Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temporal Trend of Bioactive Compounds by Year
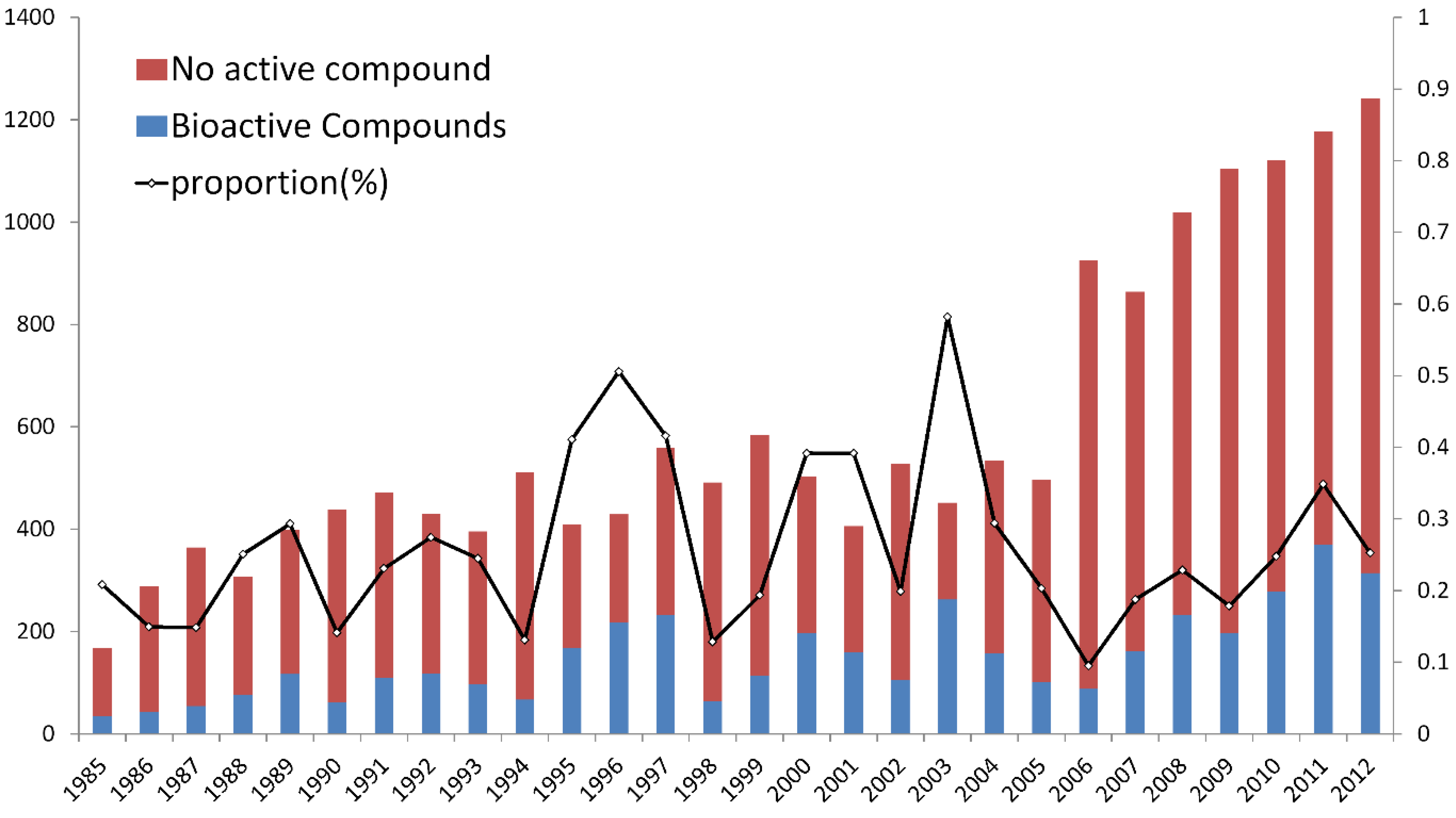
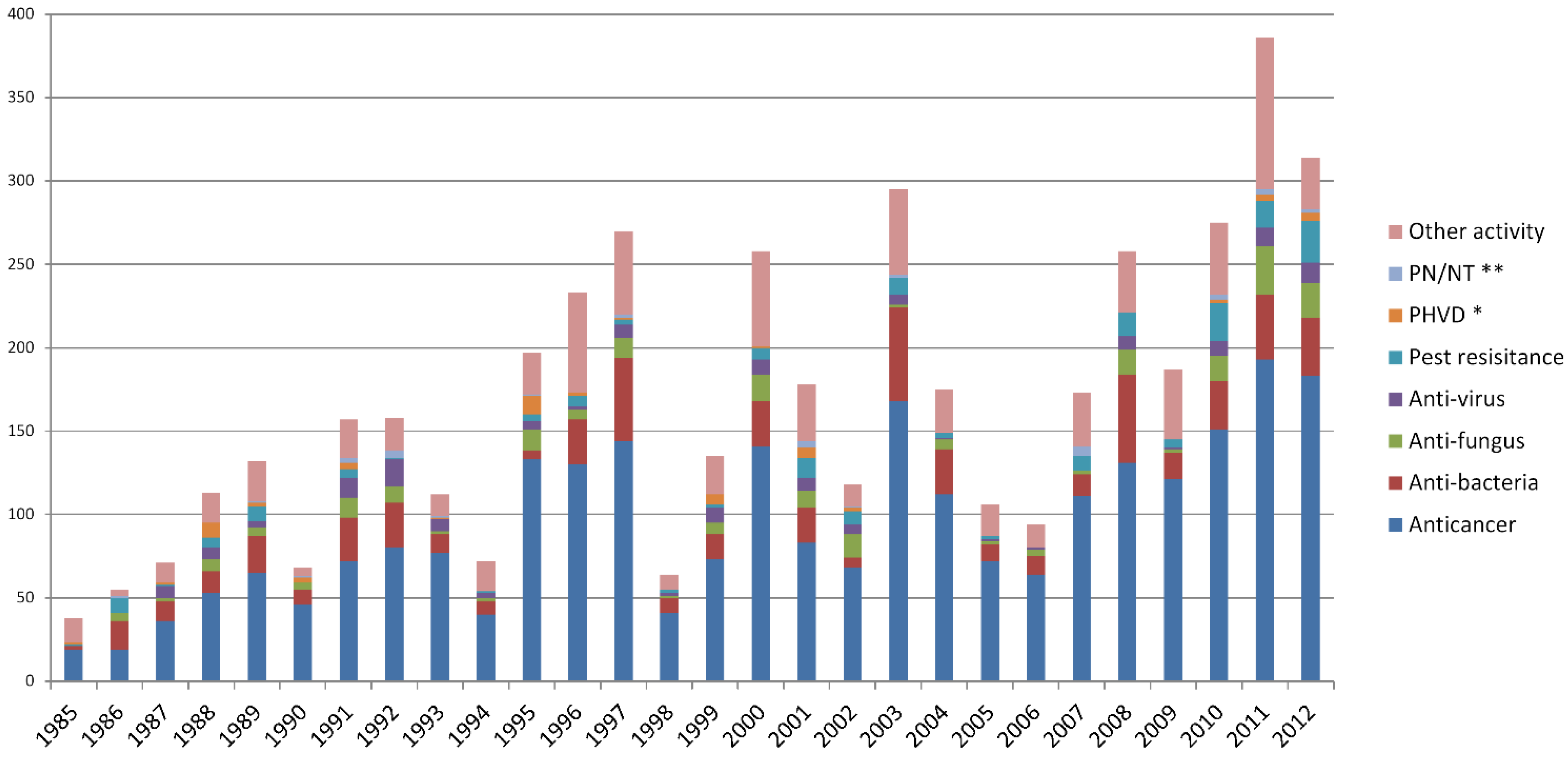
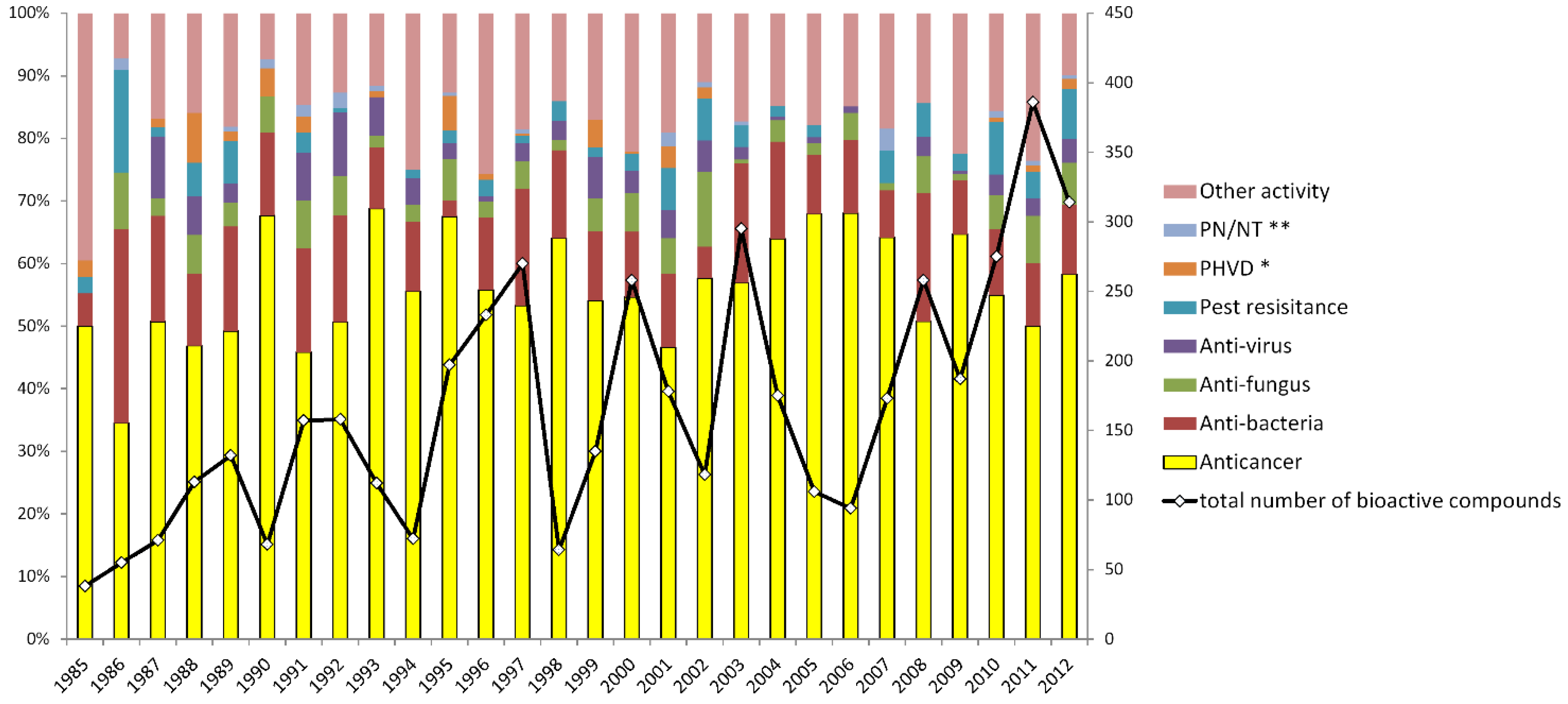
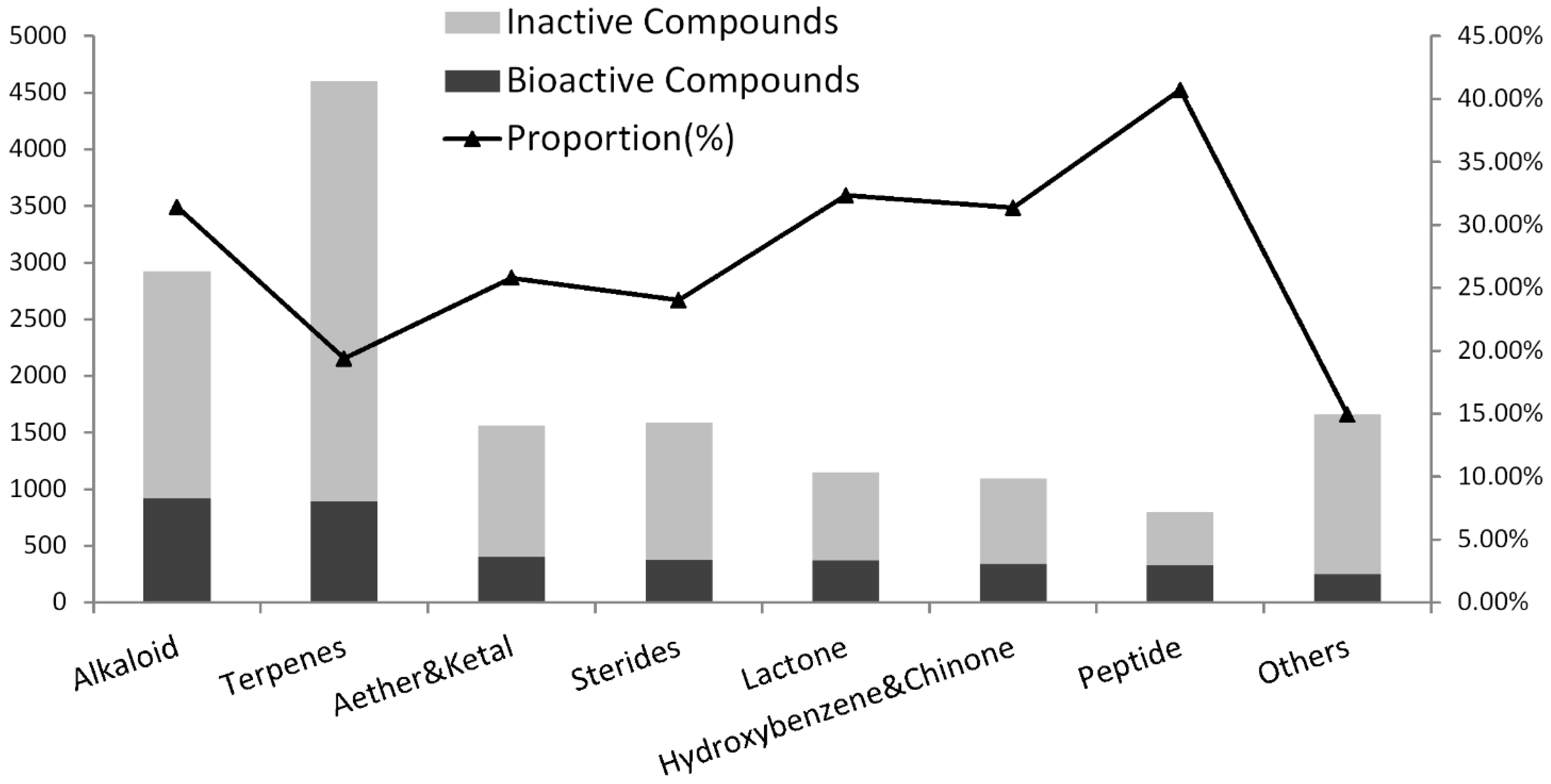
2.2. The Chemical Structure Distribution of Bioactive Compounds
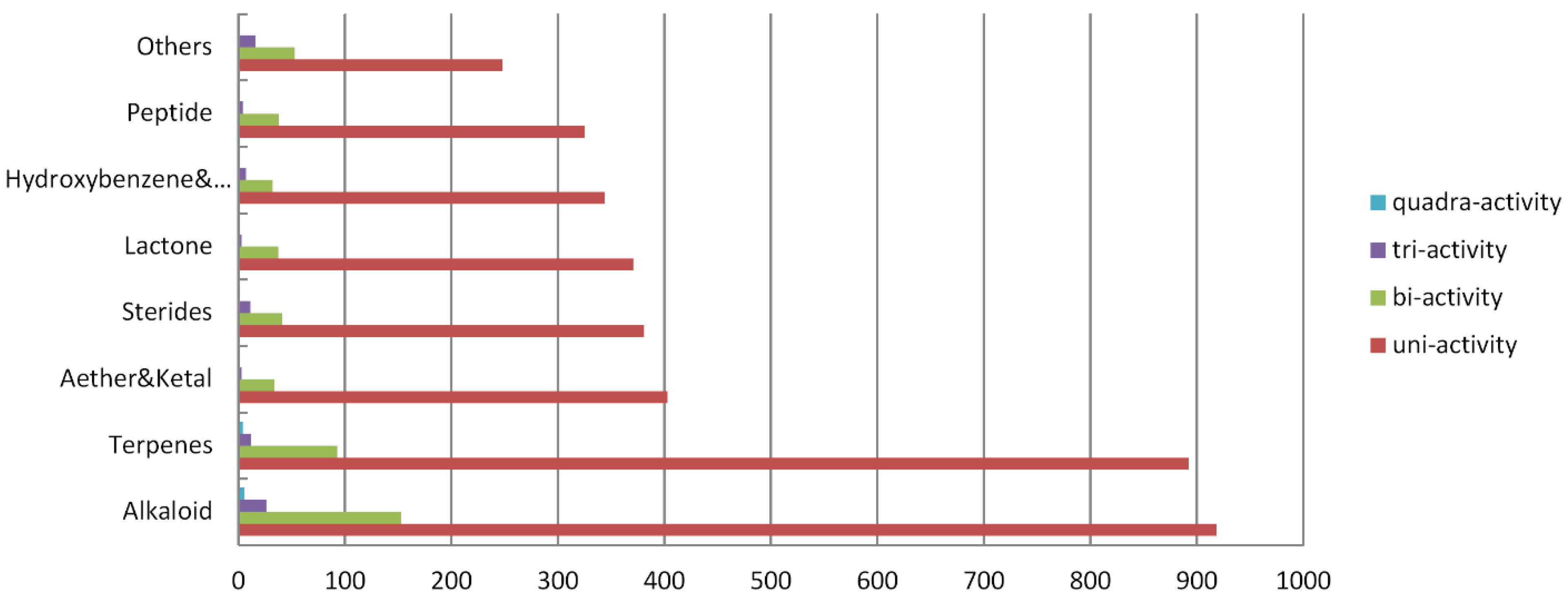
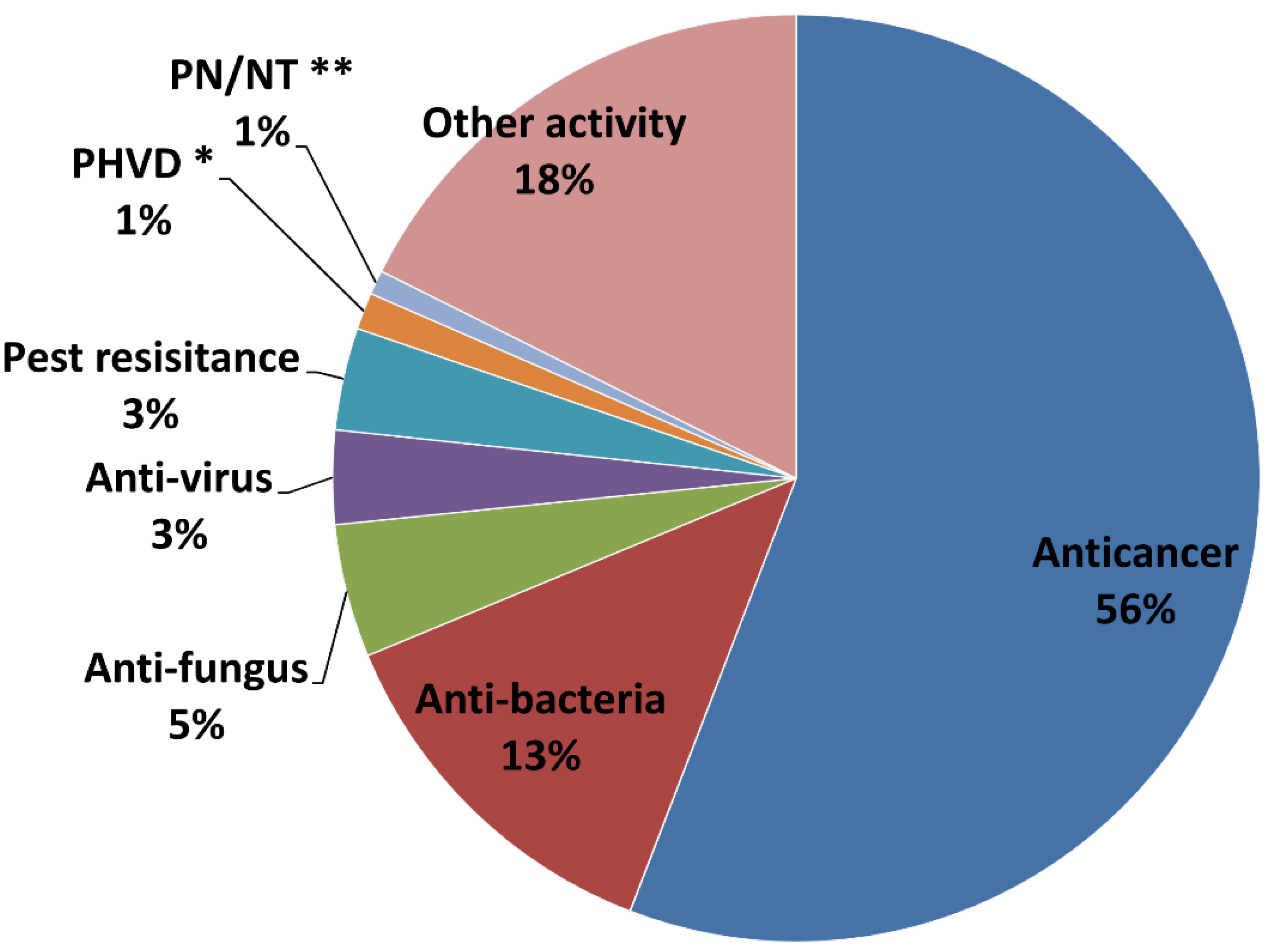
2.3. Main Bioactivity Groups of the Novel Compounds
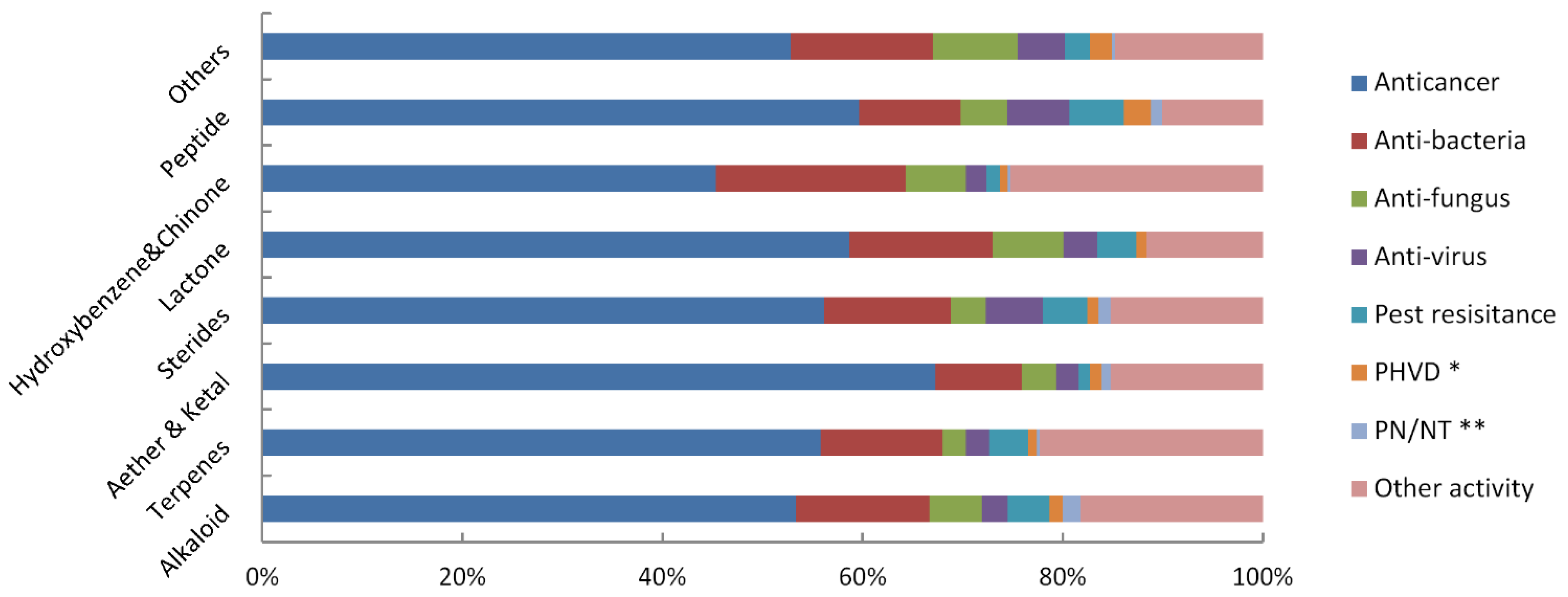
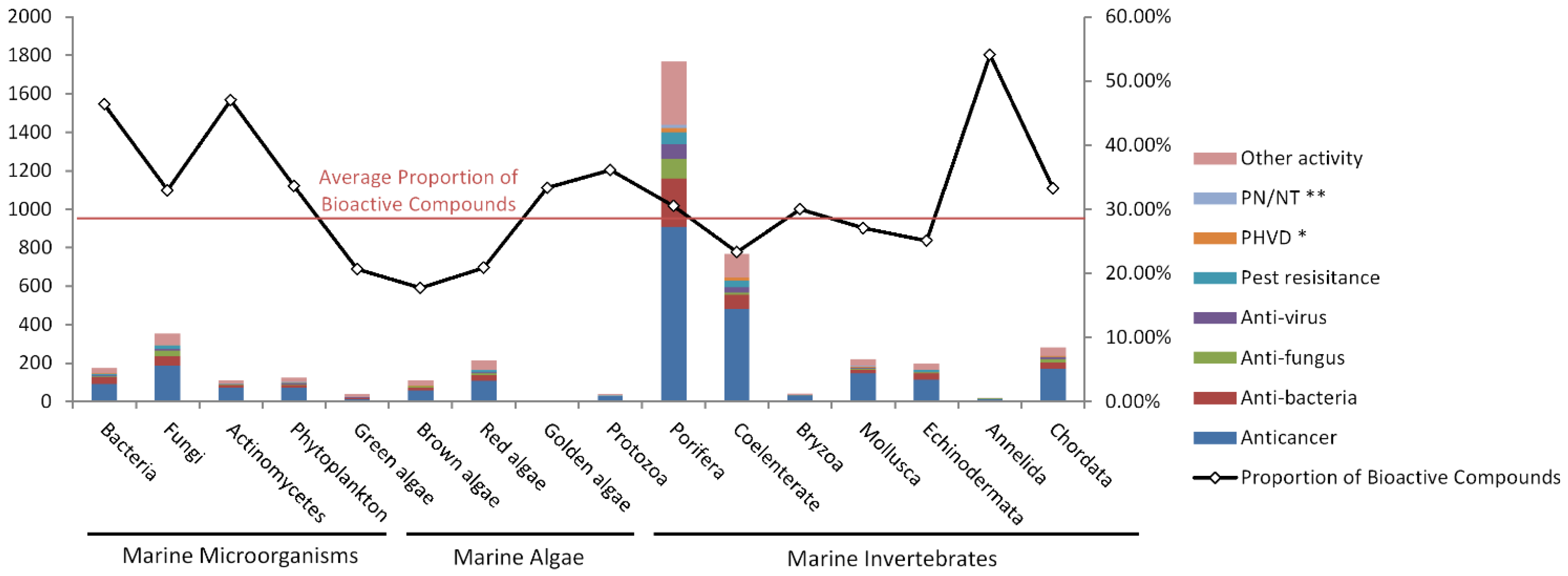
2.4. Species Distribution of the Bioactive Compounds


3. Data and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr. Med. Chem. 2004, 11, 1693–1713. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Munro, M.H.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.H.G.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J. H.; Lill, R.E.; Li, S.X.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.A.; Gerwick, L. Marine natural product drug discovery: Leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacol. Immunotoxicol. 2010, 32, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Highlights of marine natural products chemistry (1972–1999). Nat. Prod. Rep. 2000, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.B.; Mayer, A.M. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Nastrucci, C.; Cesario, A.; Russo, P. Anticancer drug discovery from the marine environment. Recent Pat. Anti Cancer Drug Discov. 2012, 7, 218–232. [Google Scholar] [CrossRef]
- Mayer, A.M.; Lehmann, V.K. Marine pharmacology in 1999: Antitumor and cytotoxic compounds. Anticancer Res. 2001, 21, 2489–2500. [Google Scholar] [PubMed]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2000: Antitumor and cytotoxic compounds. Int. J. Cancer 2003, 105, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2001–2: Antitumour and cytotoxic compounds. Eur. J. Cancer 2004, 40, 2676–2704. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2003–2004: Anti-tumour and cytotoxic compounds. Eur. J. Cancer 2006, 42, 2241–2270. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2005–2006: Antitumour and cytotoxic compounds. Eur. J. Cancer 2008, 44, 2357–2387. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.C.; Fenical, W. Antibacterials from the sea. Chemistry 2010, 16, 12512–12525. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; Hamann, M.T. Marine natural products and their potential applications as anti-infective agents. Lancet Infect. Dis. 2003, 3, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Austin, B.; Mitchell, W.J.; Morris, P.C.; Jamieson, D.J.; Adams, D.R.; Spragg, A.M.; Schweizer, M. Novel anti-infective compounds from marine bacteria. Mar. Drugs 2010, 8, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003–4: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 553–581. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007–2008: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, D.J. Marine natural products: Metabolites of marine invertebrates. Nat. Prod. Rep. 1984, 1, 551–598. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products: Metabolites of marine algae and herbivorous marine molluscs. Nat. Prod. Rep. 1984, 1, 251–280. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1986, 3, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1987, 4, 539–576. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1988, 5, 613–663. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1990, 7, 269–309. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1991, 8, 97–147. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1992, 9, 323–364. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1993, 10, 497–539. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1994, 11, 355–394. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1995, 12, 223–269. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1996, 13, 75–125. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1997, 14, 259–302. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1998, 15, 113–158. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1999, 16, 155–198. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2000, 17, 7–55. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2003, 20, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 25, 35–94. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat Prod Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.P.; Yuan, J.; Sun, L.; She, Z.G.; Wu, J.H.; Lan, X.J.; Zhu, X.; Lin, Y.C.; Chen, S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Edrada, R.A.; Lin, W.; Proksch, P. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat. Protoc. 2008, 3, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Zhang, P.; Lee, Y.; Hong, J.; Lee, C.O.; Jung, J.H. Monoindole alkaloids from a marine sponge Spongosorites sp. Mar. Drugs 2007, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Lee, Y.R.; Lee, D.S.; Kim, Y.C.; Oh, H. PTP1B inhibitory secondary metabolites from marine-derived fungal strains Penicillium spp. and Eurotium sp. J. Microbiol. Biotechnol. 2013, 23, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Lillsunde, K.E.; Festa, C.; Adel, H.; de Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; Tammela, P. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed]
- Balis, F.M. Evolution of anticancer drug discovery and the role of cell-based screening. J. Natl. Cancer Inst. 2002, 94, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Eloe, E.A.; Fadrosh, D.W.; Novotny, M.; Zeigler Allen, L.; Kim, M.; Lombardo, M.J.; Yee-Greenbaum, J.; Yooseph, S.; Allen, E.E.; Lasken, R.; et al. Going deeper: Metagenome of a hadopelagic microbial community. PLoS One 2011, 6, e20388. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.L.; Rusch, D.B.; Yooseph, S.; Lombardo, M.J.; Richter, R.A.; Valas, R.; Novotny, M.; Yee-Greenbaum, J.; Selengut, J.D.; Haft, D.H.; et al. Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J. 2012, 6, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Challis, G.L. Genome mining for novel natural product discovery. J. Med. Chem. 2008, 51, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.J.; Chabot-Fletcher, M.C. The natural product hymenialdisine inhibits interleukin-8 production in U937 cells by inhibition of nuclear factor-kappaB. J. Pharmacol. Exp. Ther. 1997, 282, 459–466. [Google Scholar] [PubMed]
- Roshak, A.; Jackson, J.R.; Chabot-Fletcher, M.; Marshall, L.A. Inhibition of NFkappaB-mediated interleukin-1beta-stimulated prostaglandin E2 formation by the marine natural product hymenialdisine. J. Pharmacol. Exp. Ther. 1997, 283, 955–961. [Google Scholar] [PubMed]
- Meijer, L.; Thunnissen, A.M.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.Z.; et al. Inhibition of cyclin-dependent kinases, GSK-3beta and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; de Rosa, S.; de Stefano, S.; Mazzarella, L.; Puliti, R.; Sodano, G. Isolation and X-ray crystal structure of a novel bromo-compound from two marine sponges. Tetrahedron Lett. 1982, 23, 767–768. [Google Scholar] [CrossRef]
- Demetri, G.D.; Chawla, S.P.; von Mehren, M.; Ritch, P.; Baker, L.H.; Blay, J.Y.; Hande, K.R.; Keohan, M.L.; Samuels, B.L.; Schuetze, S.; et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J. Clin. Oncol. 2009, 27, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- D’Incalci, M.; Galmarini, C.M. A review of trabectedin (ET-743): A unique mechanism of action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; Napolitano, A.; Frezza, A.M.; Schiavon, G.; Santini, D.; Tonini, G. Wide-spectrum characterization of trabectedin: Biology, clinical activity and future perspectives. Pharmacogenomics 2010, 11, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Herzog, T.J.; Kaye, S.B.; Krasner, C.N.; Vermorken, J.B.; Muggia, F.M.; Pujade-Lauraine, E.; Park, Y.C.; Parekh, T.V.; Poveda, A.M. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. Eur. J. Cancer 2012, 48, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Le Cesne, A. 13 years of trabectedin, 5 years of Yondelis(R): What have we learnt? Expert Rev. Anticancer Ther. 2013, 13, 11–19. [Google Scholar]
- Mascilini, F.; Amadio, G.; di Stefano, M.G.; Ludovisi, M.; di Legge, A.; Conte, C.; de Vincenzo, R.; Ricci, C.; Masciullo, V.; Salutari, V.; et al. Clinical utility of trabectedin for the treatment of ovarian cancer: Current evidence. Onco Targets Ther. 2014, 7, 1273–1284. [Google Scholar] [PubMed]
- Liu, J.; Hu, Y.; Waller, D.L.; Wang, J.; Liu, Q. Natural products as kinase inhibitors. Nat. Prod. Rep. 2012, 29, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.; Ducancel, F.; Konno, K. Natural peptides with potential applications in drug development, diagnosis, and/or biotechnology. Int. J. Pept. 2012, 2012. [Google Scholar] [CrossRef]
- Kaspar, A.A.; Reichert, J.M. Future directions for peptide therapeutics development. Drug Discov. Today 2013, 18, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. An evaluation of intrathecal ziconotide for the treatment of chronic pain. Expert Opin. Investig. Drugs 2000, 9, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A.; Perry, C. Ziconotide. CNS Drugs 2006, 20, 331–338; discussion 339–341. [Google Scholar] [CrossRef] [PubMed]
- Carletti, I.; Banaigs, B.; Amade, P. Matemone, a new bioactive bromine-containing oxindole alkaloid from the indian ocean sponge Iotrochota purpurea. J. Nat. Prod. 2000, 63, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Isolation and structure of the cytotoxin lyngbyabellin B and absolute configuration of lyngbyapeptin A from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Kalberer, J.T., Jr. Impact of the national cancer act on grant support. Cancer Res. 1975, 35, 472–481. [Google Scholar] [PubMed]
- Kanavos, P.; Sullivan, R.; Lewison, G.; Schurer, W.; Eckhouse, S.; Vlachopioti, Z. The role of funding and policies on innovation in cancer drug development. Ecancermedicalscience 2010, 4. [Google Scholar] [CrossRef]
- Hallock, Y.F.; Cragg, G.M. National cooperative drug discovery groups (NCDDGs): A successful model for public private partnerships in cancer drug discovery. Pharm. Biol. 2003, 41, 78–91. [Google Scholar] [CrossRef]
- Rae, I.D. The roche research institute of marine pharmacology, 1974–1981: Searching for drug Leads from Australian Marine Organisms. Hist. Rec. Aust. Sci. 2009, 20, 209–231. [Google Scholar] [CrossRef]
- Boyd, M. The NCI In Vitro Anticancer Drug Discovery Screen. In Anticancer Drug Development Guide, 1st ed.; Teicher, B., Ed.; Humana Press: Totowa, NJ, USA, 1997; pp. 23–42. [Google Scholar]
- Von Berlepsch, K. Drugs from marine organisms: The target of the Roche Research Institute of Marine Pharmacology in Australia. Naturwissenschaften 1980, 67, 338–342. [Google Scholar]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.N.; Shinnar, A.E.; Noecker, L.A.; Chao, T.L.; Feibush, B.; Snyder, B.; Sharkansky, I.; Sarkahian, A.; Zhang, X.; Jones, S.R.; et al. Aminosterols from the dogfish shark Squalus acanthias. J. Nat. Prod. 2000, 63, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Knight, J.C.; Collins, J.C.; Herald, D.L.; Pettit, R.K.; Boyd, M.R.; Young, V.G. Antineoplastic agents 430. Isolation and structure of cribrostatins 3, 4, and 5 from the republic of maldives cribrochalina species. J. Nat. Prod. 2000, 63, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Etahiri, S.; Bultel-Ponce, V.; Caux, C.; Guyot, M. New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2001, 64, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B. Confronting the challenges of discovery of novel antibacterial agents. Bioorg. Med. Chem. Lett. 2014, 24, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.P.; Edmond, M.B. Managing antibiotic resistance. N. Engl. J. Med. 2000, 343, 1961–1963. [Google Scholar] [CrossRef] [PubMed]
- Bromiker, R.; Arad, I.; Peleg, O.; Preminger, A.; Engelhard, D. Neonatal bacteremia: Patterns of antibiotic resistance. Infect. Control Hosp. Epidemiol. 2001, 22, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Hopwood, S.; Davies, S.C. Antimicrobial resistance: A global challenge. Sci. Transl. Med. 2014, 6, 236ed10. [Google Scholar] [CrossRef]
- Projan, S.J.; Shlaes, D.M. Antibacterial drug discovery: Is it all downhill from here? Clin. Microbiol. Infect. 2004, 10, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Projan, S.J. Why is big Pharma getting out of antibacterial drug discovery? Curr. Opin. Microbiol. 2003, 6, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, I.H. Drug discovery for neglected diseases: Molecular target-based and phenotypic approaches. J. Med. Chem. 2013, 56, 7719–7726. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.M.; Boyd, K.G.; Adams, D.R.; Burgess, J.G. Biofilm-specific cross-species induction of antimicrobial compounds in bacilli. Appl. Environ. Microbiol. 2003, 69, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.H.; Matkar, P.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Cross-Species Induction of Antimicrobial Compounds, Biosurfactants and Quorum-Sensing Inhibitors in Tropical Marine Epibiotic Bacteria by Pathogens and Biofouling Microorganisms. Curr. Microbiol. 2011, 62, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.M.; Clardy, J. Bacterial symbionts and natural products. Chem. Commun. 2011, 47, 7559–7566. [Google Scholar] [CrossRef]
- Johnson, T.A. A Comprehensive Reinvestigation Into the Bioactive Secondary Metabolites of an Indo-Pacific Marine Sponge: Cacospongia Mycofijiensis; University of California: Santa Cruz, CA, USA, 2008. [Google Scholar]
- Abdelmohsen, U.R.; Bayer, K.; Hentschel, U. Diversity, abundance and natural products of marine sponge-associated actinomycetes. Nat. Prod. Rep. 2014, 31, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Gao, A.H.; Xie, Q.Y.; Gao, H.; Zhuang, L.; Lin, H.P.; Yu, H.P.; Li, J.; Yao, X.S.; Goodfellow, M.; et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Marine Organism Metabolite Data Center. Key Laboratory of Functional Molecules from Oceanic Microorganisms (Sun Yat-sen University). Available online: http://momdc.sysu.edu.cn (accessed on 28 July 2014).
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Villoslada, P.; Moreno, B.; Melero, I.; Pablos, J.L.; Martino, G.; Uccelli, A.; Montalban, X.; Avila, J.; Rivest, S.; Acarin, L.; et al. Immunotherapy for neurological diseases. Clin. Immunol. 2008, 128, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202-221. https://doi.org/10.3390/md13010202
Hu Y, Chen J, Hu G, Yu J, Zhu X, Lin Y, Chen S, Yuan J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Marine Drugs. 2015; 13(1):202-221. https://doi.org/10.3390/md13010202
Chicago/Turabian StyleHu, Yiwen, Jiahui Chen, Guping Hu, Jianchen Yu, Xun Zhu, Yongcheng Lin, Shengping Chen, and Jie Yuan. 2015. "Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012" Marine Drugs 13, no. 1: 202-221. https://doi.org/10.3390/md13010202




