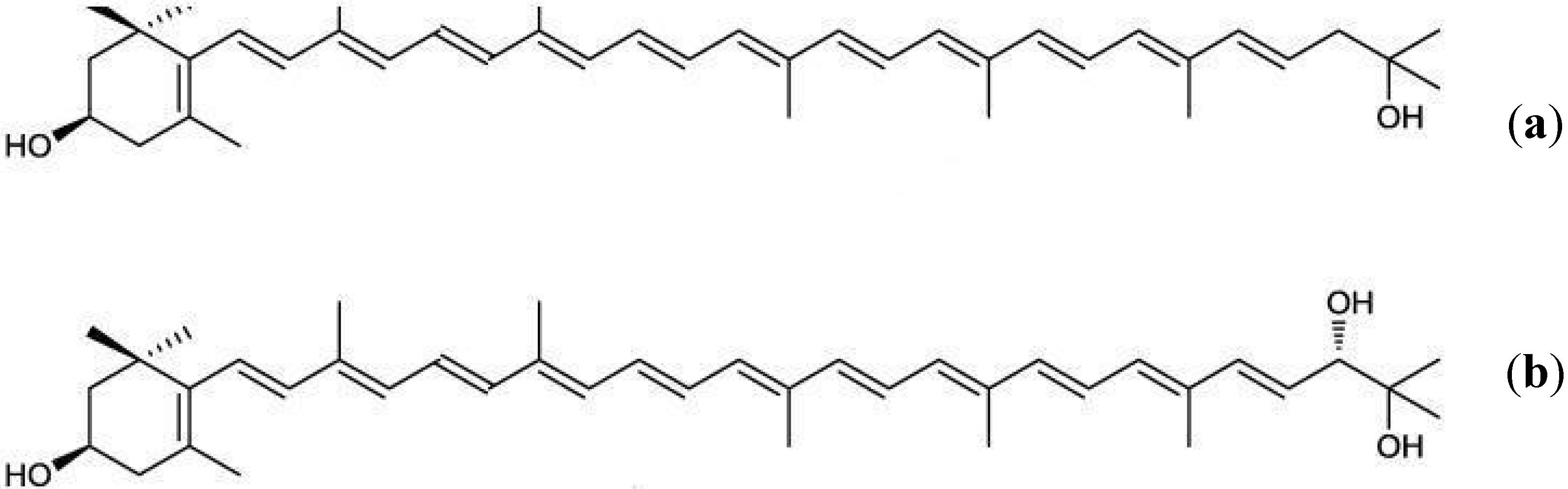
3.5. Rare Marine Carotenoids: Siphonaxanthin, Saproxanthin, and Myxol
4. Antioxidant and Pro-Oxidant Activities of Carotenoids
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gori, T.; Nzel, T.M. Oxidative stress and endothelial dysfunction: Therapeutic implications. Ann. Med. 2011, 43, 259–272. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; DeGirolamo, M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Leto, T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar] [PubMed]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: Partial reversal by feeding acetyl-l-carnitine and/or R-alpha-lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Rosenfeld, M.E.; Yla, H.S.; Gurtner, G.C.; Socher, S.S.; Butler, S.W.; Carew, T.E.; Parthasarathy, S.; Steinberg, D.; Witztum, J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA 1989, 86, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Traysman, R.J.; Kirsch, J.R.; Koehler, R.C. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J. Appl. Physiol. 1991, 71, 1185–1195. [Google Scholar]
- Erdogan, C.; Unlucerci, Y.; Turkmen, A.; Kuru, A.; Cetin, O.; Bekpinar, S. The evaluation of oxidative stress in patients with chronic renal failure. Clin. Chim. Acta 2002, 322, 157–161. [Google Scholar] [CrossRef]
- Bodamyali, T.; Kanczler, J.M.; Millar, T.M.; Stevens, C.R.; Blake, D.R. Free radicals in rheumatoid arthritis: Mediators and modulators. Oxid. Stress Dis. 2004, 10, 591–610. [Google Scholar]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo 1999, 13, 295–309. [Google Scholar] [PubMed]
- VanDenBerg, H.; Faulks, R.; Granado, H.F.; Hirschberg, J.; Olmedilla, B.; Sandmann, G.; Stahl, W.; Southon, S. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 2000, 80, 880–912. [Google Scholar] [CrossRef]
- Riccioni, G.; Speranza, L.; Pesce, M.; Cusenza, S.; D’Orazio, N. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition 2012, 28, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. Function of marine carotenoids. Forum Nutr. 2009, 61, 136–146. [Google Scholar] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, T.G. Interactions of dietary carotenoids with singlet oxygen (1O2) and free radicals: Potential effects for human health. Acta Biochim. Pol. 2012, 59, 27–30. [Google Scholar] [PubMed]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; DeLutiis, M.A. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokinessecretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Wang, S.L.; He, L.J.; He, T.B.; Han, W.; Wang, Q. Effect of astaxanthin on oxidative stress of red blood cells and peroxidation damage of membrane. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015, 23, 552–556. [Google Scholar] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; DeLutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Astaxanthin Treatment Confers Protection against Oxidative Stress in U937 Cells Stimulated with Lipopolysaccharide Reducing O2− Production. PLoS ONE 2014, 9, e8835–e8839. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar]
- Naguib, Y.M.A. Antioxidant acitivities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 1999, 70, 185–189. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.J.; Economou, M.; Wallace, A.M.; Rumley, A.; Lowe, G.; Slater, C.; Caslake, M.; Sattar, N.; Lean, M.E. Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis 2007, 193, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Akhter, S.; Hasan, A.T.; Alam, T.; Nageeb Hasan, S.M.; Saifullah, A.R.; Shohel, C. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab. Brain Dis. 2015, 27, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Rahman, M.M.; Khan, F.R.; Zaman, F.; Mahmud Reza, H. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav. Brain Res. 2015, 286, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Sajdel-Sulkowska, E.M.; Xu, M.; Koibuchi, N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum 2009, 8, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Lee, Y.-J.; Chou, M.-C.; Chang, R.; Chiu, C.-H.; Liang, Y.-J.; Wu, L.-S. Astaxanthin Protects Steroidogenesis from Hydrogen Peroxide-Induced Oxidative Stress in Mouse Leydig Cells. Mar. Drugs 2015, 13, 1375–1388. [Google Scholar] [PubMed]
- Zhang, J.; Xu, P.; Wang, Y.; Wang, M.; Li, H.; Lin, S.; Mao, C.; Wang, B.; Song, X.; Lv, C. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission. J. Cell. Mol. Med. 2015, 19, 2215–2231. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Gemello, E.; Riccioni, G.; D’Orazio, N. Marine bioactives and potential application in sports. Mar. Drugs 2014, 12, 2357–2382. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnatifida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LC-MS (APCI). Mol. Cell. Biochem. 2010, 333, 299–310. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; DeGirolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Shimomura, I.; Kihara, S.; Funahashi, T. Importance of adipokines in obesity-related diseases. Horm. Res. 2003, 60, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, M.K.; Park, Y.B.; Shin, Y.C.; Choi, M.S. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem. Toxicol. 2010, 13, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Muradian, K.; Vaiserman, A.; Min, K.J.; Fraifeld, V.E. Fucoxanthin and lipid metabolism: A minireview. Nutr. Metab. Cardiovasc. Dis. 2015, 3, S0939–S0953. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kitamura, A.; Machida, H.; Watanabe, M.; Negishi, H.; Hiraoka, J.; Nakano, T. Effect of Undaria pinnatifida on the development of cerebrovascular diseases in stroke prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Comparative effects between fucoxanthinol and its precursor fucoxanthin on viability and apoptosis of breast cancer cell lines MCF-7 and MDA-MB-231. Anticancer Res. 2015, 35, 207–219. [Google Scholar] [PubMed]
- Holden, J.M.; Eldridge, A.L.; Beecher, G.R. Carotenoid content of US foods: An update of the database. J. Food Compos. Anal. 1998, 12, 169–196. [Google Scholar] [CrossRef]
- Shahina, M.; Hameed, A.; Lin, S.Y.; Lee, R.J.; Lee, M.R.; Young, C.C. Gramella planctonica sp. nov., a zeaxanthin-producing bacterium isolated from surface seawater, and emended descriptions of Gramella aestuarii and Gramella echinicola. Antonie Van Leeuwenhoek 2014, 105, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Shahina, M.; Lin, S.Y.; Lai, W.A.; Hsu, Y.H.; Liu, Y.C.; Young, C.C. Aquibacter zeaxanthinifaciens gen. nov., sp. nov., a zeaxanthin-producing bacterium of the family Flavobacteriaceae isolated from surface seawater, and emended descriptions of the genera Aestuariibaculum and Gaetbulibacter. Int. J. Syst. Evol. Microbiol. 2014, 64, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. J. Nutr. 2002, 132, 518S–524S. [Google Scholar] [PubMed]
- Giblin, F.J. Glutathione: A vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.Y.O.; Yeum, K.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces—H2O2− induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; Sangiovanni, J.P.; Davis, M.D.; Ferris, F.L. Age-Related Eye Disease Study Research Group. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report No. 36. JAMA Ophthalmol. 2014, 132, 272–277. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Vagaggini, T.; Chen, Y.; Hu, D.N. Zeaxanthin inhibits hypoxia-induced VEGF secretion by RPE cells through decreased protein levels of hypoxia-inducible factors-1α. Biomed Res. Int. 2015, 2015, 6873–6886. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Paul-Labrador, M.J.; Fan, J. Progression of carotid intima-media thickness and plasma antioxidants: The Los Angeles Atherosclerosis Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 313–390. [Google Scholar] [CrossRef] [PubMed]
- Musch, D.C. Evidence for Including Lutein and Zeaxanthin in Oral Supplements for Age-Related Macular Degeneration. JAMA Ophthalmol. 2014, 132, 139–141. [Google Scholar] [CrossRef]
- Geisert, M.; Rose, T.; Bauer, W.; Zahn, R.K. Occurrence of carotenoids and sporopollenin in Nanochlorum eucaryotum, a novel marine alga with unusual characteristics. Biosystems 1987, 20, 133–142. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Yang, Z. Carotenoids: Epidemiology of Health Effects. In Encyclopedia of Human Nutrition, 2nd ed.; Elsevier Ltd: University of Wisconsin-Madison, Madison, WI, USA, 2005; Volume 1, pp. 339–346. [Google Scholar]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Symmons, D.P.M.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary beta-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [PubMed]
- Cerhan, J.R.; Saag, K.G.; Merlino, L.A.; Mikuls, T.R.; Criswell, L. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am. J. Epidemiol. 2003, 157, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, S.B.; Bush, A.J.; Pahor, M.; Gross, M.D. Serum carotenoids and markers of inflammation in non-smokers. Am. J. Epidemiol. 2000, 152, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ito, Y.; Ochiai, J. Relation-ship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac. J. Cancer Prev. 2003, 4, 259–266. [Google Scholar] [PubMed]
- Leoncini, E.; Edefonti, V.; Hashibe, M.; Parpinel, M.; Cadoni, G.; Ferraroni, M.; Serraino, D.; Matsuo, K.; Olshan, A.F.; Zevallos, J.P.; et al. Carotenoid intake and head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Eur. J. Epidemiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- San Millan, C.; Soldevilla, B.; Martín, P.; Gil-Calderon, B.; Compte, M.; Pérez-Sacristán, B.; Donoso, E.; Peña, C.; Romero, J.; Granado-Lorencio, F.; et al. β-Cryptoxanthin synergistically enhances the antitumoral activity of oxaliplatin through δNP73 negative regulation in colon cancer. Clin. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Yang, C.M.; Chen, J.Y.; Yueh, T.C.; Hu, M.L. Multicarotenoids at Physiological Levels Inhibit Metastasis in Human Hepatocarcinoma SK-Hep-1 Cells. Nutr. Cancer 2015, 67, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, H. Siphonaxanthin, a Green Algal Carotenoid, as a Novel Functional Compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimoto, S.; Yokono, M.; Higuchi, M.; Tomo, T.; Takaichi, S.; Murakami, A.; Mimuro, M. Solvent effects on excitation relaxation dynamics of a keto-carotenoid, siphonaxanthin. Photochem. Photobiol. Sci. 2008, 7, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qin, X.; Sang, M.; Chen, D.; Wang, K.; Lin, R.; Lu, C.; Shen, J.; Kuang, T. Spectral and functional studies on siphonaxanthin-type light-harvesting complex of photosystem II from Bryopsis corticulans. Photosynth. Res. 2013, 117, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine algal carotenoids from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K. TRAIL/Apo-2L: Mechanisms and clinical applications in cancer. Neoplasia 2001, 3, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Sugawara, T.; Matsubara, K.; Hirata, T. Inhibitory effect of carotenoids on the degranulation of mast cells via suppression of antigen-induced aggregation of high affinity IgE receptors. J. Biol. Chem. 2009, 284, 28172–28179. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigen-induced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo Sci. 2014, 63, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Atheroscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Takaichi, S. Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Mochimaru, M.; Maoka, T. Presence of free myxol and 4-hydroxymyxol and absence of myxol glycosides in Anabaena variabilis ATCC 29413, and proposal of biosynthetic pathway of carotenoids. Plant Cell Physiol. 2006, 47, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Kimura, M.; Iga, M. Potent antioxidant activity of cacalol, a sesquiterpene contained in Cacalia delphiniifolia Sleb et Zucc. Biosci. Biotechnol. Biochem. 2004, 68, 1393–1394. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar] [PubMed]
- Saw, C.L.; Yang, A.Y.; Guo, Y.; Kong, A.N. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Gammone, M.A.; Tettamanti, G.; Bergante, S.; Pulchinotta, F.; D’Orazio, N. Resveratrol and anti-atherogenic effects. Int. J. Food Sci. Nutr. 2015, 66, 603–610. [Google Scholar]
- Hubacek, J.A.; Bobkova, D. Role of cholesterol 7alpha-hydroxylase (CYP7A1) in nutrigenetics and pharmacogenetics of cholesterol lowering. Mol. Diagn. Ther. 2006, 10, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lobo, G.P.; Amengual, J.; Li, H.N.M.; Golczak, M.; Bonet, M.L.; Palczewski, K.; VonLintig, J. Beta-Carotene Decreases PPAR-alpha Activity and Reduces Lipid Storage Capacity of Adipocytes in a beta-Carotene Oxygenase 1-dependent Manner. J. Biol. Chem. 2010, 285, 27891–27899. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, V.; Jailkhani, R. Oxidative stress in non insulin dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar]
- Virtamo, J.; Pietinen, P.; Huttunen, J.K.; Korhonen, P.; Malila, N.; Virtanen, M.J.; Albanes, D.; Taylor, P.R.; Albert, P.; ATBC Study Group. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: A post-intervention follow-up. JAMA 2003, 290, 476–485. [Google Scholar] [PubMed]
- Omenn, G.S.; Goodman, G.; Thornquist, M.; Grizzle, J.; Rosenstock, L.; Barnhart, S.; Balmes, J.; Cherniack, M.G.; Cullen, M.R.; Glass, A. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994, 54, 2038S–2043S. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammone, M.A.; Riccioni, G.; D'Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226-6246. https://doi.org/10.3390/md13106226
Gammone MA, Riccioni G, D'Orazio N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Marine Drugs. 2015; 13(10):6226-6246. https://doi.org/10.3390/md13106226
Chicago/Turabian StyleGammone, Maria Alessandra, Graziano Riccioni, and Nicolantonio D'Orazio. 2015. "Marine Carotenoids against Oxidative Stress: Effects on Human Health" Marine Drugs 13, no. 10: 6226-6246. https://doi.org/10.3390/md13106226





