Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review
Abstract
:1. Introduction
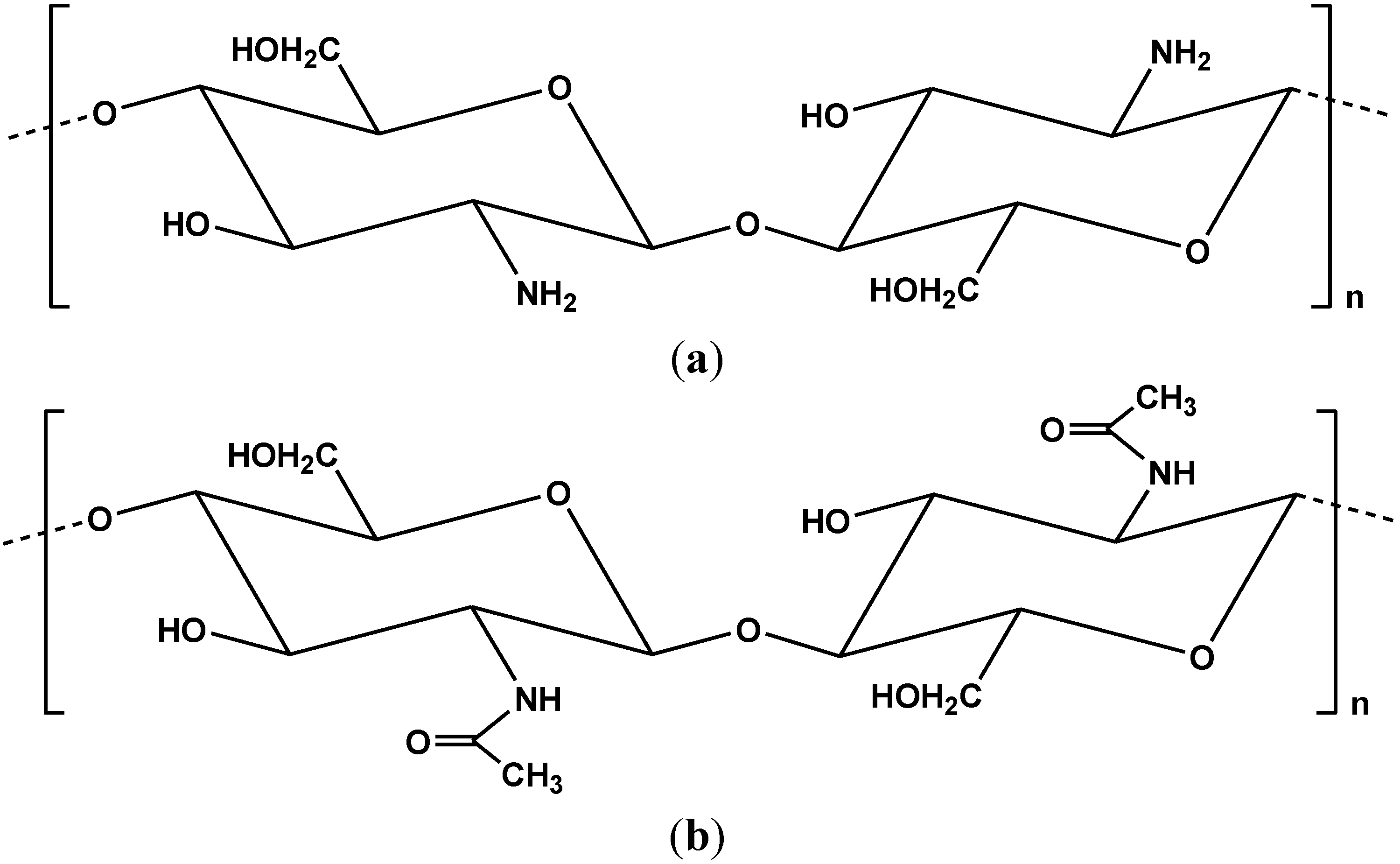
2. Modified Chitosans for Dye Adsorption

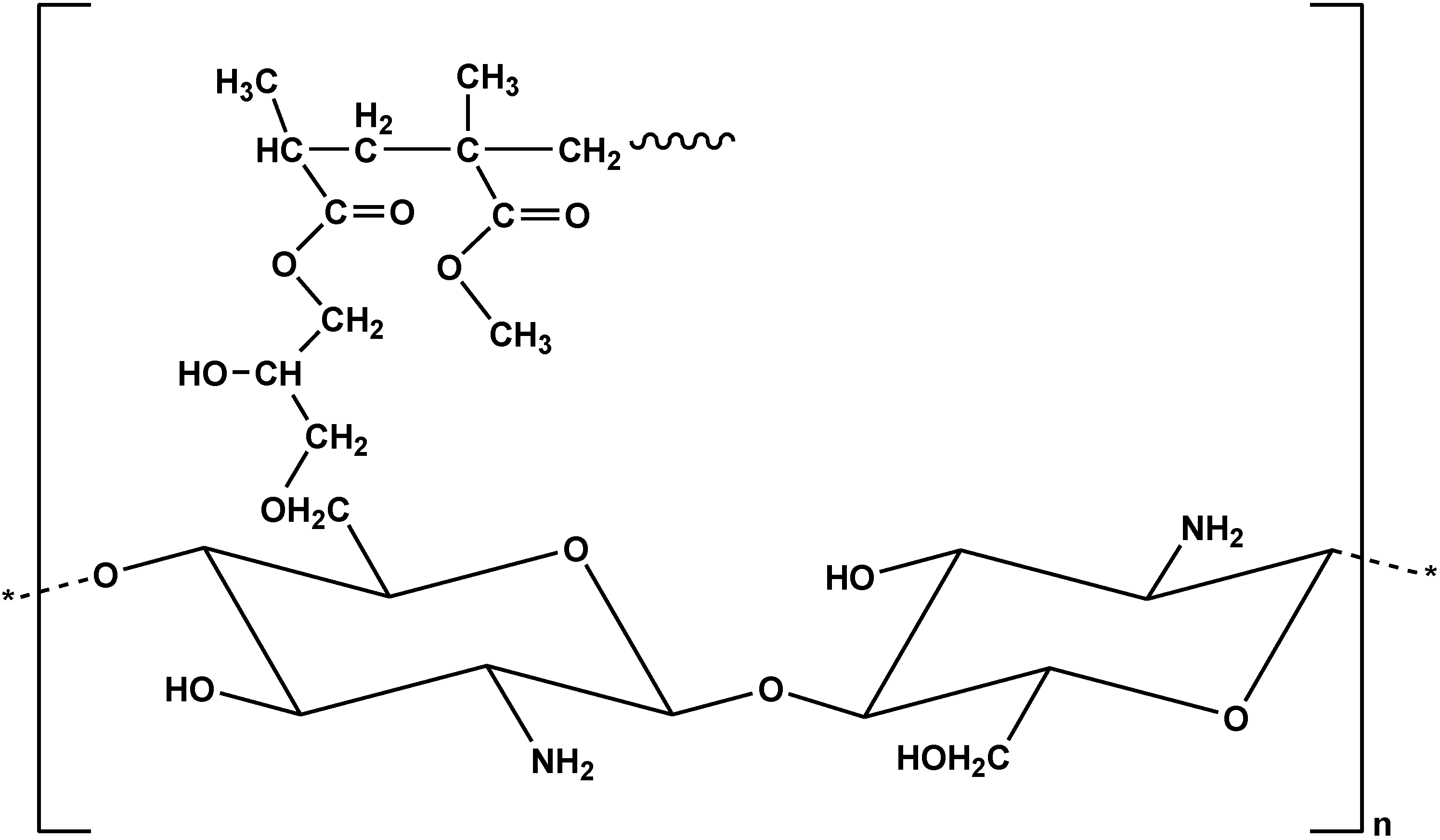

| Modified Chitosan Adsorbent | Dye | Isotherms | Qm | Ref. |
|---|---|---|---|---|
| Modified Ball clay/Chitosan composite | MB | L, F, R-P | 259.8 mg/g | [30] |
| β-cyclodextrin/chitosan magnetic nanocomposite | MB | L, F | 2788 mg/g | [31] |
| Chitosan/glutaraldehyde/3-amino-1,2,4 triazole,5-thiol resin | BBR250 | L, F | 0.8 mmol/g | [33] |
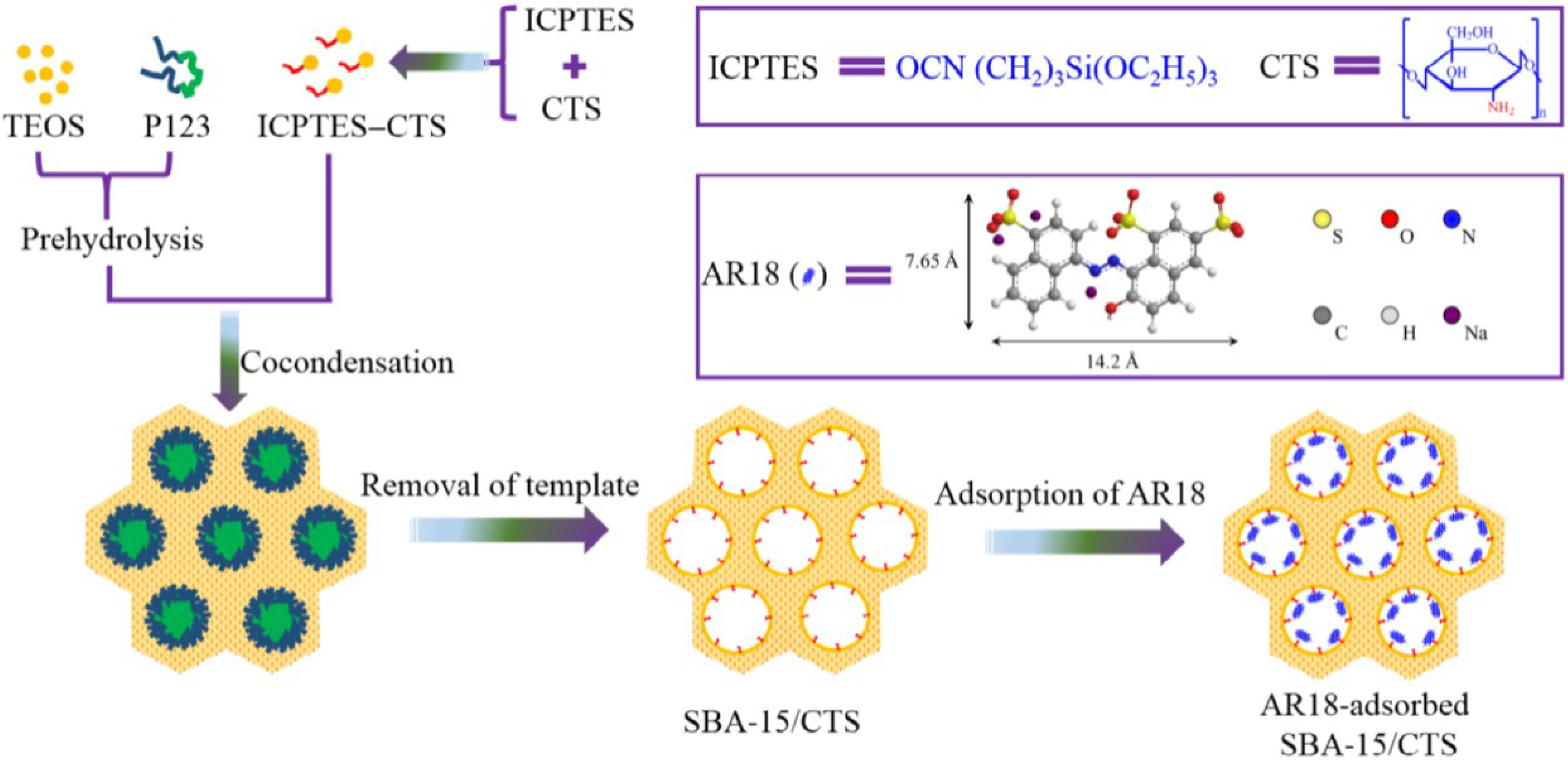
| Chitosan/SBA-15 | AR18 | L, F | 201.2 mg/g | [34] |
| Chitosan grafted with poly(methyl methacrylate) | RB19 | L | 1498 mg/g | [35] |
| Chitosan modified bentonite | WASC | H, L, F, T, D-R | 102 mg/g | [36] |
| Chitosan/CTAB modified bentonite | WASC | H, L, F, T, D-R | 175 mg/g | [36] |
| Magnetic chitosan grafted with Reactive red 120 | Lysozyme | L | 116.9 mg/g | [37] |
| Chitosan/Zeolite A | BO16 | L, F | 305.8 mg/g | [38] |
| Chitosan-modified palygorskite | RY3RS | L, F | 71.38 mg/g | [39] |
| Chitosan grafted with polypropylene imine | RB5 | L, F, T | 6250 mg/g | [40] |
| RR198 | L, F, T | 5855 mg/g | [40] | |
| Chitosan grafted with diethylenetriamine | AO7 | L, F | 6.02 mmol/g | [41] |
| AG25 | L, F | 4.37 mmol/g | [41] | |
| Chitosan-modified magnetic graphitized multi-walled carbon nanotubes | CR | L, F | 263.3 mg/g | [42] |
| Chitosan/Graphite oxide composite | RB5 | L-F | 277 mg/g | [46] |
| Magnetic chitosan/Graphite oxide nanocomposite | RB5 | L-F | 391 mg/g | [47] |
| Chitosan-Molecularly Imprinted Polymers | RR3BS | L, F | 35 mg/g | [48] |
3. Modified Chitosans for Metals/Ions Adsorption
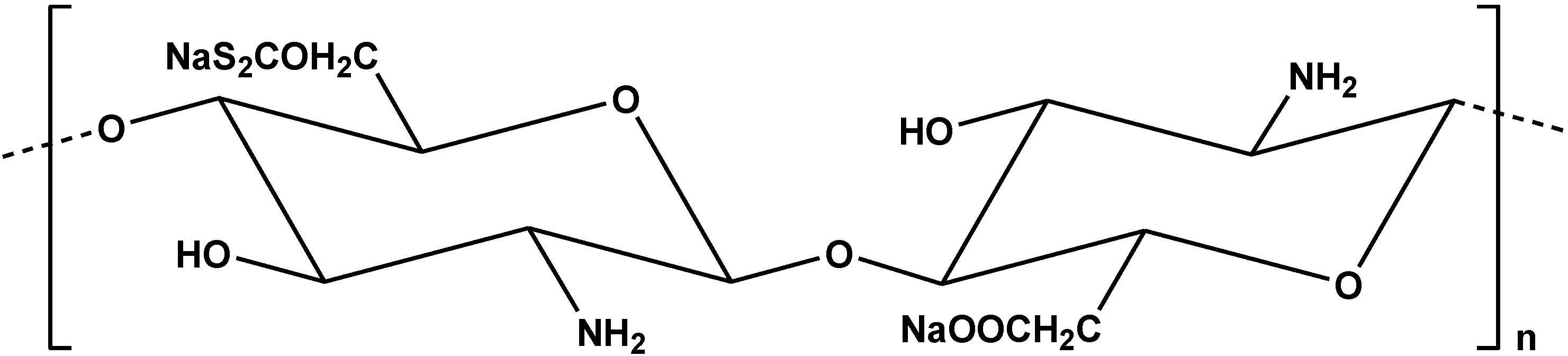
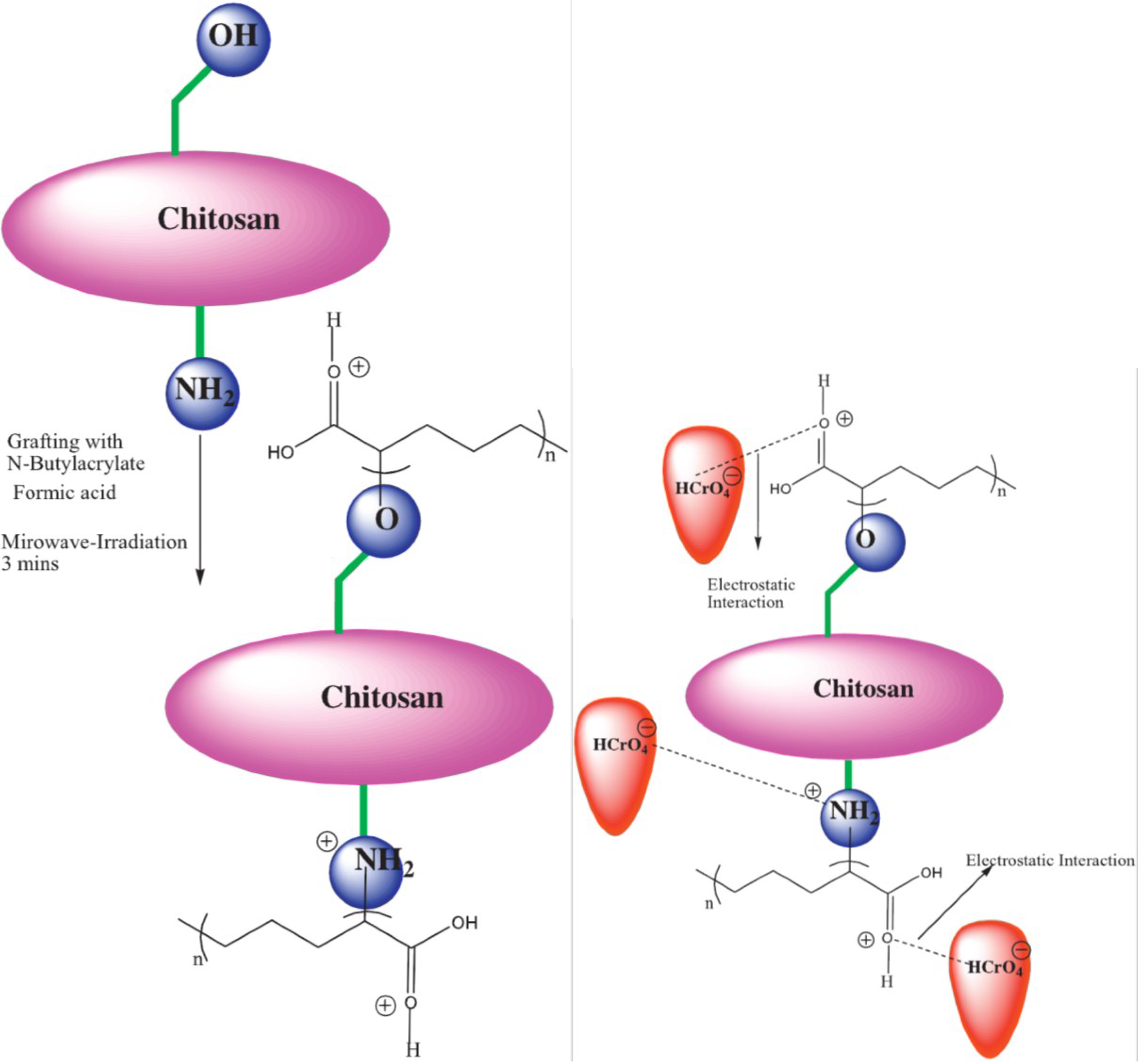
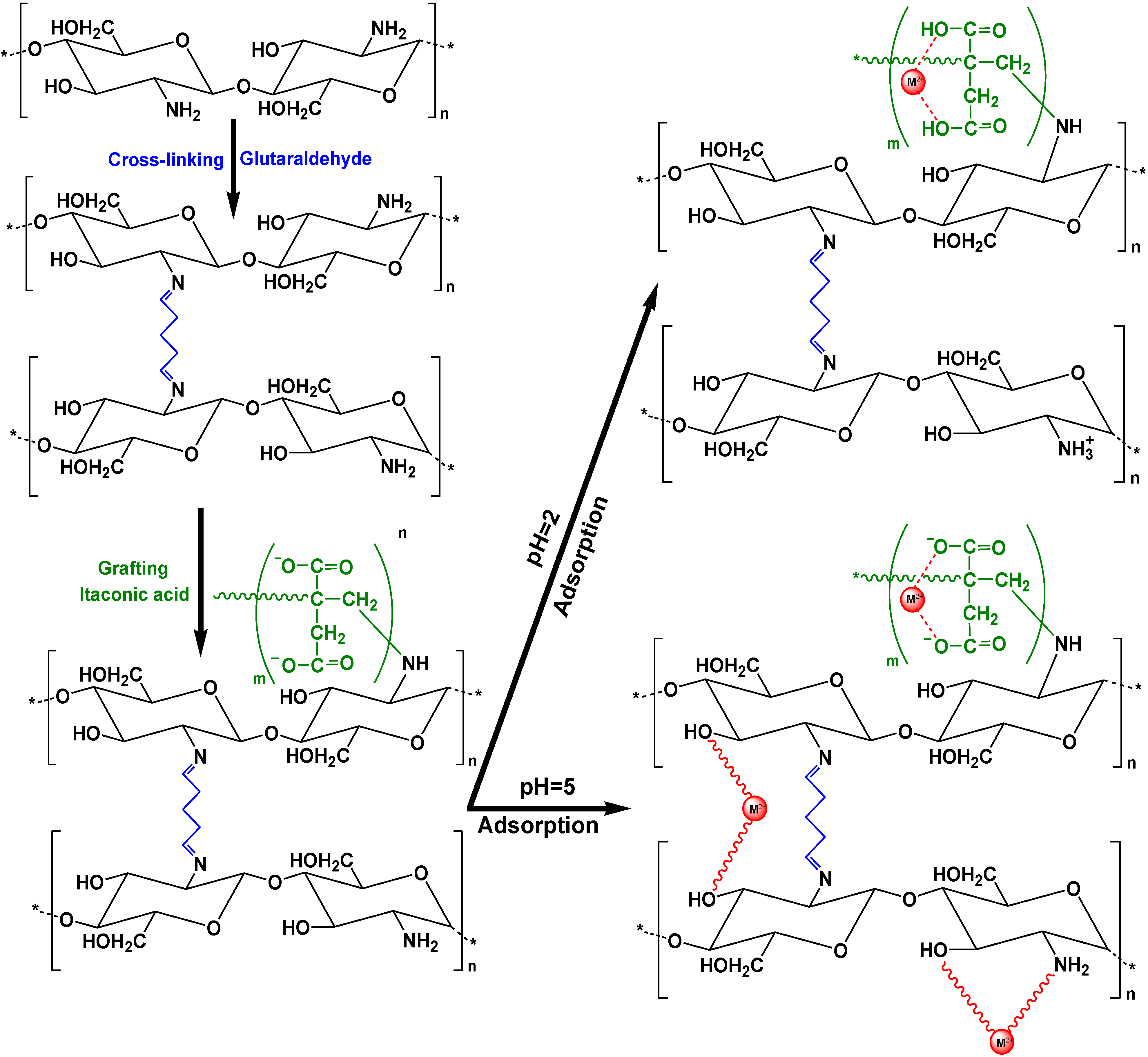
| Modified Chitosan Adsorbent | Metals/Ions | Isotherms | Qm | Ref. |
|---|---|---|---|---|
| Chitosan grafted with with 3,4-dimethoxybenzaldehyde | Cd(II) | L | 217.4 mg/g | [51] |
| Chitosan modified multiwalled carbon nanotubes | U(VI) | L | 71 mg/g | [52] |
| Xanthate-modified magnetic cross-linked chitosan | Co(II) | L, F | 18.5 mg/g | [53] |
| Ethylene-1,2-diamine-6-deoxy-chitosan | Cu(II), Pb(II), Zn(II) | L | 41.6, 31.8, 20.0 mg/g | [54] |
| Ethylene-1,2-diamine-6-deoxy-N-phthaloylchitosan | Cu(II), Pb(II), Zn(II) | L | 32.3, 28.6, 18.6 mg/g | [54] |
| Magnetically modified graphene oxide-chitosan composite | Cr(VI) | L, F, R-P | 82 mg/g | [55] |
| Magnetic cross-linked chitosan grafted with tetraethylenepentamine | UO2(II) | L, F, D-R | 486 mg/g | [57] |
| Cross-linked chitosan modified with histidine | Ni(II) | L, F | 55.6 mg/g | [58] |
| Protonated chitosan beads | Fe(III) | L, F | 7.042 mg/g | [59] |
| Carboxymethyl chitosan beads | Fe(III) | L, F | 9.346 mg/g | [59] |
| Grafted chtiosan beads | Fe(III) | L, F, T | 14.286 mg/g | [59] |
| Chitosan grafted with 4,4′-diformyl-α-ω-diphenoxy-ethane | Cu(II), Co(II), Zn(II), | L | 12, 8, 12 mg/g | [60] |
| Chitosan grafted with 4,4′-diformyl-α-ω-diphenoxy-ethane | Hg(II), Pb(II) | L | 56, 50 mg/g | [60] |
| Ethylenediamine-modified yeast biomass coated with magnetic chitosan | Pb(II) | L, F | 121.26 mg/g | [61] |
| Thiocarbohydrazide-modified chitosan | As(V), Ni(II), Cu(II) | [62] | ||
| Cd(II), Pb(II) | [62] | |||
| Thiosemicarbazide grafted chitosan | As(V), Ni(II), Cu(II) | [63] | ||
| Cd(II), Pb(II) | [63] | |||
| Thiocarbohydrazide grafted chitosan | As(V), Ni(II), Cu(II) | [63] | ||
| Cd(II), Pb(II) | [63] | |||
| Chitosan-thioglyceraldehyde Schiff’s base cross-linked magnetic resin | Hg(II), Cu(II, Zn(II) | L, F, T | 98, 76, 52 mg/g | [64] |
| Chloroacetic grafted chitosan | Co(II), Cu(II) | L | 59.1, 175.12 mg/g | [65] |
| Glycine grafted chitosan | Co(II), Cu(II) | L | 82.9, 165.91 mg/g | [65] |
| Chitosan cross-linked with glutaraldehyde | Cu(II), Hg(II) | L, F, L-F, H | 177.8, 661.5 mg/g | [66] |
| Chitosan cross-linked with epichlorohydrin | Cu(II), Hg(II) | L, F, L-F, H | 146.1, 681.7 mg/g | [66] |
| Amino terminated hyperbranched dendritic polyamidoamine 3rd generation chitosan beads | Cr(VI) | L, F, D-R | 224.2 mg/g | [67] |
| EDTA-modified chitosan | Co(II) | L, S | 79.7 mg/g | [68] |
| Chitosan grafted with n-butylacrylate | Cr(VI) | L, F, D-R, T | 17.15 mg/g | [70] |
| Xanthate carboxymethyl grafted chitosan | Cu(II), Ni(II) | L, F | 174.2, 128.4 mg/g | [69] |
| Cross-linked chitosan with citric acid | Pb(II) | L, F, T, D-R | 101.7 mg/g | [71] |
| Montmorillonite modified with chitosan | Co(II) | L, F, T | 150 mg/g | [72] |
| Triethylene-tetramine modified magnetic chitosan | Th(IV) | L, F, T | 133.3 mg/g | [73] |
| Diethylenetriamine-functionalized magnetic chitosan | U(VI) | L, F, S, D-R | 65.16 mg/g | [74] |
| Magnetic chitosan grafted with α-ketoglutaric acid | Cd(II) | L, F, T | 201.2 mg/g | [75] |
| Magnetic chitosan | Hg(II) | L, F | 155 mg/g | [76] |
| Chitosan grafted with itaconic acid | Cd(II), Pb(II) | L-F | 405, 334 mg/g | [77] |
4. Modified Chitosans for Other Species Adsorption
| Modified Chitosan Adsorbent | Pollutants | Isotherms | Qm | Ref. |
|---|---|---|---|---|
| Chitosan cross-linked with glutaraldehyde | p-nitrophenol | [78] | ||
| Magnetic chitosan | Diclofenac | L, F | 191.2 mg/g | [82] |
| Clofibric acid | L, F | 57.5mg/g | [82] | |
| Carbamazepine | L, F | - | [82] | |
| β-cyclodextrin/cross-linked chitosan | Phenol | L, F | 59.74 mg/g | [83] |
| 2-chlorophenol | L, F | 70.52 mg/g | [83] | |
| 4-chlorophenol | L, F | 96.43 mg/g | [83] | |
| 2,4-dichlorophenol | L, F | 315.46 mg/g | [83] | |
| 2,4,6-trichlorophenol | L, F | 375.94 mg/g | [83] | |
| Chitosan grafted with sulfuric acid | Pramipexole | L-F | 337 mg/g | [84] |
| Chitosan grafted with N-(2-carboxybenzyl) | Pramipexole | L-F | 307 mg/g | [84] |
| Graphite oxide/Carboxyl-grafted chitosan | Dorzolamide | L-F | 334 mg/g | [85] |
| Carboxyl-grafted chitosan | Dorzolamide | L-F | 229 mg/g | [85] |
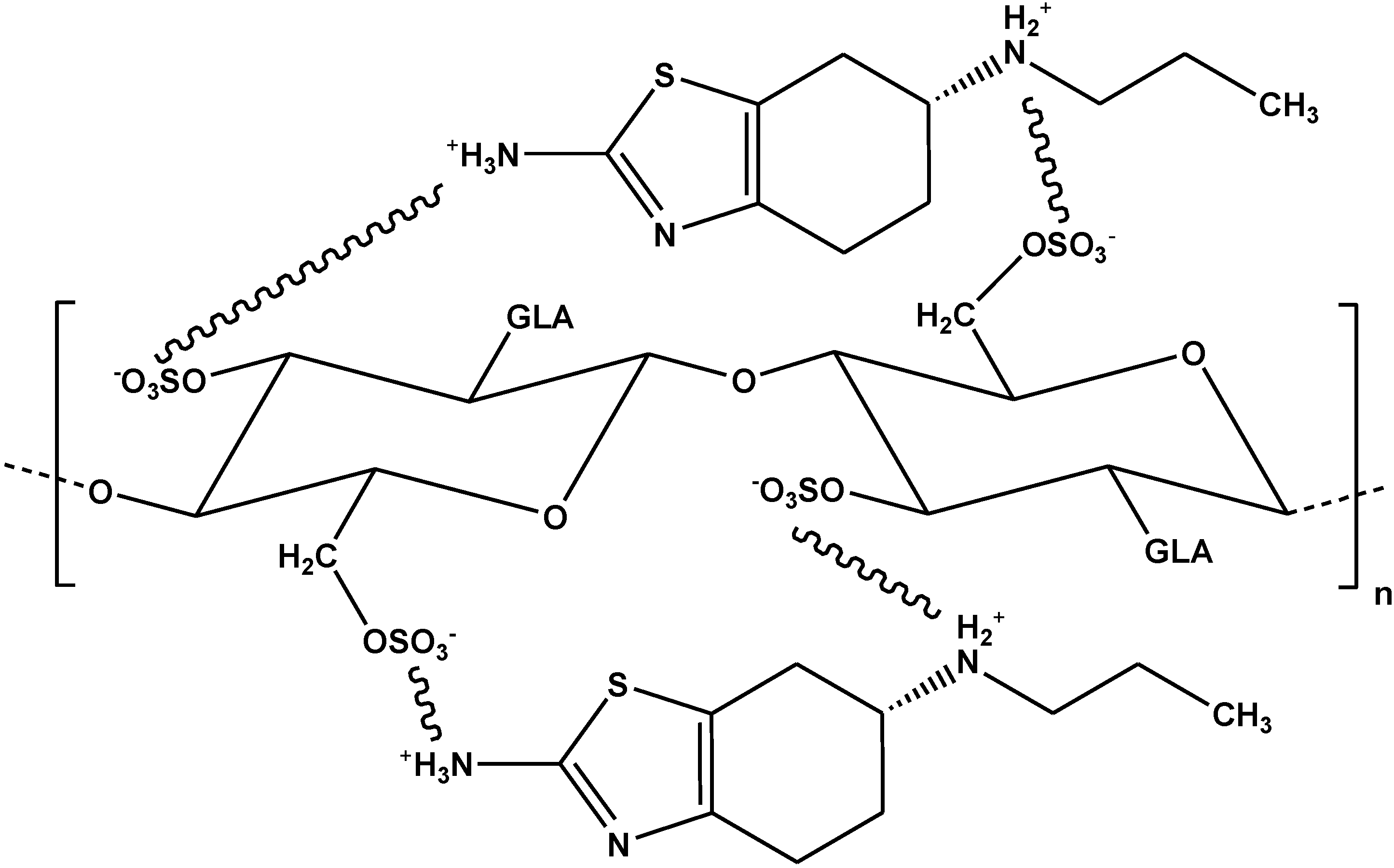

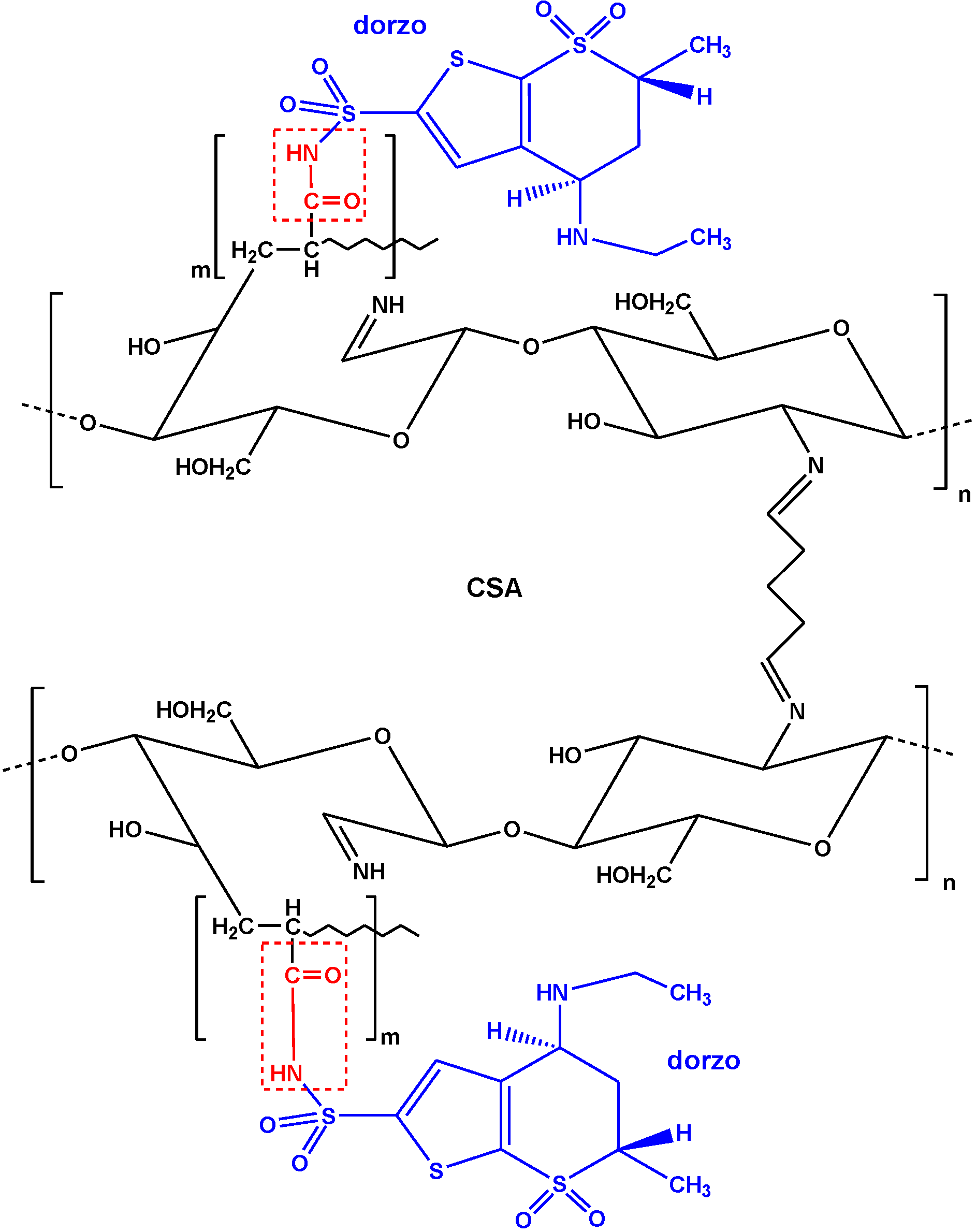
5. Critical Comparisons
6. Concluding Remarks
Acknowledgments
Nomenclature of Dyes Reported in This Study
| AG25 | Acid green 25 |
| AO7 | Acid orange 7 |
| AR18 | Acid red 18 |
| BBR250 | Brilliant blue R250 |
| BO16 | Bezactive orange 16 |
| CR | Congo red |
| MB | Methylene blue |
| RB19 | Reactive blue 19 |
| RB5 | Reactive black 5 |
| RR198 | Reactive red 198 |
| RR120 | Reactive red 120 |
| RR3BS | Remazol red 3BS |
| RY3RS | Reactive yellow 3RS |
| WASC | Weak acid scarlet |
Conflicts of Interest
References
- Clark, G.L.; Smith, A.F. X-ray diffraction studies of chitin, chitosan, and derivatives. J. Phys. Chem. 1936, 40, 863–879. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Natural Chelating Polymers: Alginic Acid, Chitin, and Chitosan; Pergamon Press: Oxford, UK, 1973; Volume 55. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lamb, G.F. The chemical constitution of chitin. Science 1916, 44, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.C. LXXII.—A polarimetric method of identifying chitin. J. Chem. Soc. Trans. 1909, 95, 564–570. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; Qu, Y.L.; OuYang, X.K.; Yang, L.Y.; Xu, Z.R. Chitosan nanoparticles attenuate hydrogen peroxide-induced stress injuryin mouse macrophage raw264.7 cells. Mar. Drugs 2013, 11, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; del González, M.P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Z.T.; Tsai, F.Y.; Yang, W.C.; Wang, J.F.; Liu, C.L.; Shen, C.R.; Yen, T.C. Preparation and characterization of ferrofluid stabilized with biocompatible chitosan and dextran sulfate hybrid biopolymer as a potential magnetic resonance imaging (MRI) T2 contrast agent. Mar. Drugs 2012, 10, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, G.; Hong, R.Y.; Li, H.Z. Preparation and characterization of chitosan poly(acrylic acid) magnetic microspheres. Mar. Drugs 2010, 8, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of glycyrrhetinic acid-modified chitosan 5-fluorouracil nanoparticles and its inhibition of liver cancer characteristics in vitro and in vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Yeh, C.H.; Wang, L.C.; Liou, T.H.; Shen, C.R.; Liu, C.L. Chitosan, the marine functional food, is a potent adsorbent of humic acid. Mar. Drugs 2011, 9, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Tubertini, O. Chitin and chitosan as chromatographic supports and adsorbents for collection of metal ions from organic and aqueous solutions and sea-water. Talanta 1969, 16, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Meyers, S.P. Application of chitosan for treatment of wastewaters. Rev. Environ. Contam. Toxicol. 2000, 163, 1–27. [Google Scholar] [PubMed]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohyd. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Guibal, E.; van Vooren, M.; Dempsey, B.A.; Roussy, J. A review of the use of chitosan for the removal of particulate and dissolved contaminants. Sep. Sci. Technol. 2006, 41, 2487–2514. [Google Scholar] [CrossRef]
- Gerente, C.; Lee, V.K.C.; Le Cloirec, P.; McKay, G. Application of chitosan for the removal of metals from wastewaters by adsorption—Mechanisms and models review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interf. Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohyd. Polym. 2011, 83, 1446–1456. [Google Scholar]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohyd. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan-clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Luo, C.; Lu, F.; Qiu, H.; Sun, M. Synthesis and characterization of magnetic β-cyclodextrin-chitosan nanoparticles as nano-adsorbents for removal of methyl blue. Int. J. Biol. Macromol. 2012, 50, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan-cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Frag. J. 2004, 19, 394–400. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Abd El-Ghaffar, M.A.; El-kousy, S.M.; El-Shorbagy, H.G. Synthesis of new ammonium chitosan derivatives and their application for dye removal from aqueous media. Chem. Eng. J. 2012, 203, 458–468. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H.; Luo, W.J.; Wang, S.; Zhou, C.G. Preparation, characterization, and adsorption evaluation of chitosan-functionalized mesoporous composites. Microporous Mesoporous Mater. 2014, 193, 15–26. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Liu, L.; Wang, S.; Tian, X. Adsorption of c.I. Reactive blue 19 from aqueous solutions by porous particles of the grafted chitosan. Chem. Eng. J. 2014, 235, 151–157. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Liu, L.; Li, B.; Yang, P.; Zhang, L.; Feng, Y. Adsorption of dye from wastewater using chitosan-ctab modified bentonites. J. Colloid Interface Sci. 2012, 382, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, M.; Zhang, W.; Liu, L.; Wang, J.; Ge, W.; Yuan, Y.; Yue, T.; Li, R.; Yu, W.W. Affinity adsorption of lysozyme with reactive red 120 modified magnetic chitosan microspheres. Food Chem. 2014, 145, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.R.; Veličković, S.J.; Antonović, D.G. Modification of chitosan by zeolite a and adsorption of bezactive orange 16 from aqueous solution. Compos. B: Eng. 2013, 53, 145–151. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, D.; Ji, J.; Kong, Y.; Wan, H.; Yao, C. Chitosan-modified palygorskite: Preparation, characterization and reactive dye removal. Appl. Clay Sci. 2013, 74, 81–86. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Arami, M.; Gharanjig, K. Dye removal from colored-textile wastewater using chitosan-PPI dendrimer hybrid as a biopolymer: Optimization, kinetic, and isotherm studies. J. Appl. Polym. Sci. 2013, 127, 2607–2619. [Google Scholar] [CrossRef]
- Yan, Y.; Xiang, B.; Li, Y.; Jia, Q. Preparation and adsorption properties of diethylenetriamine-modified chitosan beads for acid dyes. J. Appl. Polym. Sci. 2013, 130, 4090–4098. [Google Scholar]
- Zhu, H.; Fu, Y.; Jiang, R.; Yao, J.; Liu, L.; Chen, Y.; Xiao, L.; Zeng, G. Preparation, characterization and adsorption properties of chitosan modified magnetic graphitized multi-walled carbon nanotubes for highly effective removal of a carcinogenic dye from aqueous solution. Appl. Surf. Sci. 2013, 285, 865–873. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: Binding mechanism, equilibrium and kinetics. Colloid Surf. A 2007, 299, 146–152. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption properties of congo red from aqueous solution onto N,O-carboxymethyl-chitosan. Bioresour. Technol. 2008, 99, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, A. Adsorption behaviors of congo red on the N,O-carboxymethyl-chitosan/montmorillonite nanocomposite. Chem. Eng. J. 2008, 143, 43–50. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Lazaridis, N.K.; Bikiaris, D.N. Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohyd. Polym. 2013, 91, 198–208. [Google Scholar] [CrossRef]
- IUPAC. Available online: http://goldbook.iupac.org/C01012.html (accessed on 5 September 2014).
- Muzzarelli, R.A.A. Available online: http://www.chitin.it/chelating-capacity-of-chitin-and-chitosan.html (accessed on 5 September 2014).
- Arvand, M.; Pakseresht, M.A. Cadmium adsorption on modified chitosan-coated bentonite: Batch experimental studies. J. Chem. Technol. Biot. 2013, 88, 572–578. [Google Scholar] [CrossRef]
- Chen, J.H.; Lu, D.Q.; Chen, B.; Ouyang, P.K. Removal of U(VI) from aqueous solutions by using MWCNTs and chitosan modified MWCNTs. J. Radioanal. Nucl. Chem. 2013, 295, 2233–2241. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. The characteristics and mechanism of Co(II) removal from aqueous solution by a novel xanthate-modified magnetic chitosan. Nucl. Eng. Des. 2012, 242, 452–457. [Google Scholar] [CrossRef]
- Chethan, P.D.; Vishalakshi, B. Synthesis of ethylenediamine modified chitosan and evaluation for removal of divalent metal ions. Carbohyd. Polym. 2013, 97, 530–536. [Google Scholar] [CrossRef]
- Debnath, S.; Maity, A.; Pillay, K. Magnetic chitosan-go nanocomposite: Synthesis, characterization and batch adsorber design for Cr(VI) removal. J. Environ. Chem. Eng. 2014, 2, 963–973. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Atia, A.A.; Guibal, E. Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour. Technol. 2014, 160, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Eser, A.; Nüket Tirtom, V.; Aydemir, T.; Becerik, S.; Dinçer, A. Removal of nickel(II) ions by histidine modified chitosan beads. Chem. Eng. J. 2012, 210, 590–596. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Kousalya, G.N.; Meenakshi, S. Selective sorption of Fe(III) using modified forms of chitosan beads. J. Appl. Polym. Sci. 2012, 124, 1858–1865. [Google Scholar] [CrossRef]
- Kandile, N.G.; Nasr, A.S. New hydrogels based on modified chitosan as metal biosorbent agents. Int. J. Biol. Macromol. 2014, 64, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Liu, Y.G.; Peng, Q.Q.; Hu, X.J.; Liao, T.; Wang, H.; Lu, M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Li, M.L.; Li, R.H.; Xu, J.; Han, X.; Yao, T.Y.; Wang, J. Thiocarbohydrazide-modified chitosan as anticorrosion and metal ion adsorbent. J. Appl. Polym. Sci. 2014, 131, 8437–8443. [Google Scholar]
- Li, M.; Xu, J.; Li, R.; Wang, D.; Li, T.; Yuan, M.; Wang, J. Simple preparation of aminothiourea-modified chitosan as corrosion inhibitor and heavy metal ion adsorbent. J. Colloid Interface Sci. 2014, 417, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Monier, M. Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modified chitosan-thioglyceraldehyde schiff's base. Int. J. Biol. Macromol. 2012, 50, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; El Sheikh, R.; El-Farargy, A.F.; Hefni, H.H.H.; Bekhit, M. Treatment of industrial wastewater containing copper and cobalt ions using modified chitosan. J. Ind. Eng. Chem. 2014. [Google Scholar] [CrossRef]
- Rabelo, R.; Vieira, R.; Luna, F.; Guibal, E.; Beppu, M. Adsorption of copper(II) and mercury(II) ions onto chemically-modified chitosan membranes: Equilibrium and kinetic properties. Adsorpt. Sci. Technol. 2012, 30, 1–21. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Meenakshi, S. Preparation of amino terminated polyamidoamine functionalized chitosan beads and its Cr(VI) uptake studies. Carbohyd. Polym. 2013, 91, 631–637. [Google Scholar]
- Repo, E.; Koivula, R.; Harjula, R.; Sillanpää, M. Effect of edta and some other interfering species on the adsorption of Co(II) by edta-modified chitosan. Desalination 2013, 321, 93–102. [Google Scholar] [CrossRef]
- Song, Q.; Wang, C.; Zhang, Z.; Gao, J. Adsorption of Cu(II) and Ni(II) using a novel xanthated carboxymethyl chitosan. Sep. Sci. Technol. 2014, 49, 1235–1243. [Google Scholar] [CrossRef]
- Santhana Krishna Kumar, A.; Uday Kumar, C.; Rajesh, V.; Rajesh, N. Microwave assisted preparation of n-butylacrylate grafted chitosan and its application for Cr(VI) adsorption. Int. J. Biol. Macromol. 2014, 66, 135–143. [Google Scholar]
- Suc, N.V.; Ly, H.T.Y. Lead (II) removal from aqueous solution by chitosan flake modified with citric acid via crosslinking with glutaraldehyde. J. Chem. Technol. Biot. 2013, 88, 1641–1649. [Google Scholar] [CrossRef]
- Wang, H.; Tang, H.; Liu, Z.; Zhang, X.; Hao, Z.; Liu, Z. Removal of cobalt(II) ion from aqueous solution by chitosan–montmorillonite. J. Environ. Sci. 2014, 26, 1879–1884. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Jia, Y.; Liu, Z.; Adesina, A.A. Adsorption of thorium (IV) ions from aqueous solution by magnetic chitosan resins modified with triethylene-tetramine. J. Radioanal. Nucl. Chem. 2014. [Google Scholar] [CrossRef]
- Xu, J.; Chen, M.; Zhang, C.; Yi, Z. Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J. Radioanal. Nucl. Chem. 2013, 298, 1375–1383. [Google Scholar] [CrossRef]
- Yang, G.; Tang, L.; Lei, X.; Zeng, G.; Cai, Y.; Wei, X.; Zhou, Y.; Li, S.; Fang, Y.; Zhang, Y. Cd(II) removal from aqueous solution by adsorption on α-ketoglutaric acid-modified magnetic chitosan. Appl. Surf. Sci. 2014, 292, 710–716. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Siafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake. Langmuir 2014, 40, 120–131. [Google Scholar] [CrossRef]
- Poon, L.; Wilson, L.D.; Headley, J.V. Chitosan-glutaraldehyde copolymers and their sorption properties. Carbohyd. Polym. 2014, 109, 92–101. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Lazaridis, N.K. Low-swelling chitosan derivatives as biosorbents for basic dyes. Langmuir 2008, 24, 4791–4799. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Lazaridis, N.K. Reactive and basic dyes removal by sorption onto chitosan derivatives. J. Colloid Interface Sci. 2009, 331, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N.K.; Kyzas, G.Z.; Vassiliou, A.A.; Bikiaris, D.N. Chitosan derivatives as biosorbents for basic dyes. Langmuir 2007, 23, 7634–7643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Z.; Dai, C.; Zhou, X. Removal of selected pharmaceuticals from aqueous solution using magnetic chitosan: Sorption behavior and mechanism. Environ. Sci. Pollut. Res. 2014, 21, 12780–12789. [Google Scholar] [CrossRef]
- Zhou, L.C.; Meng, X.G.; Fu, J.W.; Yang, Y.C.; Yang, P.; Mi, C. Highly efficient adsorption of chlorophenols onto chemically modified chitosan. Appl. Surf. Sci. 2014, 292, 735–741. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Seredych, M.; Bandosz, T.J.; Deliyanni, E.A. Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite. Bioresour. Technol. 2014, 152, 399–406. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyzas, G.Z.; Bikiaris, D.N. Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review. Mar. Drugs 2015, 13, 312-337. https://doi.org/10.3390/md13010312
Kyzas GZ, Bikiaris DN. Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review. Marine Drugs. 2015; 13(1):312-337. https://doi.org/10.3390/md13010312
Chicago/Turabian StyleKyzas, George Z., and Dimitrios N. Bikiaris. 2015. "Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review" Marine Drugs 13, no. 1: 312-337. https://doi.org/10.3390/md13010312







