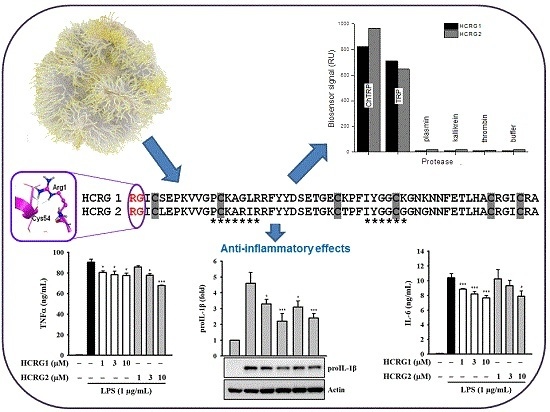
New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis crispa
Abstract
:1. Introduction
2. Results and Discussion
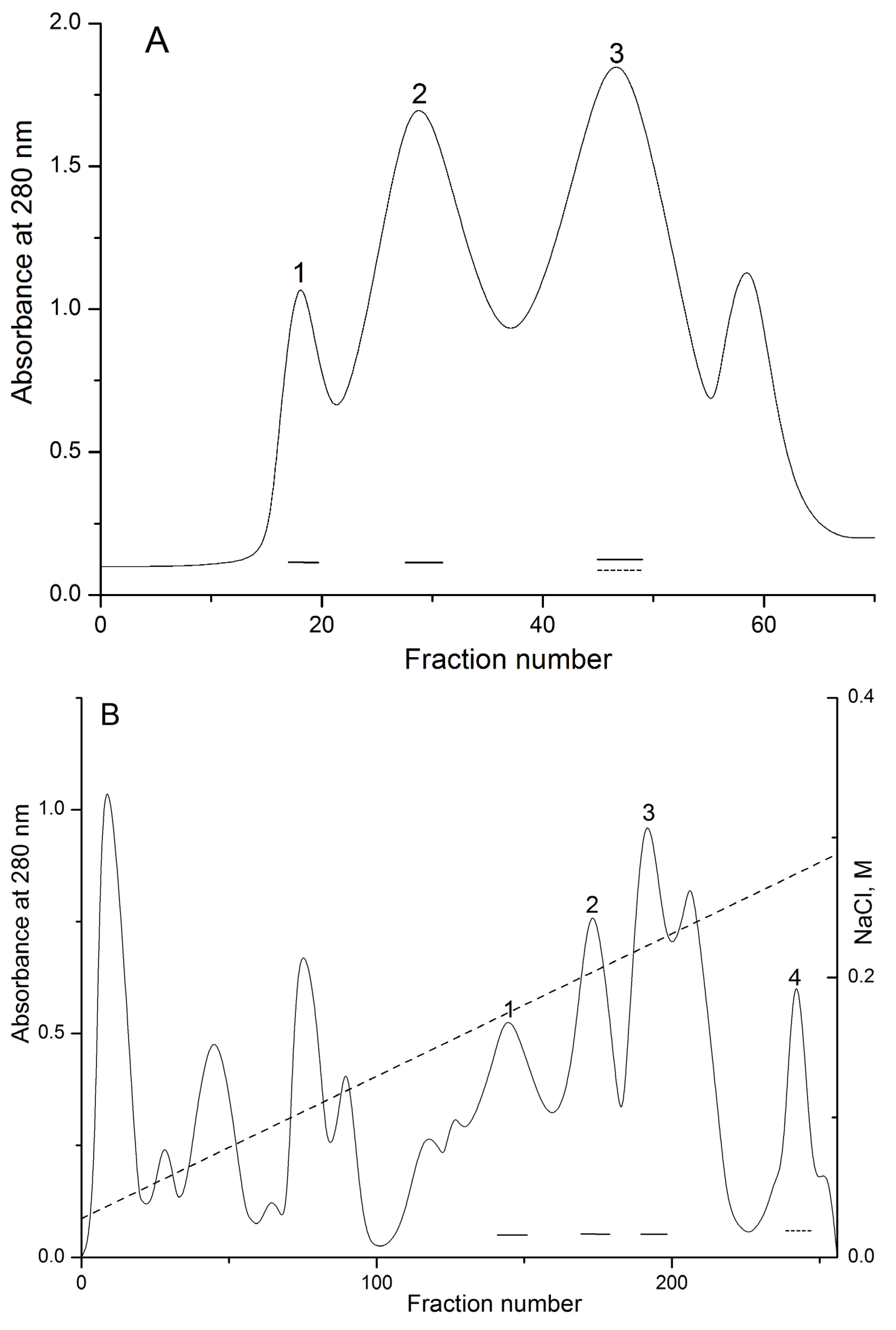
2.1. Isolation and Polypeptide Amino Acid Sequence Determination


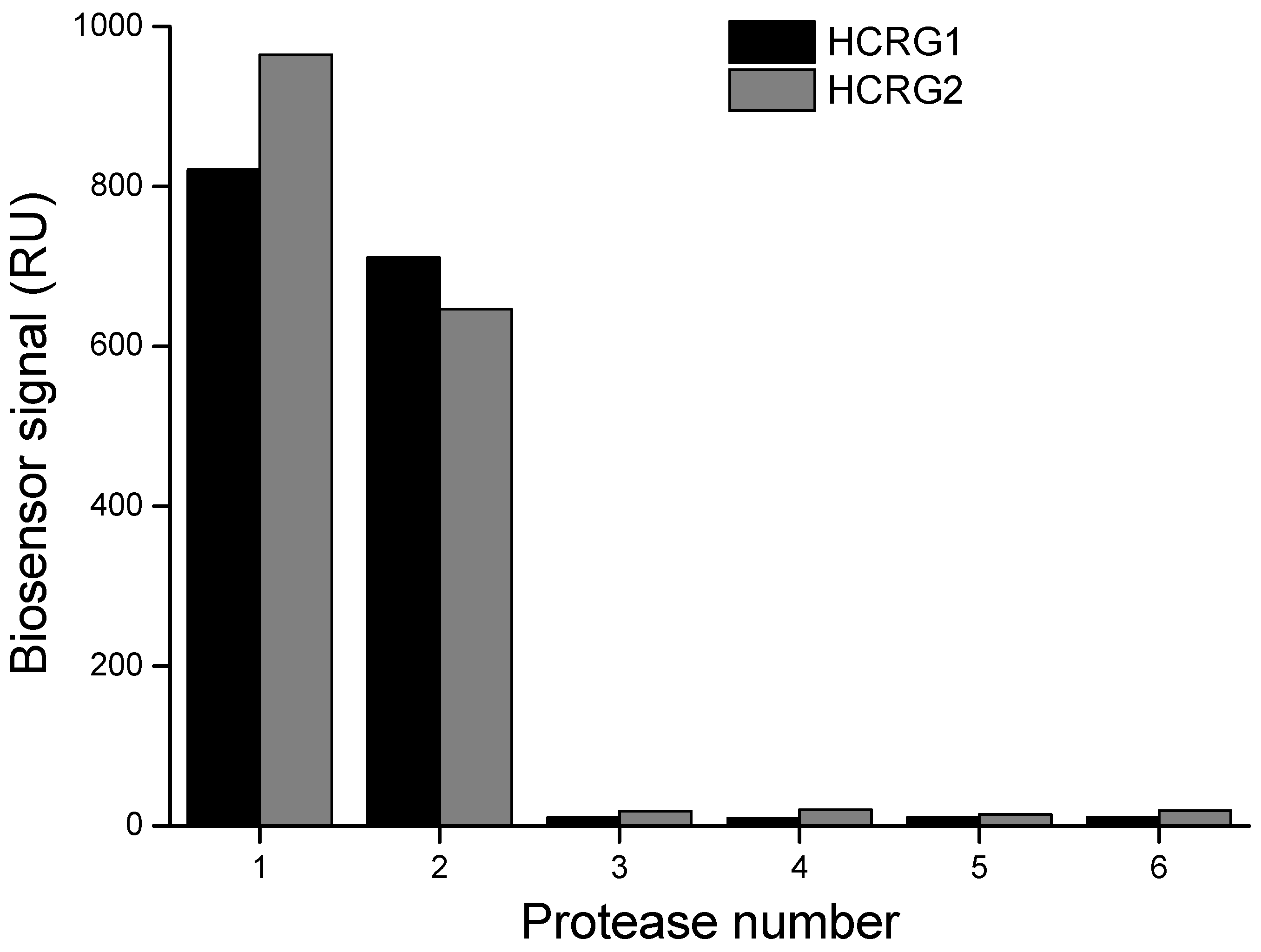
2.2. Interaction of HCRG1 and HCRG2 with Serine Proteases
| Sea Anemone | Peptide | Main Contact Site | Weak Contact Site | Inhibitory Activity against Trypsin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of aa | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 33 | 34 | 35 | 36 | 37 | 38 | ||
| H. crispa | HCRG1 | G | P | C | K | A | G | L | I | Y | G | G | C | K | 28 * |
| HCRG2 | G | P | C | K | A | R | I | I | Y | G | G | C | G | 50 * | |
| InhVJ [26] | G | P | C | T | A | Y | F | I | Y | G | G | C | E | 73.8 * | |
| Jn-IV [23] | G | P | C | T | A | Y | F | I | Y | G | G | C | E | 9.6 * | |
| APHC1 [29] | G | P | C | T | A | Y | F | I | Y | G | G | C | E | 1000 * | |
| APHC2 [30] | G | P | C | T | A | Y | F | I | Y | G | G | C | E | 900 * | |
| APHC3 [30] | G | P | C | T | A | Y | F | I | Y | G | G | C | E | 500 * | |
| S. helianthus | SHPI-1 [27] | G | R | C | K | G | Y | F | I | Y | G | G | C | G | 0.11 * |
| A. sulcata | AsKC1 [16] | G | R | C | R | A | S | H | I | Y | G | G | C | R | <30 * |
| AsKC2 [16] | G | R | C | R | A | R | H | I | Y | G | G | C | R | <30 * | |
| AsKC3 [16] | G | R | C | R | A | R | F | I | Y | G | G | C | G | <30 * | |
| S. haddoni | SHTX III [17] | P | K | C | R | G | Y | F | I | Y | G | G | C | G | 203 IU/mg ** |
| A. elegantissima | APEKTx1 [18] | G | F | C | R | A | R | F | Y | Y | G | G | C | G | 120 * |
| B. taurus | BPTI [35] | G | P | C | K | A | R | I | V | Y | G | G | C | R | 0.00006 * |

| Complex | ka, (M−1s−1) | kd, (s−1) | KD, (M) | ΔH, kJ/mol | −TΔS, kJ/mol | ΔG, kJ/mol |
|---|---|---|---|---|---|---|
| HCRG1/TRP | (1.7 ± 0.03) × 105 | (3.7 ± 0.2) × 10−5 | (2.2 ± 0.1) × 10−10 | 33 ± 4 | −88 | −55 |
| HCRG2/TRP | (1.1 ± 0.02) × 105 | (7.7 ± 0.3) × 10−5 | (6.8 ± 0.3) × 10−10 | 39 ± 5 | −92 | −53 |
| HCRG1/ChTRP | (3.6 ± 0.2) × 105 | (6.2 ± 0.1) × 10−4 | (1.8 ± 0.1) × 10−9 | 28 ± 3 | −77 | −49 |
| HCRG2/ChTRP | (6.6 ± 0.1) × 105 | (1.0 ± 0.01) × 10−3 | (1.6 ± 0.03) × 10−9 | 10 ± 2 | −60 | −50 |
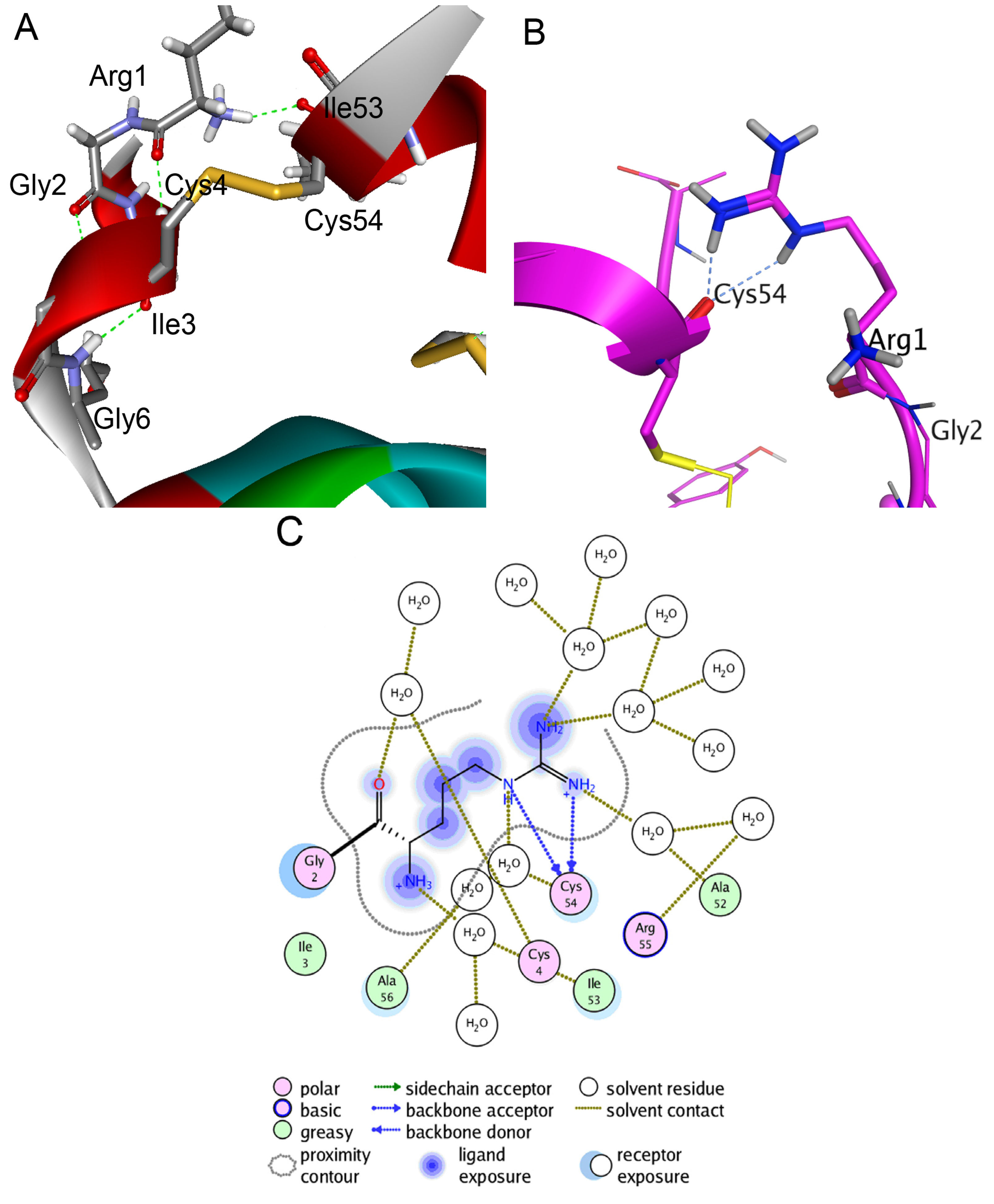
2.3. Structure Modeling

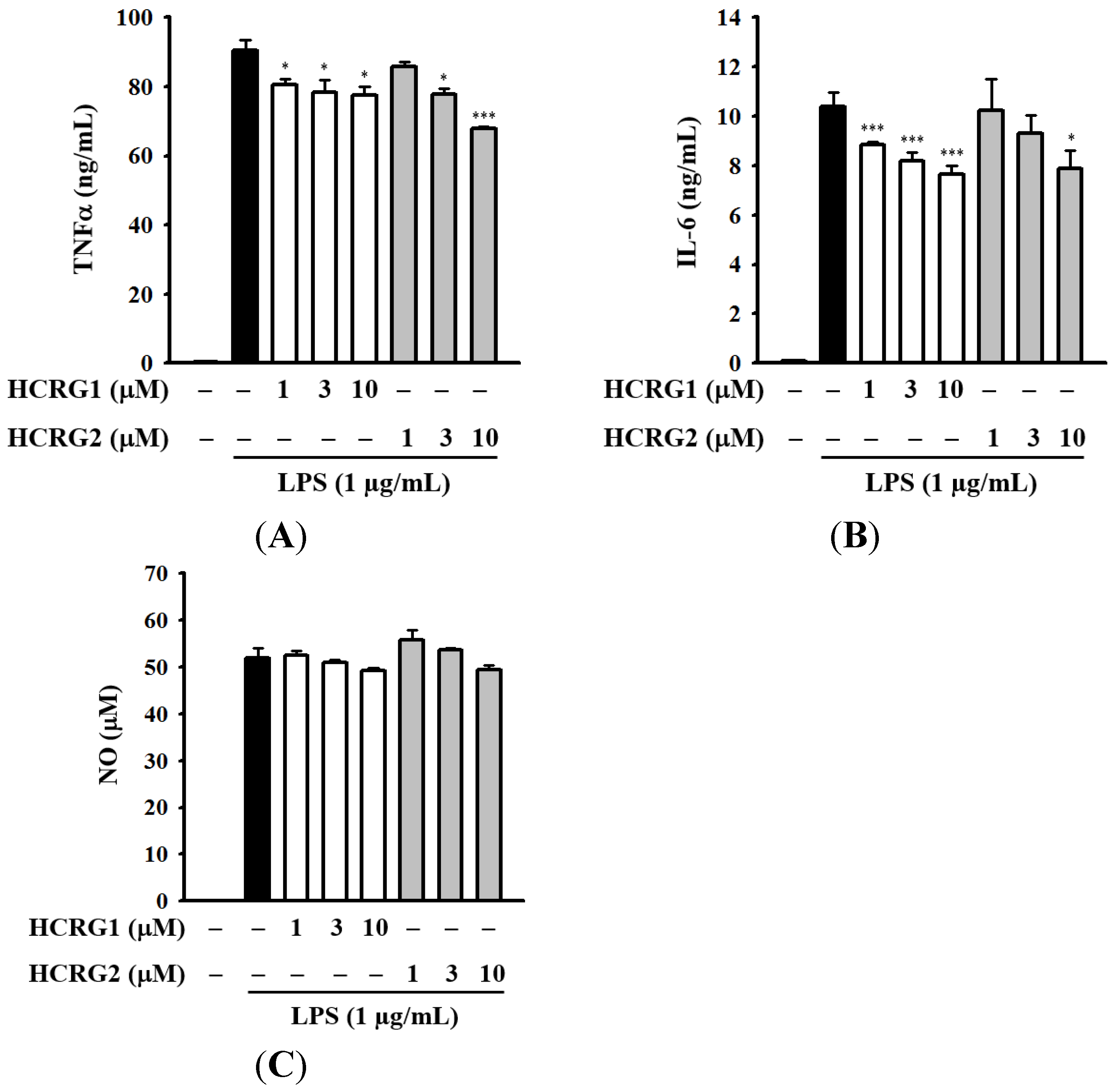
2.4. Determination of HCRG1 and HCRG2 Anti-Inflammatory Activity

3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Isolation and Polypeptide Amino Acid Sequence Determination
3.2.2. Physicochemical Characterization of HCRG1 and HCRG2
3.2.3. Hemolytic Activity
3.2.4. Trypsin Inhibitory Activity
3.2.5. SPR Measurements
3.2.6. Cell Cultures
3.2.7. Detection of proIL-1β
3.2.8. Detection of TNF-α and IL-6
3.2.9. Detection of NO
3.2.10. Statistical Analyses
3.2.11. Structure Modeling of HCRG Polypeptides and HCRGs–Serine Protease Complexes
Protein–Protein Docking
Molecular Dynamics Simulation
Computational Mutagenesis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steele, R.E.; David, C.N.; Technau, U. A genomic view of 500 million years of cnidarian evolution. Trends Genet. 2011, 27, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Frasao, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actinaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.A.; Cassoli, J.S.; Sa, F.; Dong, Z.Q.; de Freitas, J.C.; Pimenta, A.M.C.; de Lima, M.E.; Konnoe, K.; Ming Yuen Leec, S.; Garateixa, A.; et al. Peptide fingerprinting of the neurotoxic fractions isolated from the secretions of sea anemones Stichodactyla helianthus and Bunodosoma granulifera. New members of the APETx-like family identified by a 454 pyrosequencing approach. Peptides 2012, 34, 26–38. [Google Scholar]
- Dutertre, S.; Lewis, R.J. Use of Venom Peptides to Probe Ion Channel Structure and Function. J. Biol. Chem. 2011, 285, 13315–13320. [Google Scholar] [CrossRef] [PubMed]
- Кozlov, S.A.; Osmakov, D.I.; Andreev, Ia.A.; Koshelev, S.G.; Gladkikh, I.N.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Polypeptide toxin from sea anemone inhibiting proton-sensitive channel ASIC3. Russ. J. Bioorg. Chem. 2012, 38, 578–583. [Google Scholar]
- Valle, A.; Alvarado-Mesén, J.; Lanio, M.E.; Álvarez, C.; Barbosa, J.A.R.G.; Pazos, I.F. The multigene families of actinoporins (part I): Isoforms and genetic structure. Toxicon 2015, 103, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Maček, P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Pane, L. Cytotoxic and Cytolytic Cnidarian Venoms. A Review on Health Implications and Possible Therapeutic Applications. Toxins 2014, 6, 108–151. [Google Scholar] [CrossRef] [PubMed]
- Monastyrnaya, M.; Leychenko, E.; Issaeva, M.; Likhatskaya, G.; Zelepuga, E.; Kostina, E.; Trifonov, E.; Nurminski, E.; Kozlovskaya, E. Actinoporins from the sea anemones, tropical Radianthus macrodactylus and northern Oulactis orientalis: Comparative analysis of structure-function relationships. Toxicon 2010, 56, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.B.F.; Schwartz, E.F. Protease Inhibitors from Marine Venomous Animals and Their Counterparts in Terrestrial Venomous Animals. Mar. Drugs 2013, 11, 2069–2112. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; McManus, D.P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 2013, 39, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.; Dufton, M.J. Evolutionary trace analysis of the Kunitz/BPTI family of proteins: Functional divergence may have been based on conformational adjustment. J. Mol. Biol. 1999, 285, 1589–1607. [Google Scholar] [CrossRef] [PubMed]
- Kunitz, M.; Northrop, J. Isolation from beef pancreas of crystalline trypsinogen, trypsin, a trypsin inhibitor and intibular trypsin compound. J. Gen. Physiol. 1936, 19, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Czapinska, H.; Otlewski, J. Structural and energetic determinants of the S1-site specificity in serine proteases. Eur. J. Biochem. 1999, 260, 571–595. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, M.P.; Chausova, V.E.; Zelepuga, E.A.; Guzev, K.V.; Tabakmakher, V.M.; Kozlovskaya, E.P.; Monastyrnaya, M.M. A new multigene superfamily of Kunitz-type protease inhibitors from sea anemone Heteractis crispa. Peptides 2012, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemar, E.; Moinier, D.; Lancelin, J.M.; Béress, L.; Lazdunski, M. Kalicludines and Kaliseptine: Two different classes of sea anemone toxins for voltage sensitive K+ cannels. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef] [PubMed]
- Honma, T.; Kawahata, S.; Ishida, M.; Nagai, H.; Nagashima, Y.; Shiomi, K. Novel peptide toxins from the sea anemone Stichodactyla haddoni. Peptides 2008, 29, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Béress, L.; Tytgat, J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharmacol. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wunderer, G.; Machleidt, W.; Fritz, H. The broad-specificity proteinase inhibitor 5 II from the sea anemone Anemonia sulcata. Methods Enzymol. 1981, 88, 816–820. [Google Scholar]
- Minagawa, S.; Ishida, M.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Isolation and amino acid sequences of two Kunitz-type protease inhibitors from the sea anemone Anthopleura aff. xanthogrammica. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 381–386. [Google Scholar] [CrossRef]
- Minagawa, S.; Ishida, M.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Amino acid sequence and biological activities of another Kunitz-type protease inhibitor from the sea anemone Anthopleura aff. xanthogrammica. Fish Sci. 1998, 64, 155–159. [Google Scholar]
- Ishida, M.; Minagawa, S.; Miyauchi, K.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Amino acid sequences of Kunitz-type protease inhibitors from the sea anemone Actinia equina. Fish. Sci. 1997, 63, 794–798. [Google Scholar]
- Zykova, T.A.; Vinokurov, L.M.; Markova, L.F.; Kozlovskaya, E.P.; Elyakov, G.B. Amino-acid sequence of trypsin inhibitor IV from Radianthus macrodactylus. Bioorg. Khim. 1985, 11, 293–301. [Google Scholar]
- Sokotun, I.N.; Il’ina, A.P.; Monastyrnaya, M.M.; Leychenko, E.V.; Es’kov, A.A.; Anastuk, S.D.; Kozlovskaya, E.P. Proteinase inhibitors from the tropical sea anemone Radianthus macrodactylus: Isolation and characteristic. Biochem. (Mosc.) 2007, 72, 301–306. [Google Scholar] [CrossRef]
- Sokotun, I.N.; Leichenko, E.V.; Vakorina, T.I.; Es’kov, A.A.; Il’ina, A.P.; Monastyrnaia, M.M.; Kozlovskaia, E.P. A serine protease inhibitor from the anemone Radianthus macrodactylus: Isolation and physicochemical characteristics. Russ. J. Bioorg. Chem. 2007, 33, 415–422. [Google Scholar] [CrossRef]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; et al. Atypical reactive center Kunitz-type inhibitor from the sea anemone Heteractis crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef] [PubMed]
- Delfín, J.; Martínez, I.; Antuch, W.; Morera, V.; González, Y.; Rodríguez, R.; Márquez, M.; Saroyán, A.; Larionova, N.; Díaz, J.; et al. Purification, characterization and immobilization of proteinase inhibitors from Stichodactyla helianthus. Toxicon 1996, 34, 1367–1376. [Google Scholar] [CrossRef]
- Díaz, J.; Morera, V.; Delfín, J.; Huerta, V.; Lima, G.; Rodriguex de la Vega, M.; Garcia, B.; Padrón, G.; Assfalg-Machleidt, I.; Machleidt, W.; et al. Purification and partial characterization of a novel proteinase inhibitor from the sea anemone Stichodactyla helianthus. Toxicon 1998, 36, 1275–1276. [Google Scholar]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.A.; Andreev, Y.A.; Murashev, A.N.; Skobtsov, D.I.; D’yachenko, I.A.; Grishin, E.V. New polypeptide components from the Heteractis crispa sea anemone with analgesic activity. Russ. J. Bioorg. Chem. 2009, 35, 711–719. [Google Scholar] [CrossRef]
- Shigetomi, H.; Onogi, A.; Kajiwara, H.; Yoshida, S.; Furukawa, N.; Haruta, S.; Tanase, Y.; Kanayama, S.; Noguchi, T.; Yamada, Y.; et al. Anti-inflammatory actions of serine protease inhibitors containing the Kunitz domain. Inflamm. Res. 2010, 59, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bosman, M.; Royston, D. Aprotinin and renal dysfunction. Expert Opin. Drug Saf. 2008, 7, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Sintsova, O.V.; Monastyrnaya, M.M.; Pislyagin, E.A.; Menchinskaya, E.S.; Leychenko, E.V.; Aminin, D.L.; Kozlovskaya, E.P. Anti-inflammatory Activity of the Polypeptide of the Sea Anemone, Heteractis crispa. Bioorg. Chem. 2015, 41. in press. [Google Scholar]
- Monastyrnaya, M.M.; Zykova, T.A.; Apalikova, O.V.; Shwets, T.V.; Kozlovskaya, E.P. Biologically active polypeptides from the tropical sea anemone Radianthus macrodactylus. Toxicon 2002, 40, 1197–1217. [Google Scholar] [CrossRef]
- Creighton, T.E.; Charles, I.G. Sequences of the genes and polypeptide precursors for two bovine protease inhibitors. J. Mol. Biol. 1987, 194, 11–22. [Google Scholar] [CrossRef]
- Bode, W.; Huger, R. Structural basis of the endoproteinase-protein inhibitor interaction. Biochim. Biophys. Acta 2000, 1477, 241–252. [Google Scholar] [CrossRef]
- Zelepuga, E.A.; Tabakmakher, V.M.; Chausova, V.E.; Monastyrnaya, M.M.; Isaeva, M.P.; Kozlovskaya, E.P. Interaction of sea anemone Heteractis crispa Kunitz type polypeptides with pain vanilloid receptor TRPV1: In silico investigation. Russ. J. Bioorg. Chem. 2012, 38, 159–170. [Google Scholar] [CrossRef]
- García-Fernández, R.; Pons, T.; Perbandt, M.; Valiente, P.A.; Talavera, A.; González-González, Y.; Rehders, D.; Chávez, M.A.; Betzel, C.; Redecke, L. Structural insights into serine protease inhibition by a marine invertebrate BPTI Kunitz-type inhibitor. J. Struct. Biol. 2012, 180, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Scheidig, A.J.; Hynes, T.R.; Pelletier, L.A.; Wells, J.A.; Kossiakoff, A.A. Crystal structures of bovine chymotrypsin and trypsin complexed to the inhibitor domain of Alzheimer’s amyloid beta-protein precursor (APPI) and basic pancreatic trypsin inhibitor (BPTI): Engineering of inhibitors with altered specificities. Protein Sci. 1997, 6, 1806–1824. [Google Scholar] [CrossRef] [PubMed]
- Czapinska, H.; Helland, R.; Smalås, A.O.; Otlewski, J. Crystal structures of five bovine chymotrypsin complexes with P1 BPTI variants. J. Mol. Biol. 2004, 344, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Sokotun, I.N.; Gnedenko, O.V.; Leychenko, E.V.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Molnar, A.A.; Ivanov, A.S. Study of the interaction of trypsin inhibitor from the sea anemone Radianthus macrodactylus with proteases. Biochem. (Mosc.) Suppl. B Biomed. Chem. 2007, 1, 139–142. [Google Scholar] [CrossRef]
- Ivanov, A.S. The Study of Intermolecular Interactions Using Optical Biosensors Operating on the Effect of Surface Plasmon Resonance. CTM 2012, 4, 142–153. [Google Scholar]
- Chemical Computing Group Inc. Molecular Operating Environment (MOE) 2014.09; Chemical Computing Group Inc.: Montreal, QC, Canada, 2014. [Google Scholar]
- Krowarsch, D.; Cierpicki, T.; Jelen, F.; Otlewski, J. Canonical protein inhibitors of serine proteases. Cell. Mol. Life Sci. 2003, 60, 2427–2444. [Google Scholar] [CrossRef] [PubMed]
- Helland, R.; Otlewski, J.; Sundheim, O.; Dadlez, M.; Smalås, A.O. The crystal structures of the complexes between bovine beta-trypsin and ten P1 variants of BPTI. J. Mol. Biol. 1999, 287, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Astrup, T.; Nissen, U. Urinary trypsin inhibitor (Mingin): Transformation into a new trypsin inhibitor by acid hydrolysis or by sialidase. Nature 1964, 203, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Denda, K.; Kitamura, A.; Kawaguchi, T.; Kito, M.; Kondo, J.; Kagaya, S.; Qin, L.; Takata, H.; Miyazawa, K.; et al. Hepatocyte growth factor activator inhibitor, a novel Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 6370–6376. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.T.; Obiekwe, B.C.; Al-Ani, A.T.; Seppala, M.; Chard, T. Molecular heterogeneity of placental protein 5 (PP5) in late pregnancy serum and plasma: Evidence for a heparin-PP5 polymer. Clin. Chim. Acta 1980, 107, 211–215. [Google Scholar] [CrossRef]
- Nakamura, A.; Mori, Y.; Hagiwara, K.; Suzuki, T.; Sakakibara, T.; Kikuchi, T.; Igarashi, T.; Ebina, M.; Abe, T.; Miyazaki, J.; et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J. Exp. Med. 2003, 197, 669–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Yoshida, R.; Kanada, Y.; Fukuda, Y.; Yagyu, T.; Inagaki, K.; Kondo, T.; Kurita, N.; Suzuki, M.; Kanayama, N.; et al. Suppression of lipopolysaccharide induced cytokine production of gingival fibroblasts by a soybean, Kunitz trypsin inhibitor. J. Periodont. Res. 2005, 40, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Aosasa, S.; Ono, S.; Mochizuki, H.; Tsujimoto, H.; Ueno, C.; Matsumoto, A. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocytes. Shock 2001, 15, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshida, R.; Kanada, Y.; Fukuda, Y.; Yagyu, T.; Inagaki, K.; Kondo, T.; Kurita, N.; Yamada, Y.; Sado, T.; et al. A soybean Kunitz trypsin inhibitor reduces tumor necrosis factor-α production in ultraviolet-exposed primary human keratinocytes. Exp. Dermatol. 2005, 14, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Kozlovkaya, E.P.; Grishin, E.V. Analgesic effect of peptide inhibitor TRPV 1 receptor models of thermal pain stimulation. Dokl. Akad. Nauk Moskow 2009, 424, 688–691. [Google Scholar]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.A.; Murashev, N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs 2013, 16, 5100–5115. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kassell, B. Bovine trypsin-kallikrein inhibitor (Kunitz inhibitor, basic pancreatic trypsin inhibitor, polyvalent inhibitor from bovin organs). Methods Enzymol. 1970, 19, 844–852. [Google Scholar]
- Dixon, M.; Webb, E.C. Enzymes; Mir Publishing: Moscow, Russia, 1982; Volume 2, pp. 397–661. [Google Scholar]
- Eswar, N.; Marti-Renom, M.A.; Webb, B.; Madhusudhan, M.S.; Eramian, D.; Shen, M.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling With MODELLER. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Supplement 15, pp. 5.6:1–5.6:30. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–6512. [Google Scholar] [CrossRef] [PubMed]
- Antuch, W.; Berndt, D.K.; Chávez, A.M.; Delfín, J.; Wüthrich, K. The NMR solution structure of a Kunitz-type proteinase inhibitor from the sea anemone Stichodactyla helianthus. Eur. J. Biochem. 1993, 212, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK—A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Kozakov, D.; Brenke, R.; Comeau, S.R.; Vajda, S. PIPER: An FFT-based protein docking program with pairwise potentials. Proteins 2006, 65, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Comeau, S.R.; Gatchell, D.W.; Vajda, S.; Camacho, C.J. ClusPro: An automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 2004, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladkikh, I.; Monastyrnaya, M.; Zelepuga, E.; Sintsova, O.; Tabakmakher, V.; Gnedenko, O.; Ivanov, A.; Hua, K.-F.; Kozlovskaya, E. New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis crispa. Mar. Drugs 2015, 13, 6038-6063. https://doi.org/10.3390/md13106038
Gladkikh I, Monastyrnaya M, Zelepuga E, Sintsova O, Tabakmakher V, Gnedenko O, Ivanov A, Hua K-F, Kozlovskaya E. New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis crispa. Marine Drugs. 2015; 13(10):6038-6063. https://doi.org/10.3390/md13106038
Chicago/Turabian StyleGladkikh, Irina, Margarita Monastyrnaya, Elena Zelepuga, Oksana Sintsova, Valentin Tabakmakher, Oksana Gnedenko, Alexis Ivanov, Kuo-Feng Hua, and Emma Kozlovskaya. 2015. "New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis crispa" Marine Drugs 13, no. 10: 6038-6063. https://doi.org/10.3390/md13106038
APA StyleGladkikh, I., Monastyrnaya, M., Zelepuga, E., Sintsova, O., Tabakmakher, V., Gnedenko, O., Ivanov, A., Hua, K.-F., & Kozlovskaya, E. (2015). New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis crispa. Marine Drugs, 13(10), 6038-6063. https://doi.org/10.3390/md13106038