2. Results and Discussion
Five new norditerpene endoperoxides (
1–
5) were obtained from the CH
2Cl
2 extract of the sponge. The signal patterns for a 1,2-dioxane ring and a substituted methyl propionate group were acquired from NMR, establishing a scaffold of the marine-derived endoperoxide class [
10,
11]. Further analysis of the NMR spectra (
Table 1 and
Table 2) provided evidence for the existence of monoterpene substitutions different from reported derivatives [
12,
13]. The stereochemical issues were encountered either because of conformational flexibility or two distal stereogenic centers (
vide infra). Traditionally, the determination of the relative configurations of this type of compound counted on the chemical shift differences based on the empirical rule developed by Capon and Macleod [
14], and the absolute configuration primarily relied on optical rotation [
15] or conducting semisynthesis, since only three XRD (X-ray diffraction) structures in this class were reported [
6,
16,
17]. In this work, the structures of the new norditerpene endoperoxides were determined by using spectroscopic analyses and computational approaches, including DFT (density functional theory)-based molecular modeling, conformational analysis and NMR and ECD (electronic circular dichroism) calculations.
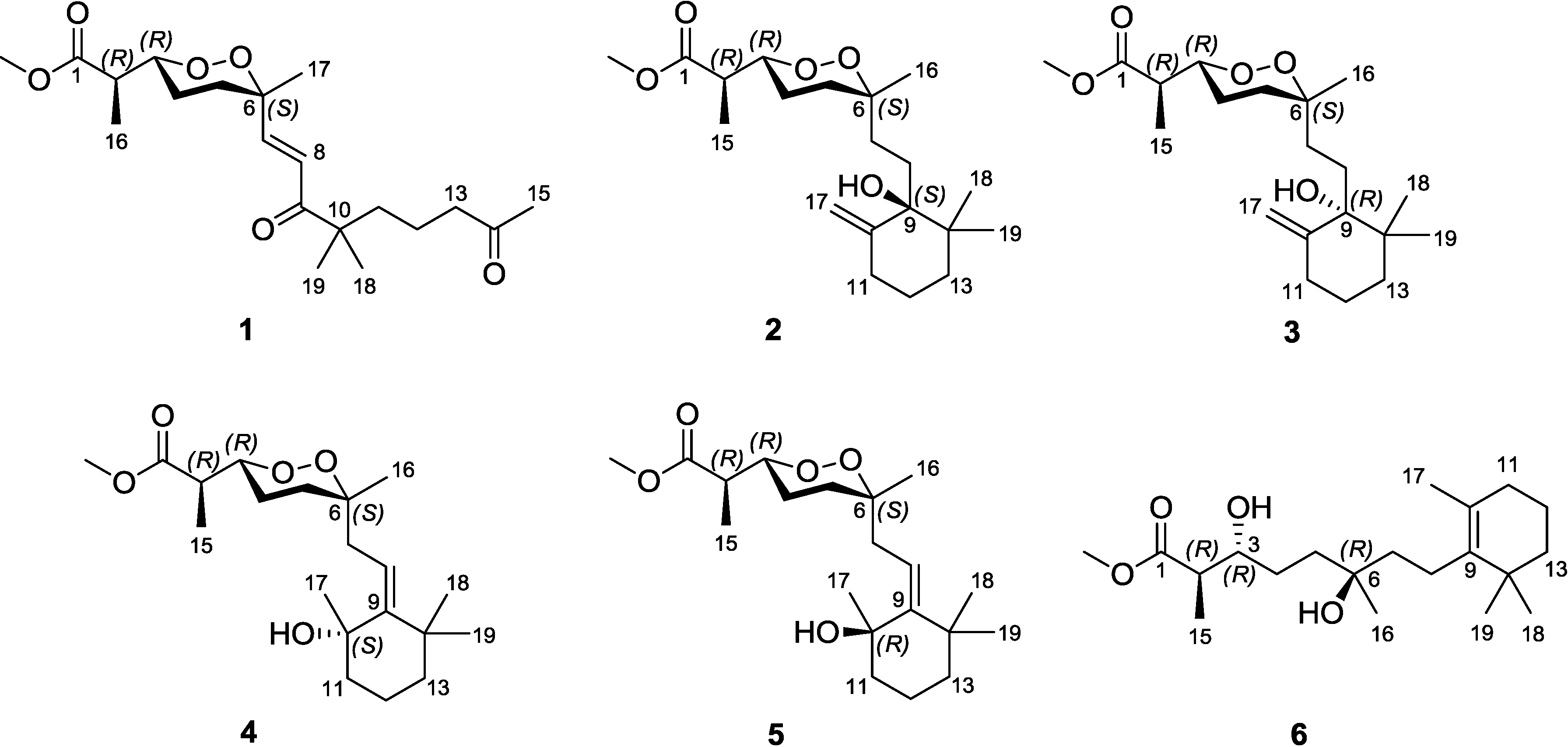
Diacarperoxide H (
1) was isolated as colorless oil. The molecular formula of C
20H
32O
6 was determined by the analysis of HRESIMS (high resolution electrospray ionization mass spectroscopy) data with five degrees of unsaturation. The
1H and
13C NMR signal patterns suggested a norditerpene endoperoxide core and established an isolated spin system, allowing the assignment of C-1–C-6, C-16 and C-17 positions [
18,
19]. Analyzing from the remaining signals in the low field region of
13C NMR, another two carbonyl functional groups (δ
C 208.5 and 203.8) and two olefinic carbons (δ
C 147.6 and 124.3) were deduced. The sp
2 carbons (δ
C 124.3 and 147.6) were proven to be located at the α and β positions of the carbonyl (δ
C 203.8), based on the HMBC correlations from the corresponding olefinic protons H-7 (δ
H 6.88) and H-8 (δ
H 6.75) to the carbonyl C-9 (δ
C 203.8) (
Figure 2). This olefinic functional group was attached to the quaternary C-6 (δ
C 80.9) on the endoperoxide core, which was deduced from the HMBC correlations from H-7 (δ
H 6.88) and H-8 (δ
H 6.75) to C-6, thus assigning the positions of C-7 and C-8, respectively. The large coupling constant of the two olefinic protons (15.6 Hz) and the NOESY correlation between H-8 (δ
H 6.75) and 17-methyl protons (δ
H 1.24) determined a
trans double bond in an isolated spin system. Another quaternary carbon (C-10, δ
C 46.5) with geminal dimethyl groups was connected to the carbonyl C-9 according to the HMBC correlations from six overlapped methyl protons (δ
H 1.14) to C-9. The remaining one covalent connection of C-10 elongated an unbranched alkyl chain through three consecutive methylene groups to a terminal acetyl functional group, establishing the last spin system, on the basis of COSY correlations of 11-CH
2 and 12-CH
2 (δ
H 1.51) with 13-CH
2 (δ
H 2.41). The HMBC correlations from dimethyl protons 18-CH
3 and 19-CH
3 (δ
H 1.14) to the methylene carbon C-11 (δ
C 38.8) and from the methylene protons 13-CH
2 and methyl protons 15-CH
3 (δ
H 2.12) to the carbonyl C-14 (δ
C 208.5), aligning the positions C-11–C-15. Attaching to the endoperoxide core, the acyclic side chain (C-7–C-15) was elucidated furnishing the gross structure of
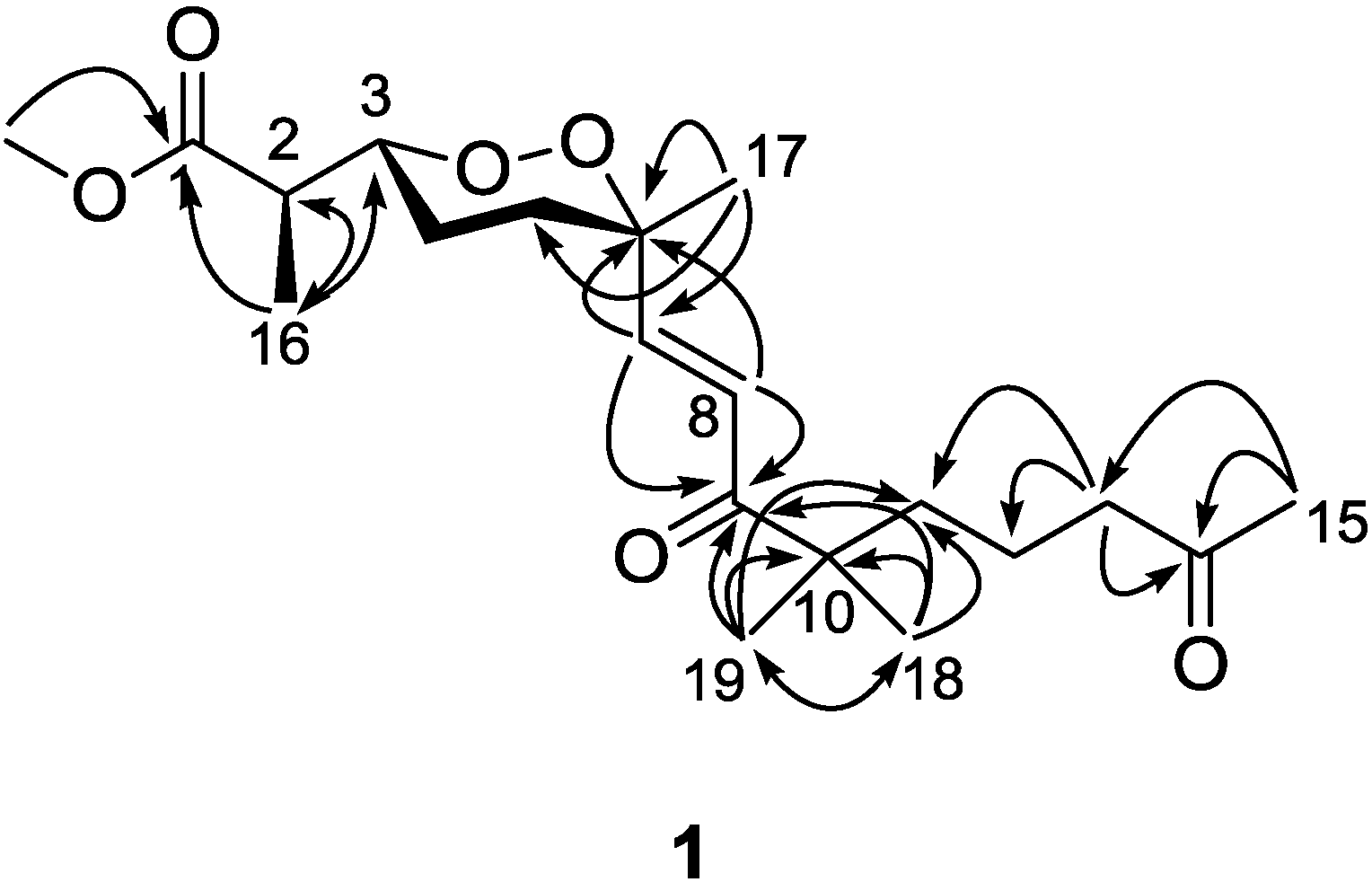
1. The signal splitting pattern of H-3 (δ
H 4.32,
J = 9.0, 2.5 Hz) suggested
anti and
gauche relationships between H-3-H-4
ax and H-3-H-4
eq, respectively. According to the Karplus equation [
20], H-3 was therefore in the axial position representing major conformations. These spatial relationships were then confirmed by the strong NOESY correlations of H-3 (δ
H 4.32)/H-4b (δ
H 1.68) and H-5a (δ
H 1.83). On the other side, the olefinic C-7 was in the axial position based on the NOESY correlation between H-7 (δ
H 6.88) and H-5b (δ
H 2.03), H-5a (δ
H 1.83) and Me-17 (δ
H 1.24), H-5b and H-8 (δ
H 6.75), H-4a (δ
H 1.51) and H-8 (
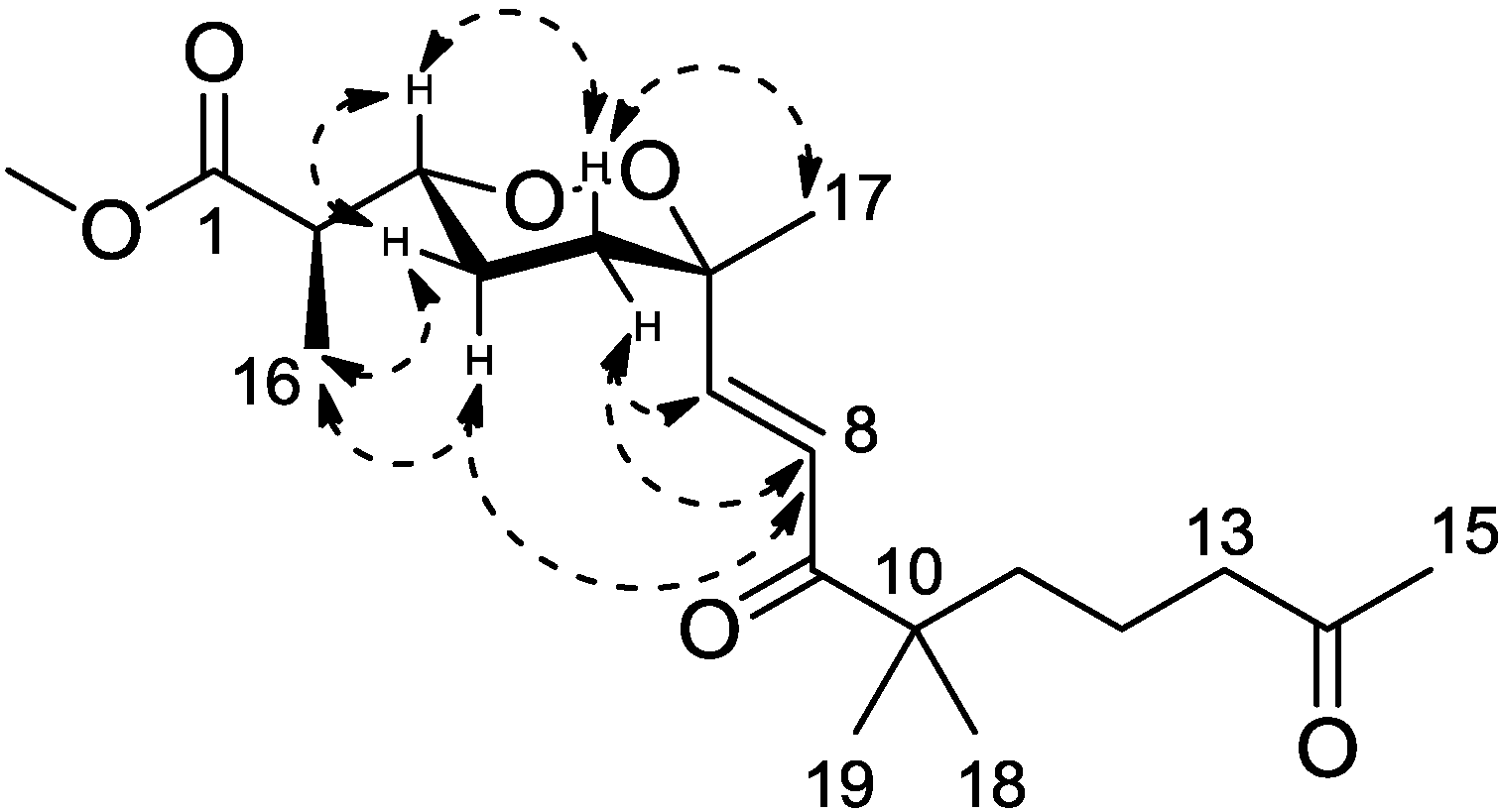
Figure 3). Calculations were then conducted to confirm this conformation. Resulting from conformational analyses and DFT calculations, an averaged
A-value of 1.8 kcal/mol was attributed to
1 from our model study (
Figure 4) [
21].
Table 1.
1H NMR data of Compounds 1–6 in CDCl3 (500 MHz).
Table 1.
1H NMR data of Compounds 1–6 in CDCl3 (500 MHz).
| Position | 1, δH, mult. (J in Hz) | 2, δH, mult. (J in Hz) | 3, δH, mult. (J in Hz) | 4, δH, mult. (J in Hz) | 5, δH, mult. (J in Hz) | 6, δH, mult. (J in Hz) |
|---|
| 2 | 2.41, t (7.2) | 2.54, quin (6.5) | 2.60, quin (7.5) | 2.59, quin (7.5) | 2.65, quin (7.5) | 2.56, quin (7.0) |
| 3 | 4.32, td (9.0, 2.5) | 4.23, td (9.0, 2.5) | 4.24, td (9.0, 1.5) | 4.23, ddd (8.0, 6.5, 2.0) | 4.23, ddd (8.0, 6.5, 1.5) | 3.70, m |
| 4 | H4a(ax), 1.51, m | 1.67, m | 1.64, m | 1.72, m | 1.72, m | H4a, 1.56, m |
| H4b(eq), 1.68, m | H4b, 1.69, m |
| 5 | H5a(ax), 1.83, td (13.2, 4.5) | 1.67, m | 1.64, m | H5a(ax), 1.72, m | 1.72, m | H5a, 1.59, m |
| H5b(eq), 2.03, dt (13.5, 3.6) | H5b(eq), 1.83, m | 1.83, m | H5b, 1.69, m |
| 7 | 6.88, d (15.6) | H7a, 1.30, td (8.0, 4.5) | H7a, 1.18, m | H7a, 2.42, dd (15.5, 8.0) | H7a, 2.83, dd (15.5, 7.0) | 1.56, m |
| H7b, 1.67, m | H7b, 1.90, m | H7b, 3.36, dd (16.0, 7.0) | H7b, 2.97, dd (15.0, 7.5) |
| 8 | 6.75, d (15.6) | H8a, 1.51, m | H8a, 1.64, m | 5.54, t (7.5) | 5.59, t (7.5) | 2.03, dd (11.0, 5.0) |
| H8b, 1.87, td (13.0, 3.0) | H8b, 1.90, m |
| 11 | 1.51, m | H11a(ax), 2.11, td (13.0, 3.0) | H11a(ax), 1.77, td (13.5, 5.5) | H11a(ax),1.56, m | H11a(ax),1.56, m | 1.90, t (6.0) |
| H11b(eq), 2.27, br.d (13.0) | H11b(eq), 2.30, br.d (13.5) | H11b(eq),1.91, m | H11b(eq),1.83, m |
| 12 | 1.51, m | 1.51, m | 1.64, m | 1.56, m | 1.56, m | H12a, 1.41, m |
| H12b, 1.56, m |
| 13 | 2.41, t (7.2) | H13a(ax), 1.36, m | H13a(ax), 1.38, br.d (13.0) | 1.41, m | 1.41, m | 1.41, m |
| H13b(eq), 1.67, m | H13b(eq), 1.64, m |
| 15 | 2.12, s | 1.12, d (7.0) | 1.14, d (7.0) | 1.12, d (7.0) | 1.14, d (7.0) | 1.21, d (7.2) |
| 16 | 1.10, d (7.2) | 1.09, s | 1.09, s | 1.13, s | 1.13, s | 1.21, s |
| 17 | 1.24, s | H17a, 4.81, br.s | H17a, 4.89, br.s | 1.42, s | 1.41, s | 1.59, s |
| H17b, 4.89, br.s | H17b, 4.91, br.s |
| 18 | 1.14, s | 0.97, s | 0.98, s | 1.18, s | 1.17, s | 0.99, s |
| 19 | 1.14, s | 0.89, s | 0.91, s | 1.07, s | 1.08, s | 0.99, s |
| 1-OCH3 | 3.70, s | 3.69, s | 3.70, s | 3.70, s | 3.70, s | 3.71, s |
Table 2.
13C NMR data and DEPT analysis of Compounds 1–6 in CDCl3 (125 MHz).
Table 2.
13C NMR data and DEPT analysis of Compounds 1–6 in CDCl3 (125 MHz).
| Position | 1, δC, DEPT | 2, δC, DEPT | 3, δC, DEPT | 4, δC, DEPT | 5, δC, DEPT | 6, δC, DEPT |
|---|
| 1 | 174.1, C | 174.3, C | 174.3, C | 174.6, C | 174.6, C | 176.4, C |
| 2 | 42.5, CH | 42.6, CH | 42.5, CH | 42.5, CH | 42.5, CH | 45.4, CH |
| 3 | 81.6, CH | 81.0, CH | 81.2, CH | 81.6, CH | 81.4, CH | 73.7, CH |
| 4 | 23.5, CH2 | 22.4, CH2 | 22.4, CH2 | 22.7, CH2 | 22.5, CH2 | 28.8, CH2 |
| 5 | 33.5, CH2 | 33.3, CH2 | 32.9, CH2 | 32.3, CH2 | 32.4, CH2 | 37.3, CH2 |
| 6 | 80.9, C | 79.9, C | 79.9, C | 80.2, C | 80.4, C | 72.7, C |
| 7 | 147.6, CH | 28.3, CH2 | 28.4, CH2 | 37.5, CH2 | 37.5, CH2 | 42.2, CH2 |
| 8 | 124.3, CH | 25.6, CH2 | 26.1, CH2 | 120.1, CH | 120.6, CH | 22.8, CH2 |
| 9 | 203.8, C | 80.0, C | 79.6, C | 152.6, C | 151.6, C | 136.7, C |
| 10 | 46.5, C | 151.3, C | 150.3, C | 73.9, C | 74.0, C | 127.0, C |
| 11 | 38.8, CH2 | 33.8, CH2 | 34.0, CH2 | 44.2, CH2 | 43.9, CH2 | 32.8, CH2 |
| 12 | 19.0, CH2 | 22.8, CH2 | 22.8, CH2 | 19.4, CH2 | 19.3, CH2 | 19.5, CH2 |
| 13 | 43.9, CH2 | 38.0, CH2 | 38.1, CH2 | 39.9, CH2 | 39.9, CH2 | 39.9, CH2 |
| 14 | 208.5, C | 39.9, C | 39.8, C | 37.5, C | 37.5, C | 35.1, C |
| 15 | 29.8, CH3 | 12.5, CH3 | 12.8, CH3 | 13.0, CH3 | 13.0, CH3 | 14.2, CH3 |
| 16 | 12.6, CH3 | 23.9, CH3 | 23.9, CH3 | 23.8, CH3 | 24.2, CH3 | 26.6, CH3 |
| 17 | 26.1, CH3 | 107.9,CH2 | 108.6, CH2 | 29.2, CH3 | 29.4, CH3 | 19.8, CH3 |
| 18 | 24.1, CH3 | 24.1, CH3 | 24.2, CH3 | 32.3, CH3 | 32.4, CH3 | 28.7, CH3 |
| 19 | 23.9, CH3 | 22.2, CH3 | 22.4, CH3 | 32.0, CH3 | 32.0, CH3 | 28.7, CH3 |
| 20 | 51.9, OCH3 | 51.8, OCH3 | 51.9, OCH3 | 51.9, OCH3 | 51.9, OCH3 | 51.7, OCH3 |
Figure 2.
Key HMBC correlations for Compound 1.
Figure 2.
Key HMBC correlations for Compound 1.
Figure 3.
Key NOESY correlations for Compound 1.
Figure 3.
Key NOESY correlations for Compound 1.
Figure 4.
A-value determination of the endoperoxide core.
Figure 4.
A-value determination of the endoperoxide core.
From the assigned conformation, the relative configuration of C-2/C-3 was then suggested as
erythro (2
R,3
R or 2
S,3
S) by the empirical method, which was based on the
1H NMR chemical shift difference of the methyl group C-16 between the
erythro and
threo configuration [
14]. To backup this method, NMR calculations were performed at different theoretical levels [
22]. The calculated chemical shift for H-16 (δ
calc. 1.08/δ
exp. 1.10) at the B3LYP/6-31G(d,p) level matched precisely with our experimental value; while for the supposed
threo configuration (2
R,3
S or 2
S,3
R), the calculated value for H-16 (δ
calc. epimer 1.31/δ
exp. 1.24–1.26) showed further downfield from Capon’s report [
14]. These results confirmed the
erythro configuration of C-2 with C-3 and secured Capon’s method. Not being mentioned by the report, probably because of the trivial differences, C-16 chemical shifts for those
threo configurations were slightly upfield compared with the
erythro ones. DFT calculations were able to track this trend and resolve the small differences, although the relative chemical shifts for
13C NMR could not be predicted as accurately as for the proton NMR, resulting in C-16 (δ
C 12.4–12.8/δ
calc. 15.7–16.1 for
erythro, and δ
C 13.2–13.5/δ
calc. 17.0–18.1 for
threo) [
14]. NMR calculations for
2–
5 (
vide infra) were also conducted and matched well with the experiments (
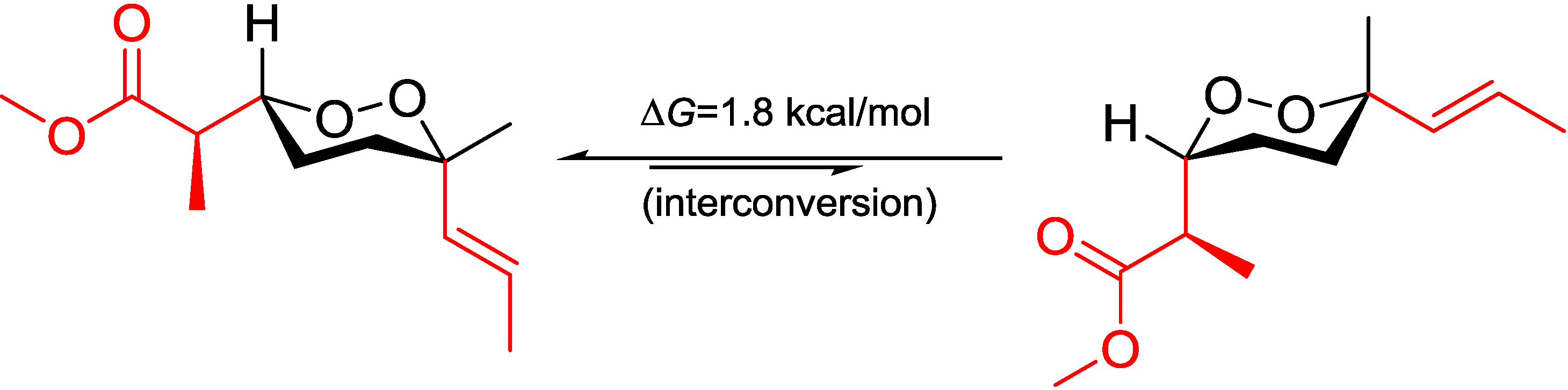
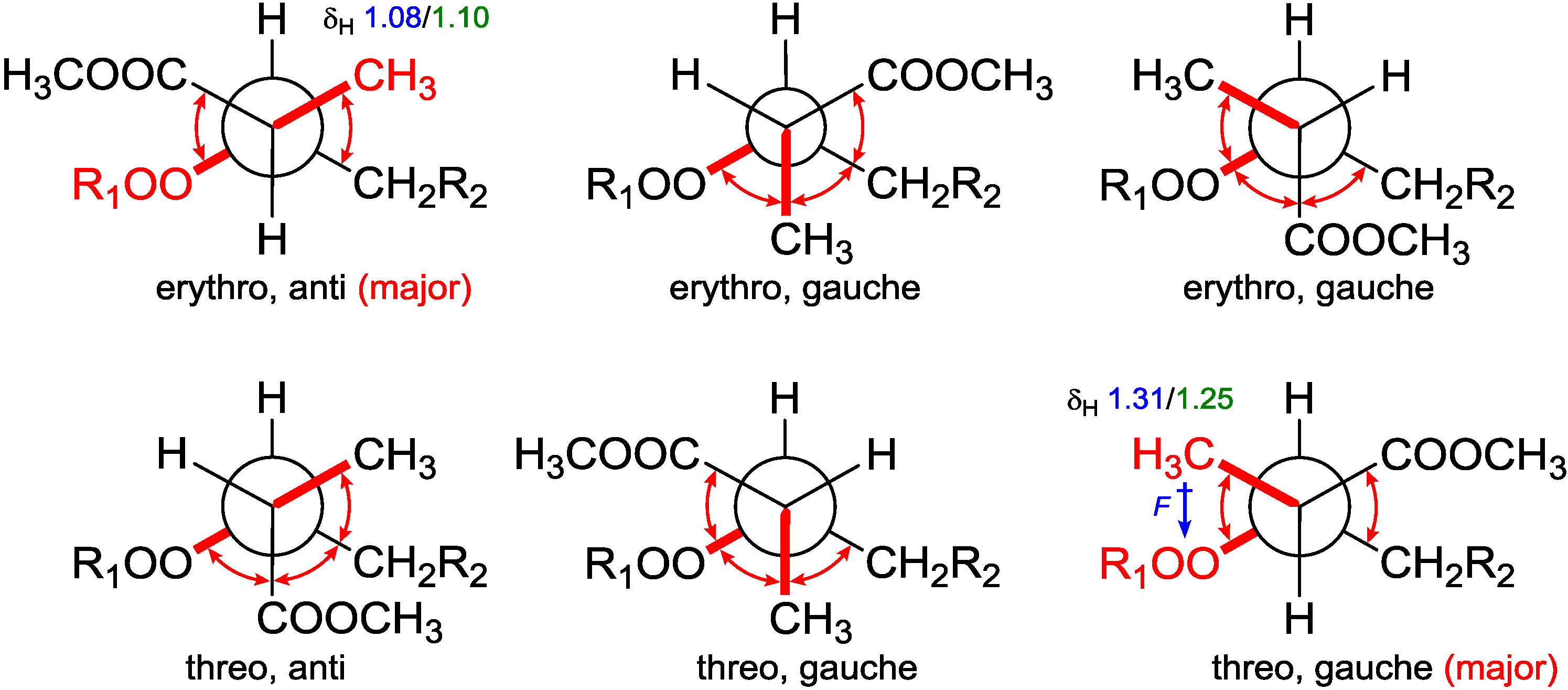
Table 3). The chemical shift differences between the two possible diastereomers revealed that C-16 was anti to the peroxide group in the
erythro configuration, whereas it was gauche in the
threo configuration representing the major conformations. These preferred arrangements were due to minimizing the unfavored gauche interactions (
Figure 5). Calculations for the minor conformations of
erythro/gauche and
threo/anti showed mismatching (
Table 3).
Table 3.
Calculated NMR chemical shifts for the predominant conformations of Compounds 1–5 using different methods applying the PCM (polarizable continuum model) solvation model.
Table 3.
Calculated NMR chemical shifts for the predominant conformations of Compounds 1–5 using different methods applying the PCM (polarizable continuum model) solvation model.
| Species | B3LYP | MPW1PW91 | Experimental | B3LYP | MPW1PW91 | Experimental |
|---|
| (erythro/threo) | (erythro/threo) | (erythro/threo) | (erythro/threo) |
|---|
| 1 | δH16 1.08/1.31 | δH16 1.01/1.25 | δH16 1.10 | δC16 15.8/17.5 | δC16 15.0/16.7 | δC16 12.6 |
| 2 | δH15 1.11/1.29 | δH15 1.03/1.24 | δH15 1.09 | δC15 16.1/17.0 | δC15 15.0/16.2 | δC15 12.5 |
| 3 | δH15 1.13/1.35 | δH15 1.06/1.29 | δH15 1.09 | δC15 15.9/17.9 | δC15 15.0/17.0 | δC15 12.8 |
| 4 | δH15 1.11/1.34 | δH15 1.04/1.26 | δH15 1.13 | δC15 15.7/17.9 | δC15 15.0/16.6 | δC15 13.0 |
| 5 | δH15 1.12/1.36 | δH15 1.05/1.30 | δH15 1.13 | δC15 16.1/18.1 | δC15 15.3/17.2 | δC15 13.0 |
Figure 5.
Conformational analysis of C-2–C-3 using Newman projection (blue and green numeric numbers indicating calculated and experimental values, respectively; curved red arrows indicating gauche interactions).
Figure 5.
Conformational analysis of C-2–C-3 using Newman projection (blue and green numeric numbers indicating calculated and experimental values, respectively; curved red arrows indicating gauche interactions).
On the grounds of these results, we believed that it is the field effect produced by the peroxide moiety withdrawing electrons from the methyl C-16 in the
threo configuration, thus deshield it to low field, whereas C-16 in the
erythro configuration is distal from the peroxy; thus, no deshielding was observed (
Figure 5). Although no XRD structure of
1 could be obtained, our calculations showed the same conformation (
Figure S52 in Supplementary Information) of the endoperoxide core as all of the three closely-related crystal structures [
12,
13,
14]. Back to our calculations, it is the configurationally dependent conformations that thus dictate the chemical shift differences and underlie the empirical rule.
With the relative configuration and major conformations of
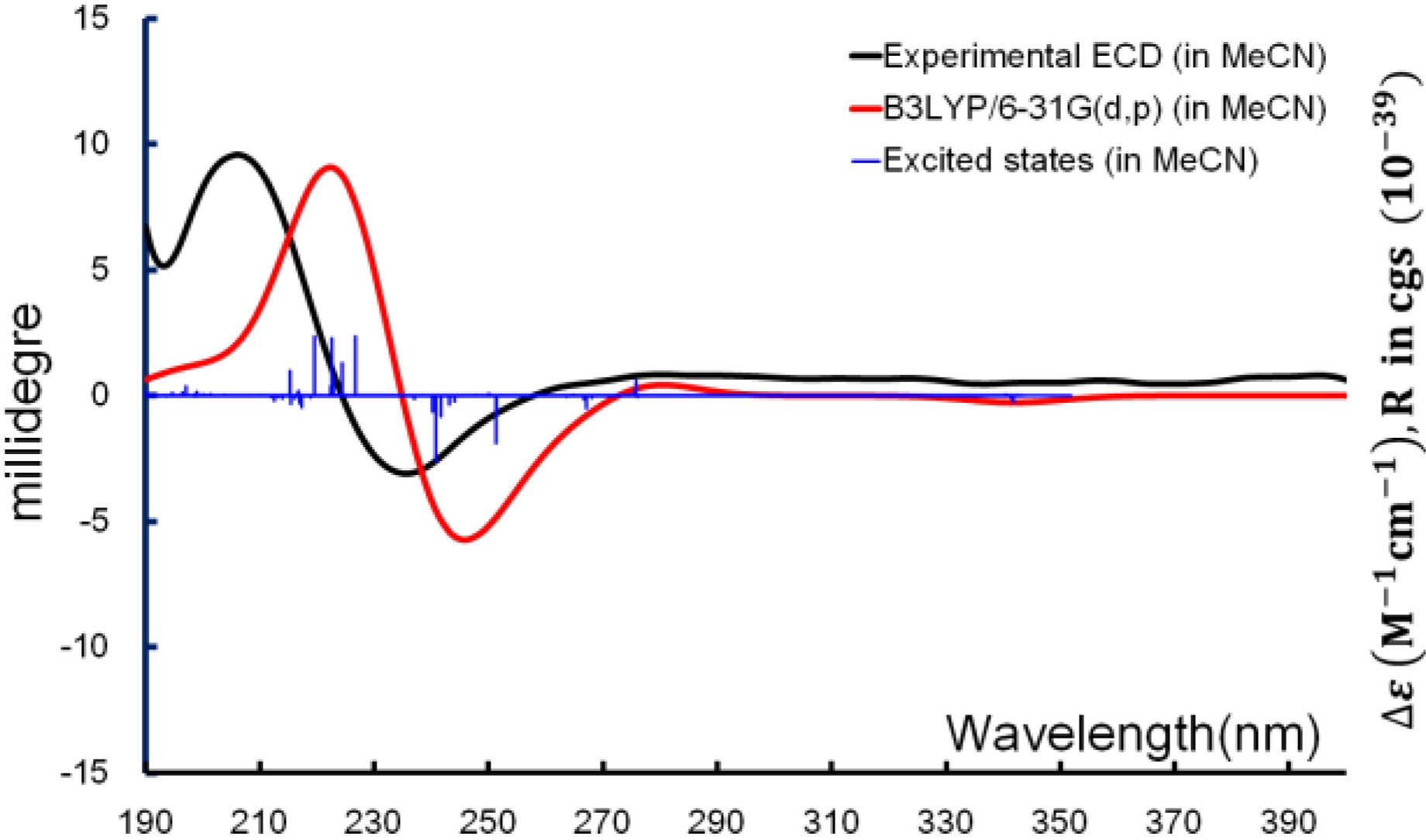
1 established, ECD computation was used for determining the absolute configuration. Time-dependent density function theory (TDDFT) was applied for the excited-state calculations, and the experimental ECD spectrum was used for comparison. The exciton from π→π* transition of the α,β-unsaturated carbonyl producing the CE (Cotton effect) around 240 nm was able to be reproduced
in silico. From the overlaid spectra (
Figure 6), the absolute configuration of
1 was thus elucidated as 2
R,3
R,6
S.
Figure 6.
Boltzmann averaged ECD of diacarperoxide H (1).
Figure 6.
Boltzmann averaged ECD of diacarperoxide H (1).
Diacarperoxide I (2) was obtained as a colorless oil with a molecular formula of C20H34O5 deduced from the HRESIMS data, implying four degrees of unsaturation. Three degrees were assigned to a carbonyl group (δC 174.3), a pair of olefinic carbons (δC 151.3, 107.9) and a typical endoperoxide ring characterized by two carbons (δC 81.0/δH 4.23, δC 79.9), while the remaining one degree of unsaturation suggested an additional ring closure. It was demonstrated containing the endoperoxide core as in 1 by comparing their 1D and 2D NMR data. The attachment on C-6 was, however, significantly different from 1; instead of an olefinic substitution, a group of two consecutive methylene was attached to C-6, supported by the HMBC correlations of H-16 (δH 1.09)/C-7 (δC 28.3) and H-7 (δH 1.30)/C-16 (δC 23.9), in addition to the COSY correlations of H-7a (δH 1.30) with CH2-8 (δH 1.51, 1.87), together assigning the methylene C-7 (δC 28.3) and C-8 (δC 25.6). Another three methylenes, a dimethylated quaternary carbon, a disubstituted olefinic carbon and an oxyl quaternary carbon lynchpin tethered a six-membered ring. This arrangement was elucidated starting from the strong HMBC cross-peaks from two methyl protons 18-CH3 (δH 0.97, s) and 19-CH3 (δH 0.89, s) to the quaternary C-14 (δC 39.9) to the methyl carbon of each other and to the quaternary carbon C-9 (δC 80.0); and then, verified by the HMBC correlations from the axial H-13a (δH 1.36) to the geminal dimethyl C-18 (δC 24.1) and C-19 (δC 22.2) and from the terminal olefinic CH2-17 (δH 4.81, 4.89) to C-9 (δH 80.0) and C-11 (δH 33.8); and finally, confirmed by the COSY correlation of a well resolved proton resonance H-11b (δH 2.27) with CH2-12 (δH 1.51), fixing the positions of C-9–C-19. This enclosure together with C-8 formed a monoterpene motif attaching to the endoperoxide core.
Relative configurations for the three stereogenic centers in the endoperoxide core were determined using the same method as for
1, but the existence of an additional center, C-9, with a tertiary hydroxyl group attachment challenged us in two different manners. First, the configuration of C-9 was difficult to be spatially related to the other three centers because of the remote distance. Second, it was difficult to handle with the limited amount of our sample by applying degradation or modification on this chemical environment. It was fortunate that a closely related compound, diacarperoxide J (
3), was purified in our hands with the gross structure elucidated by comparing the spectroscopic data with
2. Notably, all of the
1H and
13C NMR chemical shifts of
3 were almost identical to
2, except for the nuclei surround C-9 (
Table 1 and
Table 2), which suggested a different relative configuration, but we still cannot conclude that
3 was the C-9 epimer of
2, since the opposite stereogenicity of each center on the marine-derived endoperoxide class has been reported before [
6,
23,
24]. Theoretically, there were four possibilities regarding the absolute configurations, e.g., for the diastereomeric-pair Compounds
2 and
3. However, based on the fact that Compounds
2 and
3 were diastereomers other than enantiomers, there were only two possible combinations, which were 2
R, 3
R, 6
S, 9
S/2
R, 3
R, 6
S, 9
R and 2
S, 3
S, 6
R, 9
R/2
S, 3
S, 6
R, 9
S, and the Compounds
2 and
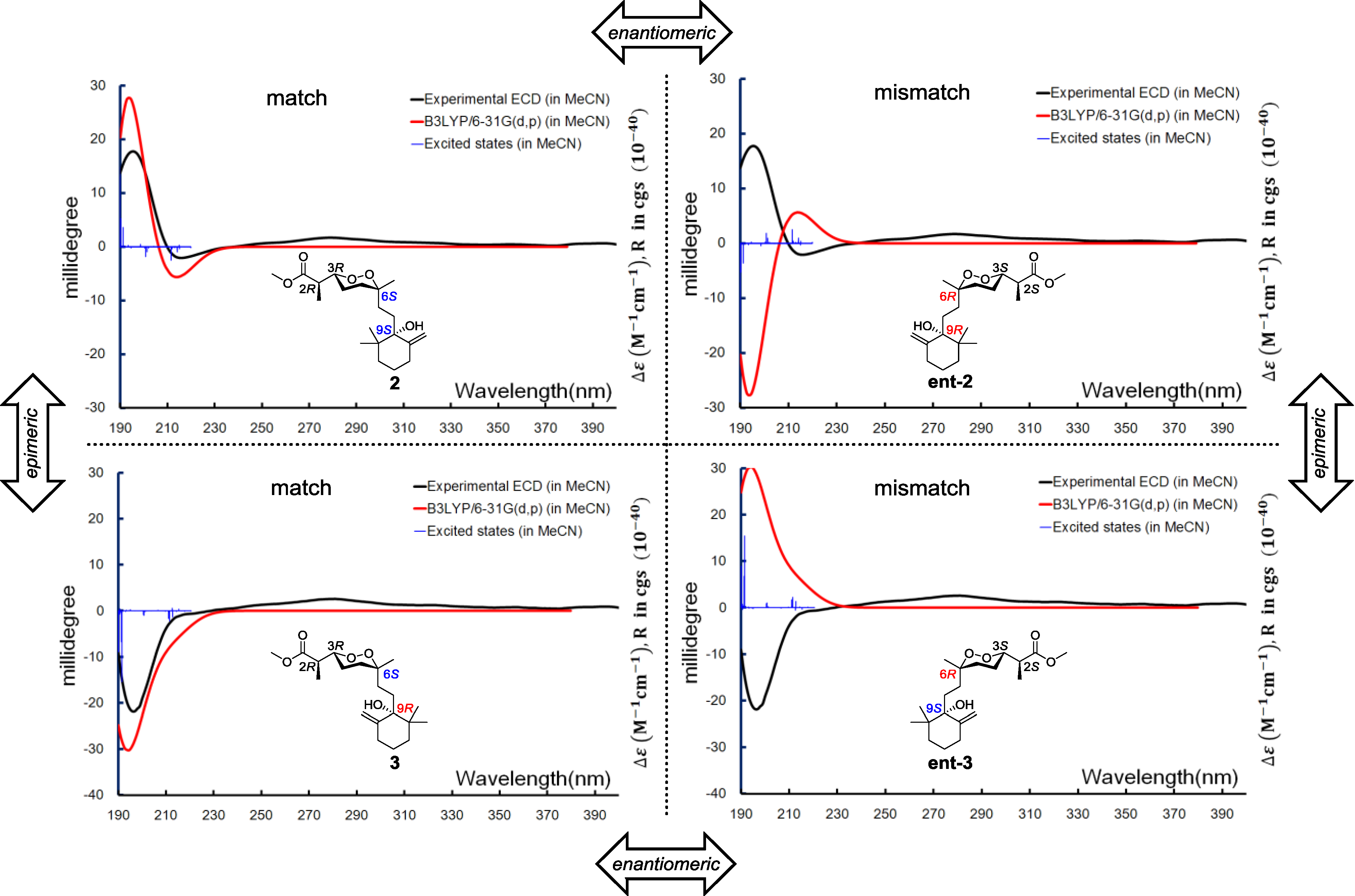
3 have to be one of those combinations. Anisotropic CEs (200 nm, positive; 210 nm, negative) of
2 and
3 were recorded by the ECD experiments, which prompted us to use exited-state calculations investigating the absolute configurations directly before establishing the relative configurations. A combination of four possible absolute configurations of
2 and
3 were modeled, and the subsequential excited-state calculations reproduced the Cotton effect, therefore assigning the absolute configurations of
2 and
3 as 2
R,3
R,6
S,9
S and 2
R,3
R,6
S,9
R, respectively (
Figure 7).
Figure 7.
Boltzmann averaged ECD of diacarperoxides I and J (2 and 3).
Figure 7.
Boltzmann averaged ECD of diacarperoxides I and J (2 and 3).
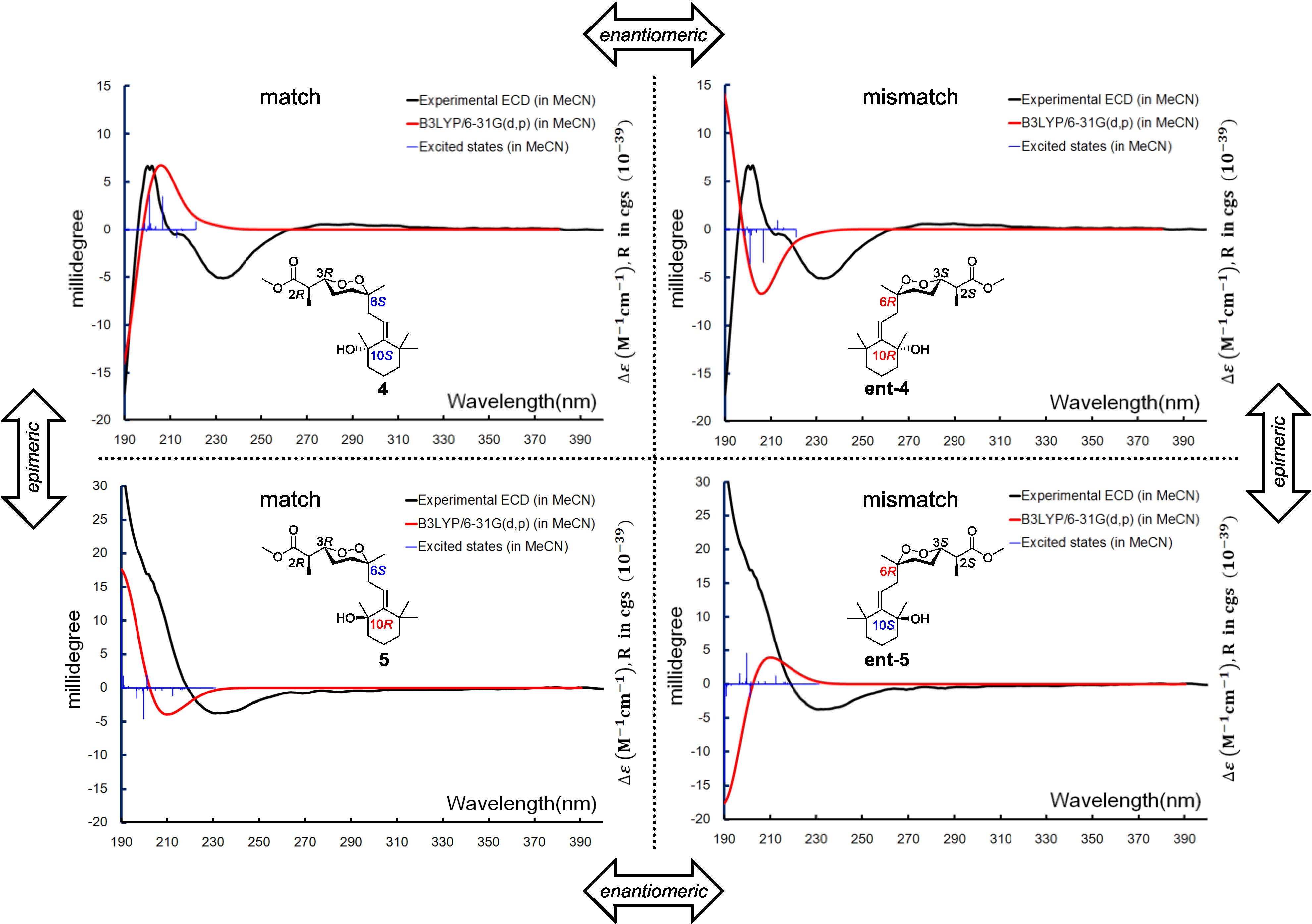
Diacarperoxide K (
4) was determined as a structural isomer of
2 and
3 by comparing the spectroscopic data. The differences were an extra methyl group showing 3H (δ
H 1.42, s) and C (δ
C 29.2), and a monosubstituted olefinic carbon showing C-8 (δ
C 120.1) and H-8 (δ
H 5.54, t,
J = 7.5 Hz); in addition to the absence of C-8 methylene resonance together postulated an E1 on the tertiary hydroxyl group and a hydrolysis of the olefinic functional group of
2 or
3. By analyzing the NMR spectra, the position of a trisubstituted olefin, assigned as C-8 and C-9, could be fixed from the COSY correlations of CH
2-7 (δ
H 2.42, 3.36) with H-8 (δ
H 5.54). The extra tertiary methyl group showed HMBC correlations from 17-CH
3 (δ
H 1.42) to C-11 (δ
C 44.2), C-10 (δ
C 73.9) and C-9 (δ
C 152.6), determining its position as C-17 attached to the oxyl quaternary carbon C-10 (δ
C 73.9), which was vicinal to the disubstituted sp
2 C-9 (δ
C 152.6). The
Z configuration of the double bond was determined by the NOESY correlations between geminal dimethyl H-18 (δ
H 1.18) and H-19 (δ
H 1.07) and the olefinic proton H-8. The remaining gross structure of
4 was unchanged from
2 and
3 by comparing the 1D and 2D NMR spectra. The same case as
2 and
3, for the diastereomeric-pair Compounds
4 and
5, there were only two possible combinations, which were 2
R, 3
R, 6
S, 10
S/2
R, 3
R, 6
S, 10
R and 2
S, 3
S, 6
R, 10
R/2
S, 3
S, 6
R, 10
S. The similar stereochemistry issue of C-10 was solved by comparing with the ECD spectrum of isolated epimer diacarperoxide L (
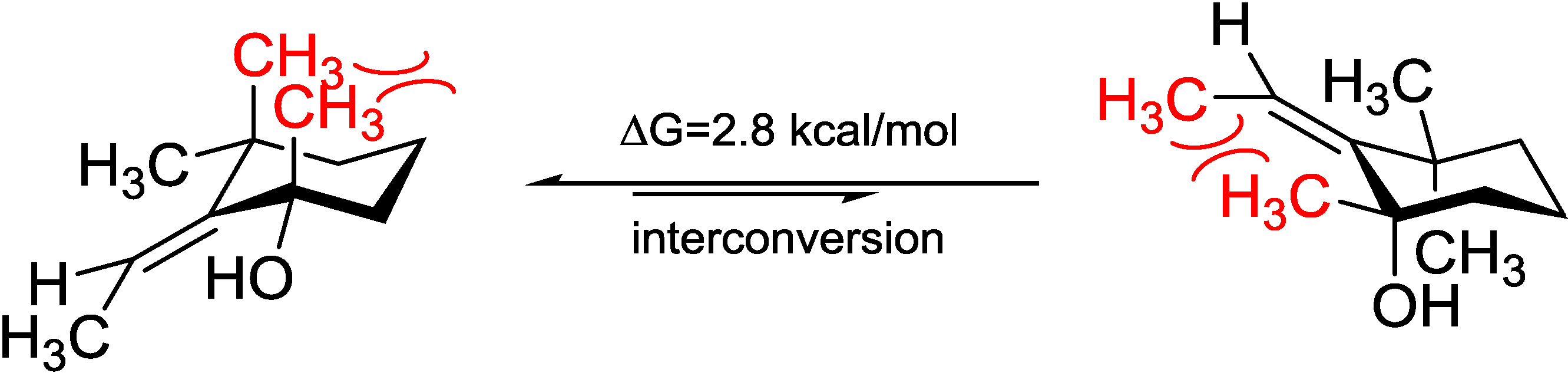
5). Acquiring from the major conformations (
Figure S52 in Supplementary Information) and the diaxial NOESY correlations of
4 and
5, hydroxyl groups were in the equatorial positions, whereas the two methyl groups were surprisingly in the axil positions, producing inevitable 1,3-diaxial interactions, but compromising with higher
A-values and avoiding more unfavored 1,3-allylic strains (
Figure 8). Comparing the computed ECD with the experiments, the absolute configurations of
4 and
5 were thus assigned as 2
R,3
R,6
S,10
S and 2
R,3
R,6
S,10
R, respectively (
Figure 9).
Figure 8.
Model study of the 1,3-diaxial interaction vs. 1,3-allylic strain of Compounds 4 and 5 applying the solvation model.
Figure 8.
Model study of the 1,3-diaxial interaction vs. 1,3-allylic strain of Compounds 4 and 5 applying the solvation model.
Figure 9.
Boltzmann averaged ECD of diacarperoxides K and L (4 and 5).
Figure 9.
Boltzmann averaged ECD of diacarperoxides K and L (4 and 5).
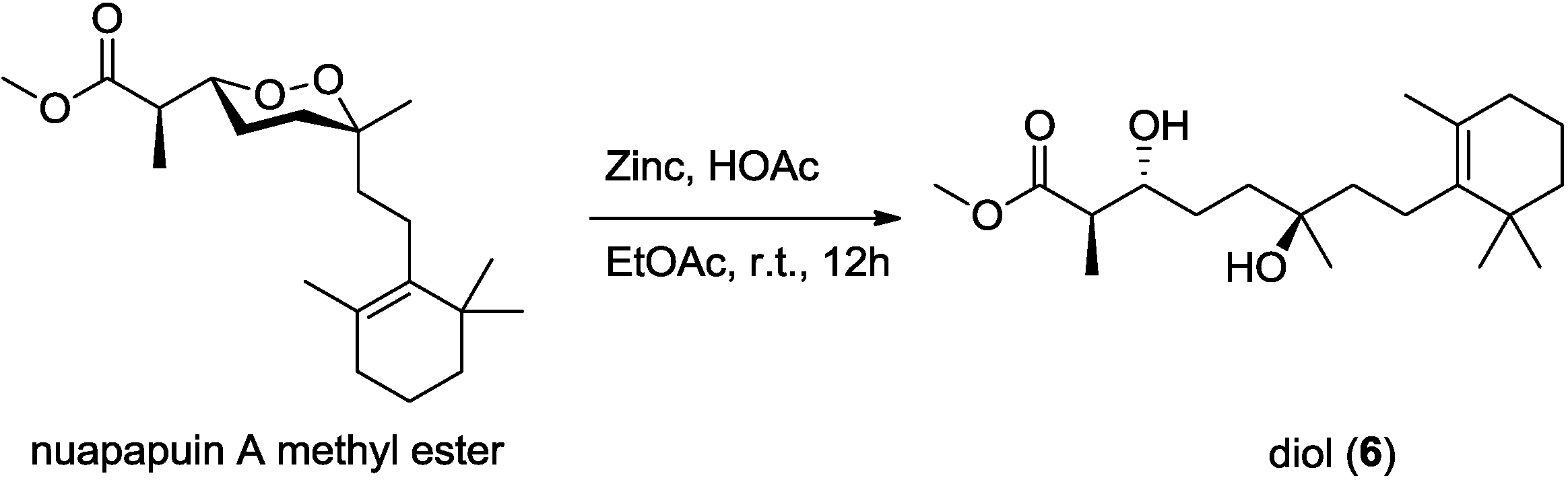
Diacardiol B (
6) was obtained as a white solid. The HRESIMS data suggested a molecular formula of C
20H
36O
4 with three degrees of unsaturation. Comparing the NMR spectra with
1–
5, the carbon chemical shifts presumably belonging to the endoperoxide core changed significantly, in which C-3 and C-6 were upfield from 81 and 80 ppm to 73.7 and 72.7 ppm, but C-4 and C-5 were downfield from 22 and 33 ppm to 28.8 and 37.3 ppm, respectively. Compound
6 was likely generated from homolysis of the peroxy. The cyclic monoterpene part was also changed for the absence of the quaternary oxyl carbon resonance and the emergence of a tetrasubstituted olefinic C-9 (δ
C 136.7) and C-10 (δ
C 127.0). The olefin was at the C-9–C-10 position, determined by the HMBC correlations from four allylic methylene protons 8-CH
2 (δ
H 2.03), 11-CH
2 (δ
H 1.90) and from the vinylic methyl protons 17-CH
3 (δ
H 1.59) to the olefinic carbons. Compound
6 was likely the acyclic form of nuapapuin A methyl ester. We applied chemical degradation on nuapapuin A methyl ester (which was also obtained by us from the same sponge) under reductive condition (
Figure 10), and the product showed identical
1H NMR spectrum and similar optical rotation with
6, verifying the structures of both of these two natural products [
12,
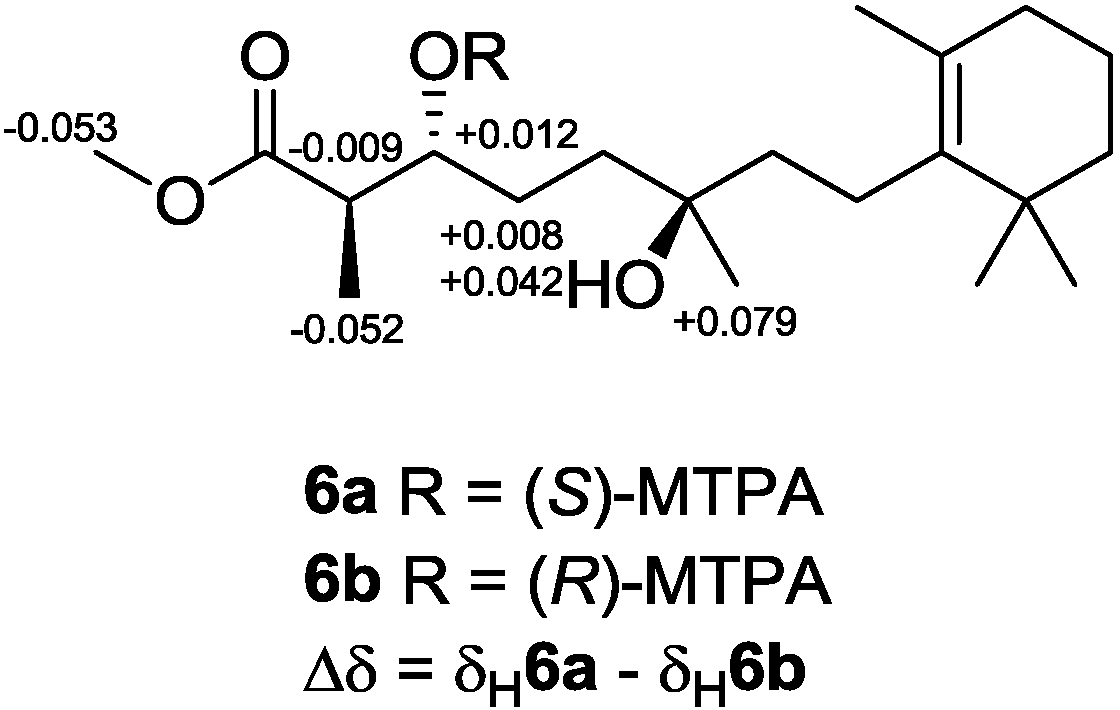
25]. To establish the absolute configuration of
6, modified Mosher’s method [
26,
27] was applied, and NMR anisotropic analysis of corresponding product was used to assign the absolute configuration of
6 as 2
R,3
R,6
R (
Figure 11).
Figure 10.
Reductive cleavage of the peroxide in nuapapuin A methyl ester.
Figure 10.
Reductive cleavage of the peroxide in nuapapuin A methyl ester.
Figure 11.
Determining the absolute configuration of diacardiol B (6) using the NMR anisotropy method.
Figure 11.
Determining the absolute configuration of diacardiol B (6) using the NMR anisotropy method.
Diacarperoxides H–J (
1–
3) showed antimalarial activity against
Plasmodium falciparum (W2 clones)
in vitro with IC
50 values of 12.9, 4.8 and 1.8 μM, while
2 and
3 exhibited antimalarial activity
in vitro against
P. falciparum (D6 clones) with IC
50 values of 7.9 and 1.6 μM, respectively (
Table 4); the IC
50 values of the control drug artemisinin were 0.071 (D6 clones) and 0.14 μM (W2 clones). The cytotoxicity against human cancer cell lines HeLa (cervical cancer), QGY-7703 (hepatocarcinoma), MDA-MB-231 (breast adenocarcinoma) and A549 (lung carcinoma) were tested for
1–
6 (
Tables S17–S20 in Supplementary Information); none of them showed significant cytotoxicity (IC
50 > 40 μM). Interestingly, there is no significant difference between Compounds
2 and
3 in sensitivity to the two
Plasmodium strains above, while Compound
1 is sensitive to the W2 clone strain; this may result from the acyclic side chain (C-7–C-15). Although the security index is relatively small, this type of marine endoperoxides could still be considered as potential drug candidates acting against malaria, and structure-activity relationships (SAR) and biological studies should be encouraged to be conducted in the future.
Table 4.
In vitro antimalarial activity of Compounds 1–3.
Table 4.
In vitro antimalarial activity of Compounds 1–3.
| Compound | Plasmodium falciparum |
|---|
| W2 Clone | D6 Clone |
|---|
| IC50 (μM) | SI a | IC50 (μM) | SI |
|---|
| 1 | 12.9 | 1.0 | not active | |
| 2 | 4.8 | 2.8 | 7.9 | 1.7 |
| 3 | 1.8 | 7.4 | 1.6 | 8.2 |
3. Experimental Section
3.1. General Experimental Procedures
NMR experiments were performed on a 500 MHz instrument (Bruker Biospin Corp., Billerica, MA, USA). High-resolution mass spectroscopic data were acquired using a TOFESIMS (Waters Corp., Milford, MA, USA). ECD spectra were recorded on a CD spectropolarimeter (Jasco Inc., Tokyo, Japan) with the path length of 1 cm and a scan width of 190 to 400 nm, and optical rotation data were measured on a general polarimeter (Perkin-Elmer Inc., Waltham, MA, USA) with a sodium lamp. Reversed-phase HPLC was performed on a C18 column (250 × 10 mm, 5 μm) using a preparative HPLC instrument (Waters Corp., Milford, MA, USA) with a UV detector (Waters Corp., Milford, MA, USA). Column chromatography (CC) was performed on Sephadex LH-20 (Pharmacia Fine Chemicals, Piscataway, NJ, USA) and ODS-A (50 μm, YMC Co. Ltd., Kyoto, Japan) columns, and vacuum liquid chromatography (VLC) was conducted on a column packed with silica gel (200–300 mesh, Qingdao Ocean Chemical Co., Jinan, China).
3.2. Animal Material
The Diacarnus megaspinorhabdosa sample was collected from Woody Island in the South China Sea during April, 2010, and identified by Prof. Jin-He Li (Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China). A voucher sample (No. XD-2010008) was deposited in the Laboratory of Marine Drugs, Department of Pharmacy, Changzheng Hospital, Second Military Medical University, China.
3.3. Extraction and Isolation
The sponge (2 kg, dry weight) was cut and extracted with 95% aqueous EtOH. The crude extraction was concentrated in vacuo, yielding a brown gum, which was then suspended in H2O and participated with EtOAc and n-BuOH to afford EtOAc and n-BuOH extracts, respectively. The EtOAc extract was dissolved in MeOH/H2O (9:1, v/v) extracted with petroleum ether to yield a brownish-red oil (30 g). The aqueous MeOH phase was then diluted to MeOH/H2O (3:2, v/v) and extracted with CH2Cl2 to afford a crude extract (18 g), which was then subjected to VLC packed with silica gel gradiently eluted with an n-hexane/acetone system to yield eight fractions (Fr. A–H). Fraction C (4.9 g) was further separated on a reversed-phase C18 silica gel column to obtain four subfractions (Fr. C1–C4), and subfraction C2 (210 mg) was purified by HPLC (YMC-Pack Pro C18 RS, 5 μm, 250 × 10 mm, 1.5 mL/min, UV detection at 210 and 202 nm) eluting with CH3OH/H2O (75:25) to yield 2 (16 mg) and 3 (20 mg). Similarly, subfraction C3 (150 mg) was purified by HPLC eluted with CH3OH/H2O (70:30) to yield 4 (10 mg) and 5 (7 mg). Fraction D (5.2 g) was purified by silica gel column eluted with CH2Cl2/EtOAc (8:1 to 1:1, v/v) to yield six subfractions (Fr. D1–D6), and subfraction D2 (100 mg) was isolated using a Sephadex LH-20 column eluted with CH2Cl2/MeOH (1:1, v/v) and then purified by HPLC to obtain 1 (30 mg). Fraction E (450 mg) was separated by silica gel column chromatography eluted with CH2Cl2/EtOAc (7:1–1:1, v/v) to afford 6 (18 mg).
Diacarperoxide H (
1): colorless oil; [α]
20D +52.7 (
c 0.28, CHCl
3); CD (CH
3CN,
c 2.2 × 10
−4) λ
max (millidegree), 192 (5.34) nm, 204 (9.43) nm, 234 (−3.05) nm; UV (CH
3CN), λ
max (log ε): 196 (2.66), 224 (3.25) nm; IR (KBr) ν
max: 3463, 2953, 1740, 1715, 1693, 1627, 1459, 1364, 1266, 1200, 1167, 1078, 1009, 982, 950, 860, 738, 477 cm
−1;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) data, see
Table 1 and
Table 2; HRESIMS
m/
z 391.2092 (calcd. for C
20H
32O
6Na, 391.2091).
Diacarperoxide I (
2): colorless oil; [α]
20D +36.5 (
c 0.28, CHCl
3); CD (CH
3CN,
c 2.2 × 10
−4) λ
max (millidegree), 195 (17.75) nm, 215 (−2.07) nm; UV (CH
3CN), λ
max (log ε): 196 (3.23), 246 (sh, 1.16) nm; IR (KBr) ν
max: 3552, 2934, 2864, 1740, 1456, 1337, 1261, 1200, 1160, 1060, 1013, 898, 872, 802, 692, 621, 472 cm
−1;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) data, see
Table 1 and
Table 2; HRESIMS
m/
z 377.2296 (calcd. for C
20H
34O
5Na, 377.2299).
Diacarperoxide J (
3): colorless oil; [α]
20D +59.1 (
c 0.64, CHCl
3); CD (CH
3CN,
c 2.2 × 10
−4) λ
max (millidegree), 196 (−21.89) nm; UV (CH
3CN), λ
max (log ε): 204 (3.64) nm; IR (KBr) ν
max: 3557, 2974, 2938, 2865, 1740, 1457, 1378, 1267, 1201, 1162, 1061, 1014, 901 cm
−1;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) data, see
Table 1 and
Table 2; HRESIMS
m/
z 377.2301 (calcd. for C
20H
34O
5Na, 377.2299).
Diacarperoxide K (
4): colorless oil; [α]
20D +41.0 (
c 0.20, CHCl
3); CD (CH
3CN,
c 2.2 × 10
−4) λ
max (millidegree), 200 (6.65) nm, 232 (−5.08) nm; UV (CH
3CN), λ
max (log ε): 196 (3.23), 222 (sh, 2.72) nm; IR (KBr) ν
max: 3458, 2930, 2873, 1740, 1458, 1374, 1265, 1199, 1164, 1074, 1012, 872 cm
−1;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) data, see
Table 1 and
Table 2; HRESIMS
m/
z 377.2297 (calcd. for C
20H
34O
5Na, 377.2299).
Diacarperoxide L (
5): colorless oil; [α]
20D +23.6 (
c 0.38, CHCl
3); CD (CH
3CN,
c 2.2 × 10
−4) λ
max (millidegree), 230 (−3.73) nm; UV (CH
3CN), λ
max (log ε): 198 (3.25), 222 (sh, 2.64) nm; IR (KBr) ν
max: 3396, 2944, 2877, 1739, 1455, 1376, 1264, 1199, 1164, 1058, 1009, 904 cm
−1;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) data, see
Table 1 and
Table 2; HRESIMS
m/
z 377.2301 (calcd. for C
20H
34O
5Na, 377.2299).
Diacardiol B (
6): colorless oil; [α]
20D −2.4 (
c 0.50, CHCl
3); UV (CH
3CN), λ
max (log ε): 210 (3.73) nm; IR (KBr) ν
max: 3319, 3208, 2949, 2927, 2870, 1740, 1465, 1435, 1358, 1199, 1167, 1123, 1087, 1043, 1025, 983, 968, 908, 886, 853, 754 cm
−1;
1H NMR (CDCl
3, 500 MHz) and
13C NMR (CDCl
3, 125 MHz) data, see
Table 1 and
Table 2; HRESIMS
m/
z 363.2506 (calcd. for C
20H
36O
4Na, 363.25058).
Nuapapuin A methyl ester: Colorless oil; [α]20D +71.2 (c 0.67, CHCl3); 1H NMR (CDCl3, 500 MHz): δ 2.58 (1H, q, J = 7.5 Hz, H-2), 4.27 (1H, td, J = 8.5, 3.0 Hz, H-3), 1.17 (3H, d, J = 7.5 Hz, 2-Me), 1.16 (3H, s, 6-Me), 3.70 (3H, OCH3); 13C NMR (CDCl3, 125 MHz): δ 174.3 (C-1), 42.5 (C-2), 81.0 (C-3), 32.8 (C-4), 35.1 (C-5), 80.2 (C-6), 22.5 (C-7), 22.2 (C-8), 136.7 (C-9), 127.1 (C-10), 32.7 (C-11), 19.6 (C-12), 39.9 (C-13), 34.8 (C-14), 12.4 (C-15), 23.6 (C-16), 19.7 (C-17), 28.5 (C-18), 28.7 (C-19), 51.9 (OCH3).
3.4. Reductive Cleavage of Nuapapuin A Methyl Ester
A mixture of nuapapuin A methyl ester (30 mg, 88.6 μmol), HOAc (1.5 mL, 26.2 mmol) and zinc (900 mg, 13.8 mmol) in EtOAc (15 mL) was stirred at room temperature for 12 h. The crude reaction mixture was then filtered, evaporated and purified on a flash silica gel column eluted with n-hexane-EtOAc (1:1) to afford the corresponding diol 6 (27 mg, 89%) showing the identical proton NMR data and similar specific rotation, [α]20D −2.0 (c 0.50, CHCl3), with 6.
3.5. Preparation of (S)- and (R)-MTPA Esters of 6
Two samples of diacardiol B (6; 1.1 mg, 3.2 μmol and 0.9 mg, 2.6 μmol) were mixed with S-(+)- or R-(−)-MTPA-Cl (15 μL, 80.1 μmol), respectively, in freshly distilled dry pyridine (500 μL) and then stirred under N2 atmosphere at room temperature for 18 h, and the solvent was then removed in vacuo. The products were purified by using a minicolumn chromatography packed with silica gel (200 mesh; petroleum ether/EtOAc, 1:1, v/v) to afford S-(+)- and R-(−)-MTPA esters 6a (0.7 mg, 78%) and 6b (0.8 mg, 73%), respectively. The anisotropic analysis was done by acquiring the interfered resonances for 1H NMR (CDCl3, 500 MHz) of 6a: δ 5.406 (1H, m, H-3), 3.598 (3H, s, COOMe), 2.866 (1H, m, H-2), 1.902 (1H, m, H-4a), 1.975 (1H, m, H-4b), 1.138 (3H, d, J = 6.1 Hz, H-15), and 1.157 (3H, s, H-16); and the interfered resonances for 1H NMR (CDCl3, 500 MHz) of 6b: δ 5.394 (1H, m, H-3), 3.651 (3H, s, COOMe), 2.875 (1H, m, H-2), 1.894 (1H, m, H-4a), 1.933 (1H, m, H-4b), 1.190 (3H, d, J = 6.1 Hz, H-15), and 1.078 (3H, s, H-16).
3.6. Antimalarial Assay
Antimalarial activities were determined against chloroquine sensitive (D6, Sierra Leone) and resistant (W2, Indo China) strains of Plasmodium falciparum in vitro by measuring plasmodial LDH activity. Each testing group dissolved in DMSO with a concentration of 2 mg/mL. A 200-μL suspension of P. falciparum culture (2% parasitemia and 2% hematocrit in RPMI 1640 medium supplemented with 10% human serum and 60 μg/mL amikacin) was added to each well, containing 10 μL of serially diluted samples, of a 96-well plate. The plate was flushed with a gas mixture of 90% N2, 5% O2 and 5% CO2 and incubated at 37 °C for 72 h in a modular incubation chamber. Plasmodial LDH activity was determined by using Malstat reagent. Briefly, 20 μL of the incubation mixture were mixed with 100 μL of the Malstat reagent and incubated for 30 min. Twenty microliters of a 1:1 mixture of NBT/PES were then added, and the plate was further incubated in dark environment for 1 h. The test was ended by adding 100 μL of a 5% acetic acid solution. The plate was monitored at 650 nm. Artemisinin was used as the control drug.
3.7. MTT Cytotoxicity Assay
Briefly, human cancer cells in the exponential growth phase were harvested and then seeded into a flat-bottomed 96-well plate, and each well contained 2.0 × 103 cells in 100 μL of solution. After incubation for 12 h in a 5% humidified CO2 incubator at 37 °C, the compound for testing was added (in triplicate experiments) at six different concentrations. After 72 h, incubating at 37 °C, 20 μL of MTT were added to each well, and the plate was incubated again at 37 °C for 3 h. Absorption was then measured by using a SpectraMAX 340 reader (Molecular Devices, Sunnyvale, CA, USA) at 550 nm, with a reference filter at 690 nm, and IC50 values were calculated on the basis of the percentage of inhibition using the linear regression method.
3.8. Computational Calculation
The MMFF (Merck molecular force field) minimized structures of Compounds
1–
5 were used for the DFT calculations. DFT calculations were performed by applying the PCM solvation models with the dielectric constant representing acetonitrile by using the Gaussian 09 program (Revision A.1, Gaussian, Inc., Wallingford, CT, USA, 2009). Low energy conformations were optimized by using the B3LYP/6-31G(d,p) or MPWLPW91/6-31G(d,p) methods applying the PCM solvation model, and the frequency calculations were performed at the same theoretical levels to verify the true located energy minimal and to generate sets of thermodynamic data at standard condition. The optimized geometries were used for TDDFT calculations at the B3LYP/6-31G(d,p) level applying the same solvation model. The generated excitation energies and rotational strengths were Boltzmann averaged and then fitted to Gaussian functions to generate computed ECD spectra normalized and overlaid with the experimental spectra for comparison [
28,
29]. The NMR calculations were performed by using the Gauge-invariant atomic orbital (GIAO) method at the B3LYP/6-31G(d,p) and MPW1PW91/6-31G(d,p) levels, and the calculated chemical shifts for TMS at the corresponding levels were used as references (computational details for Compounds
1–
5 are provided in the
Supplementary Information).