Inactivation of Heparin by Cationically Modified Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. In Vitro Experiments
2.4. In Vivo Experiments
2.5. Hemostatic Parameters
2.6. Pathomorphological Examination of the Liver
2.7. Statistical Analysis
3. Results
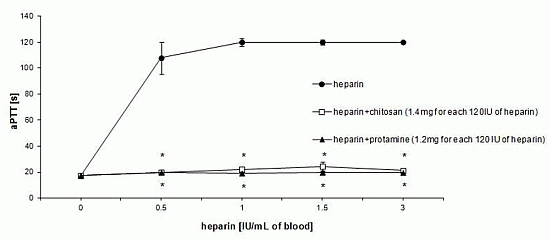
3.1. Effect of HTCC on Hemostatic Parameters of Rat Blood in Vitro


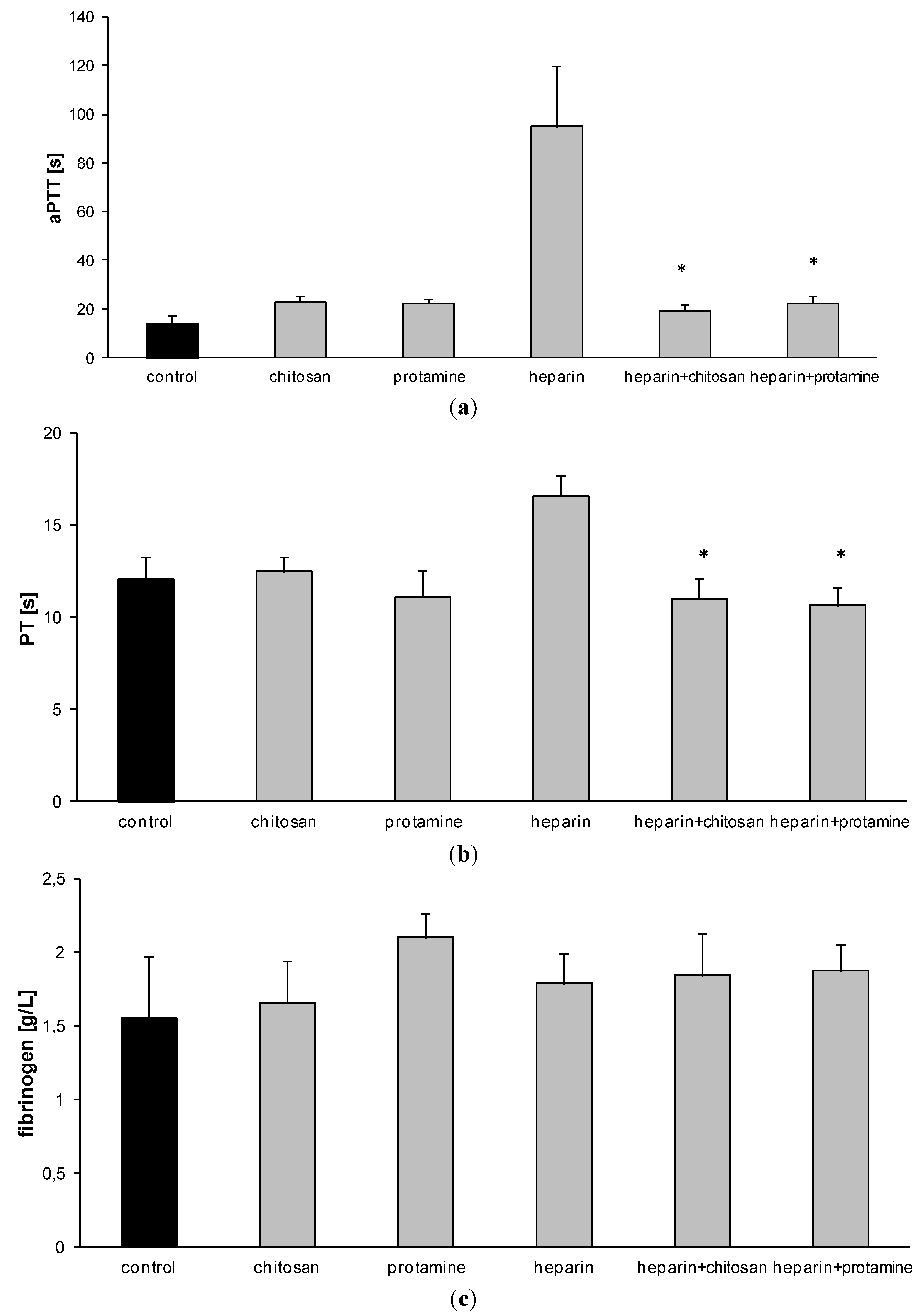
3.2. Hemostatic Effects of HTCC in Rats in Vivo


3.3. Histopathological Findings
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, L.C.; Liang, J.F.; Lee, H.F.; Lee, L.M.; Yang, V.C. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (II): In vitro evaluation of efficacy and toxicity. AAPS Pharm. Sci. 2001, 3, 15–23. [Google Scholar] [CrossRef]
- Lever, R.; Mulloy, B.; Page, C.P. Heparin—A century of progress. Handb. Exp. Pharmacol. 2012, 207, 265–276. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan—As a biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar]
- Kato, Y.; Onishi, H.; Machida, Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr. Pharm. Biotechnol. 2003, 4, 303–309. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, S.U.; Kim, J.H. Modification of chitosan to improve its hypocholesterolemic capacity. Biosci. Biotechnol. Biochem. 1999, 63, 833–839. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef]
- Kamiński, K.; Szczubiałka, K.; Zazakowny, K.; Lach, R.; Nowakowska, M. Chitosan derivatives as novel potential heparin reversal agents. J. Med. Chem. 2010, 53, 4141–4147. [Google Scholar] [CrossRef]
- Kamiński, K.; Zazakowny, K.; Szczubiałka, K.; Nowakowska, M. pH-Sensitive genipin-cross-linked chitosan microspheres for heparin removal. Biomacromolecules 2008, 9, 3127–3132. [Google Scholar] [CrossRef]
- García-Manzano, A.; González-Llaven, J.; Lemini, C.; Rubio-Póo, C. Standardization of rat blood clotting tests with reagents used for humans. Proc. West. Pharmacol. Soc. 2001, 44, 153–155. [Google Scholar]
- Lemini, C.; Jaimez, R.; Franco, Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb. Res. 2007, 120, 415–419. [Google Scholar] [CrossRef]
- Nam, K.-S.; Kim, M.-K.; Shon, Y.-H. Chemopreventive effect of chitosan oligosaccharide against colon carcinogenesis. J. Microbiol. Biotechnol. 2007, 17, 1546–1549. [Google Scholar]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterisation of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Niederhofer, A.; Müller, B.W. A method for direct preparation of chitosan with low molecular weight from fungi. Eur. J. Pharm. Biopharm. 2004, 57, 101–105. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Lin, S.-B.; Lin, Y.-C.; Chen, H.-H. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterisation and antibacterial activity. Food Chem. 2009, 116, 47–53. [Google Scholar] [CrossRef]
- Lee, C.G. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med. J. 2009, 50, 22–30. [Google Scholar] [CrossRef]
- Chou, T.-C.; Fu, E.; Wu, C.-J.; Yeh, J.-H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef]
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Krewraing, J.; Prateepasen, R. In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. J. Appl. Polym. Sci. 2006, 102, 445–451. [Google Scholar]
- Okamoto, Y.; Yano, R.; Miyatake, K.; Tomohiro, I.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on blood coagulation. Carbohydr. Polym. 2003, 53, 337–342. [Google Scholar]
- Benesch, J.; Tengvall, P. Blood protein adsorption onto chitosan. Biomaterials 2002, 23, 2561–2568. [Google Scholar] [CrossRef]
- Lord, M.S.; Cheng, B.; McCarthy, S.J.; Jung, M.; Whitelock, J.M. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 2011, 32, 6655–6662. [Google Scholar] [CrossRef]
- Dowling, M.B.; Kumar, R.; Keibler, M.A.; Hess, J.R.; Bochicchio, G.V.; Raghavan, S.R. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials 2011, 32, 3351–3357. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.; Chen, S. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84B, 131–137. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral Maxillofac. Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Schipper, N.; Vårum, K.; Artursson, P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar]
- Richardson, S.C.; Kolbe, H.V.; Duncan, R. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Omara, E.A.; Aly, H.F.; Nada, S.A. Chitosan induced hepato-nephrotoxicity in mice with special reference to gender effect in glycolytic enzymes activities. Regul. Toxicol. Pharmacol. 2012, 62, 29–40. [Google Scholar] [CrossRef]
- Loh, J.W.; Yeoh, G.; Saunders, M.; Lim, L.-Y. Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol. Appl. Pharmacol. 2010, 249, 148–157. [Google Scholar] [CrossRef]
- Farouk, M.; Atiat, M.; Abd El-Rahman, M.; Ashraf, A.; Abd El-Karim, I. Effect of some drugs and herbs on experimental rats suffering from obesity. Res. J. Specif. Educ. 2011, 697–724. [Google Scholar]
- Sethumadhavan Santhosh, T.K.S. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur. J. Pharmacol. 2007, 572, 69–73. [Google Scholar] [CrossRef]
- Zhou, G.-D.; Li, M.-R.; Zhang, J.; Pan, D.; Zhao, S.-X.; Yang, J.-F.; Yu, J.; Zhao, J.-M. Chitosan ameliorates the severity of steatohepatitis induced by high fat diet in rats. Scand. J. Gastroenterol. 2008, 43, 1371–1377. [Google Scholar] [CrossRef]
- Sobol, M.; Bartkowiak, A.; de Haan, B.; de Vos, P. Cytotoxicity study of novel water-soluble chitosan derivatives applied as membrane material of alginate microcapsules. J. Biomed. Mater. Res. A 2012, 100A, 1907–1914. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tanioka, S.; Tanaka, M.; Tanigawa, T.; Kitamura, Y.; Minami, S.; Okamoto, Y.; Miyashita, M.; Nanno, M. Effects of chitin and chitosan particles on BALB/c mice by oral and parenteral administration. Biomaterials 1997, 18, 591–595. [Google Scholar] [CrossRef]
- Zwischenberger, J.B.; Vertrees, R.A.; Brunston, R.L., Jr.; Tao, W.; Alpard, S.K.; Brown, P.S., Jr. Application of a heparin removal device in patients with known protamine hypersensitivity. J. Thorac. Cardiovasc. Surg. 1998, 115, 729–731. [Google Scholar] [CrossRef]
- Kikura, M.; Lee, M.K.; Levy, J.H. Heparin neutralization with methylene blue, hexadimethrine, or vancomycin after cardiopulmonary bypass. Anesth. Analg. 1996, 83, 223–227. [Google Scholar]
- Despotis, G.J.; Summerfield, A.L.; Joist, J.H.; Goodnough, L.T.; Santoro, S.A.; Zimmermann, J.J.; Lappas, D.G. In vitro reversal of heparin effect with heparinase: Evaluation with whole blood prothrombin time and activated partial thromboplastin time in cardiac surgical patients. Anesth. Analg. 1994, 79, 670–674. [Google Scholar]
- Stafford-Smith, M.; Lefrak, E.A.; Qazi, A.G.; Welsby, I.J.; Barber, L.; Hoeft, A.; Dorenbaum, A.; Mathias, J.; Rochon, J.J.; Newman, M.F. Efficacy and safety of heparinase I vs. protamine in patients undergoing coronary artery bypass grafting with and without cardiopulmonary bypass. Anesthesiology 2005, 103, 229–240. [Google Scholar] [CrossRef]
- Dehmer, G.J.; Fisher, M.; Tate, D.A.; Teo, S.; Bonnem, E.M. Reversal of heparin anticoagulation by recombinant platelet factor 4 in humans. Circulation 1995, 91, 2188–2194. [Google Scholar] [CrossRef]
- Schick, B.P.; Maslow, D.; Moshinski, A.; San Antonio, J.D. Novel concatameric heparin-binding peptides reverse heparin and low-molecular-weight heparin anticoagulant activities in patient plasma in vitro and in rats in vivo. Blood 2004, 103, 1356–1363. [Google Scholar]
- Kurata, M.; Sasayama, Y.; Yamasaki, N.; Kitazawa, I.; Hamada, Y.; Horii, I. Mechanism for shortening PT and APTT in dogs and rats—effect of fibrinogen on PT and APTT. J. Toxicol. Sci. 2003, 28, 439–443. [Google Scholar] [CrossRef]
- Tabata, H.; Nakamura, S.; Matsuzawa, T. Some species differences in the false prolongation of prothrombin times and activated partial thromboplastin times in toxicology. Comp. Haematol. Int. 1995, 5, 140–144. [Google Scholar] [CrossRef]
- Dempfle, C.-E.; Borggrefe, M. Do we need thrombin generation assays for monitoring anticoagulation? Thromb. Haemost. 2008, 100, 179–180. [Google Scholar]
- Nielsen, V.G. The detection of changes in heparin activity in the rabbit: A comparison of anti-xa activity, thrombelastography, activated partial thromboplastin time, and activated coagulation time. Anesth. Analg. 2002, 95, 1503–1506. [Google Scholar] [CrossRef]
- Fuller, J.; Woodman, D.D. Evaluation of a 2-stage automated coagulometer for multispecies studies. Lab. Anim. 1981, 15, 53–55. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lorkowska-Zawicka, B.; Kamiński, K.; Ciejka, J.; Szczubiałka, K.; Białas, M.; Okoń, K.; Adamek, D.; Nowakowska, M.; Jawień, J.; Olszanecki, R.; et al. Inactivation of Heparin by Cationically Modified Chitosan. Mar. Drugs 2014, 12, 3953-3969. https://doi.org/10.3390/md12073953
Lorkowska-Zawicka B, Kamiński K, Ciejka J, Szczubiałka K, Białas M, Okoń K, Adamek D, Nowakowska M, Jawień J, Olszanecki R, et al. Inactivation of Heparin by Cationically Modified Chitosan. Marine Drugs. 2014; 12(7):3953-3969. https://doi.org/10.3390/md12073953
Chicago/Turabian StyleLorkowska-Zawicka, Barbara, Kamil Kamiński, Justyna Ciejka, Krzysztof Szczubiałka, Magdalena Białas, Krzysztof Okoń, Dariusz Adamek, Maria Nowakowska, Jacek Jawień, Rafał Olszanecki, and et al. 2014. "Inactivation of Heparin by Cationically Modified Chitosan" Marine Drugs 12, no. 7: 3953-3969. https://doi.org/10.3390/md12073953
APA StyleLorkowska-Zawicka, B., Kamiński, K., Ciejka, J., Szczubiałka, K., Białas, M., Okoń, K., Adamek, D., Nowakowska, M., Jawień, J., Olszanecki, R., & Korbut, R. (2014). Inactivation of Heparin by Cationically Modified Chitosan. Marine Drugs, 12(7), 3953-3969. https://doi.org/10.3390/md12073953