Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A from Sea Urchin Scaphechinus mirabilis
Abstract
:1. Introduction
2. Results and Discussion
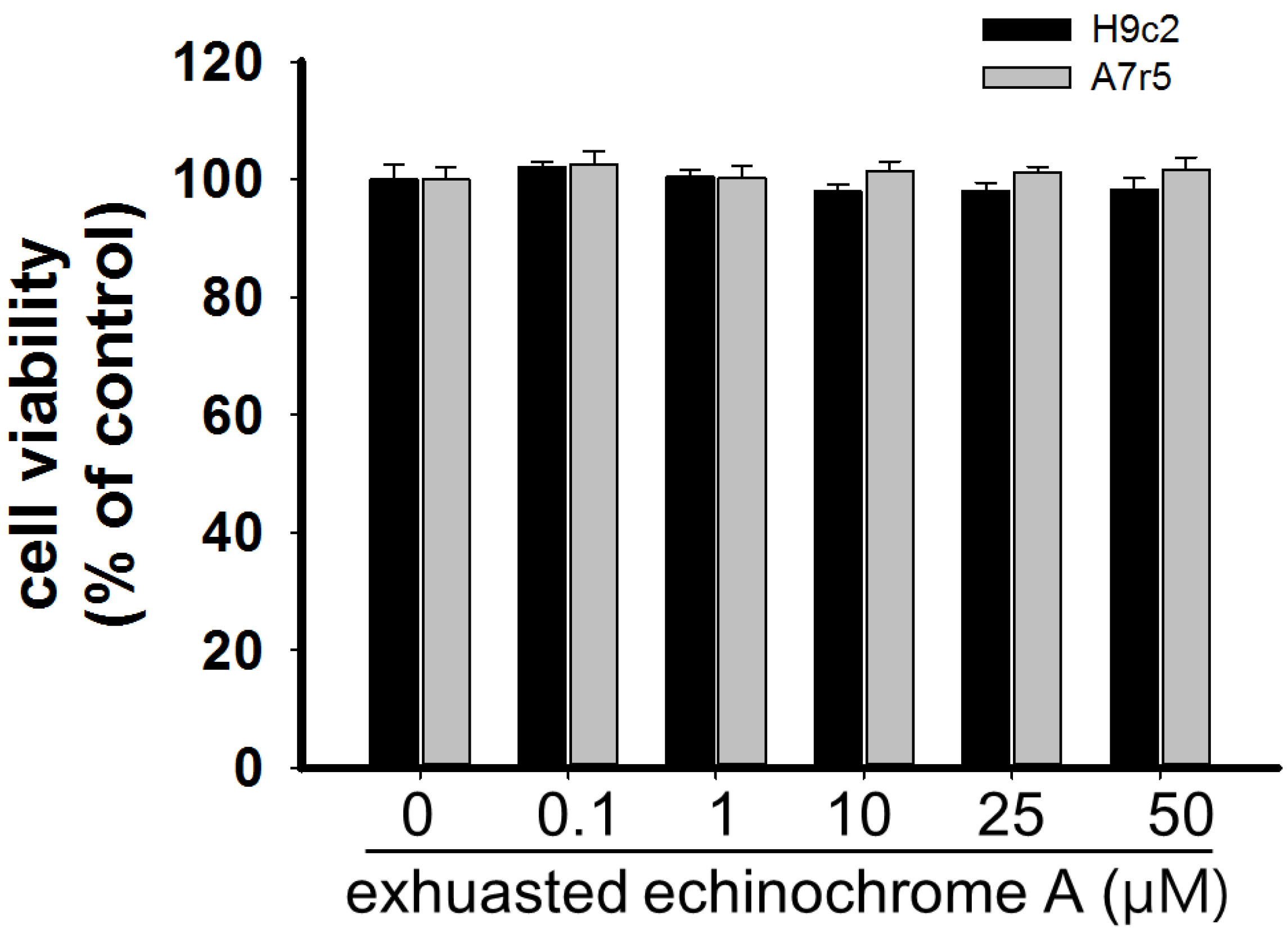
2.1. Cytotoxicity of Echinochrome A
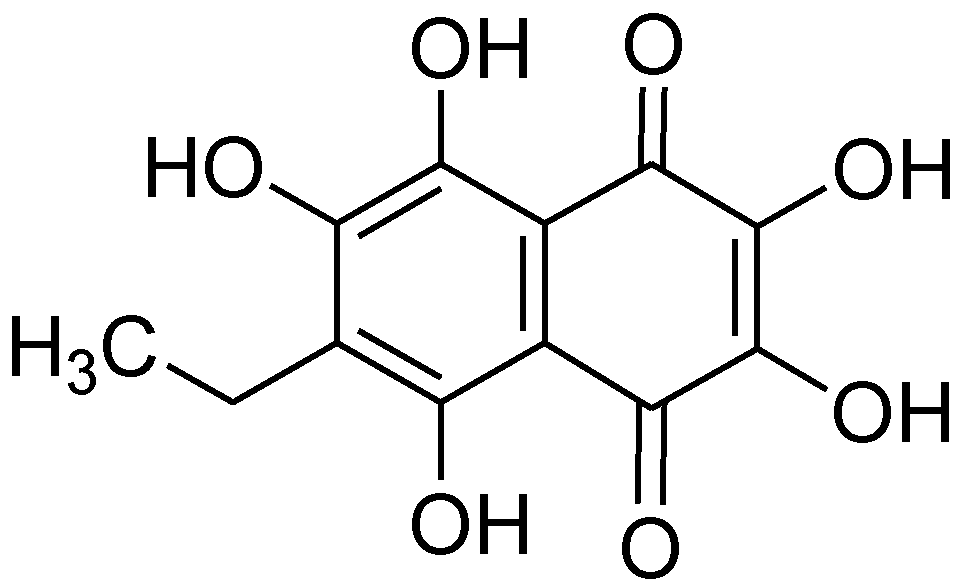

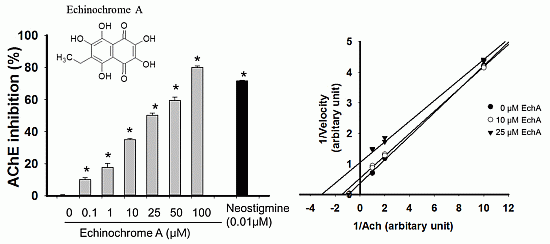
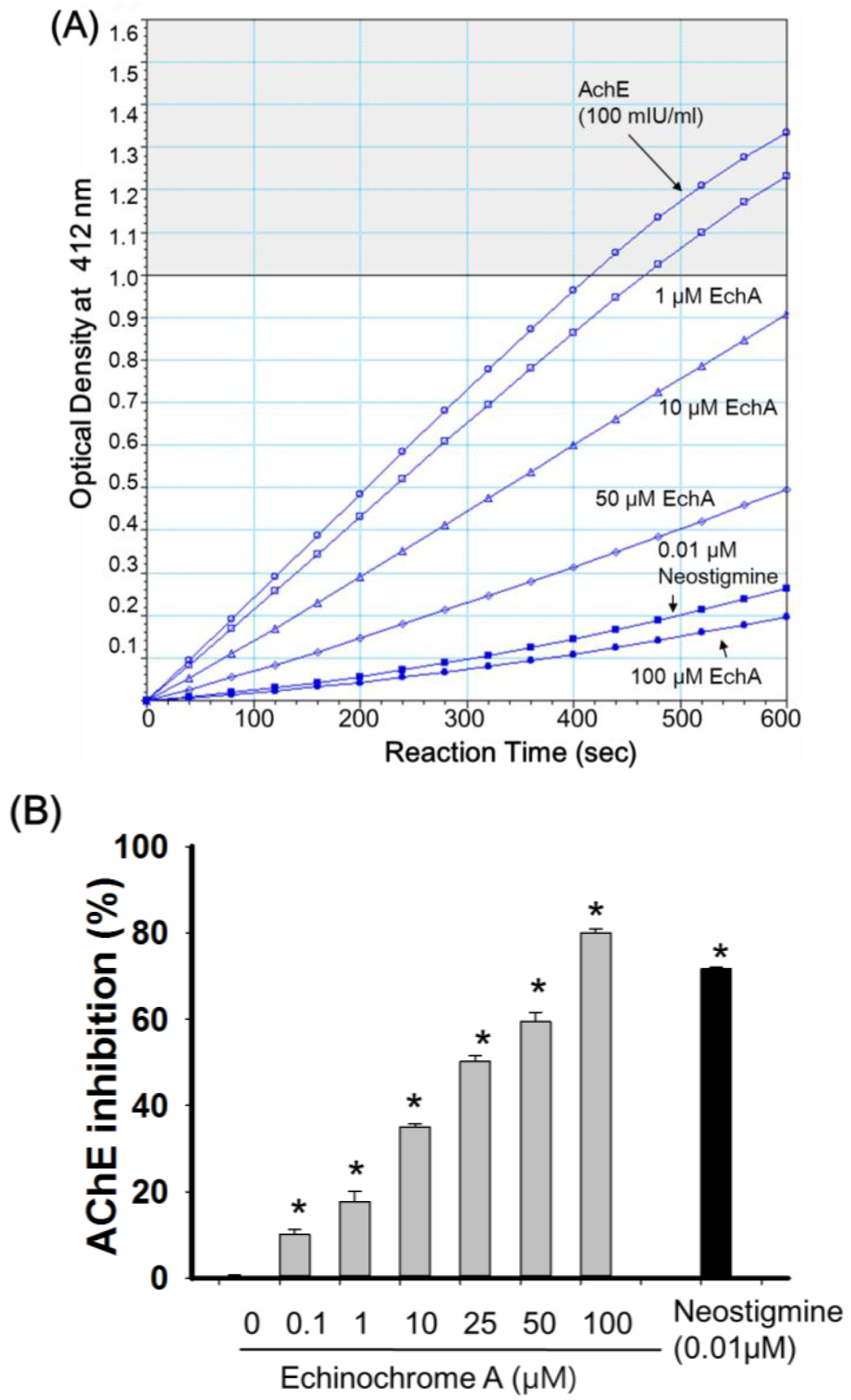
2.2. Inhibitory Effect of Echinochrome A on Acetylcholinesterase
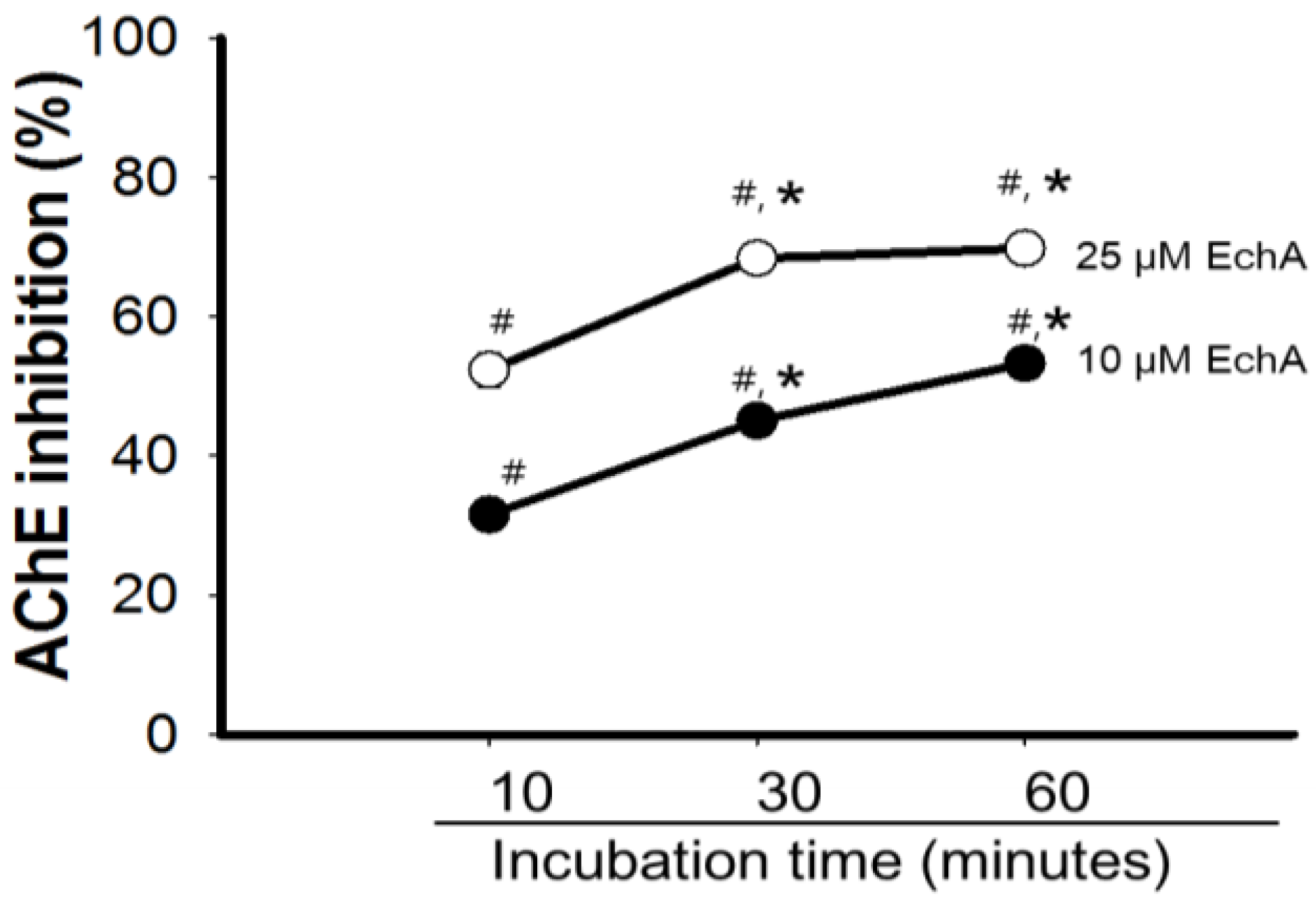
2.3. Mode of Inhibition of Echinochrome A on Acetylcholinesterase
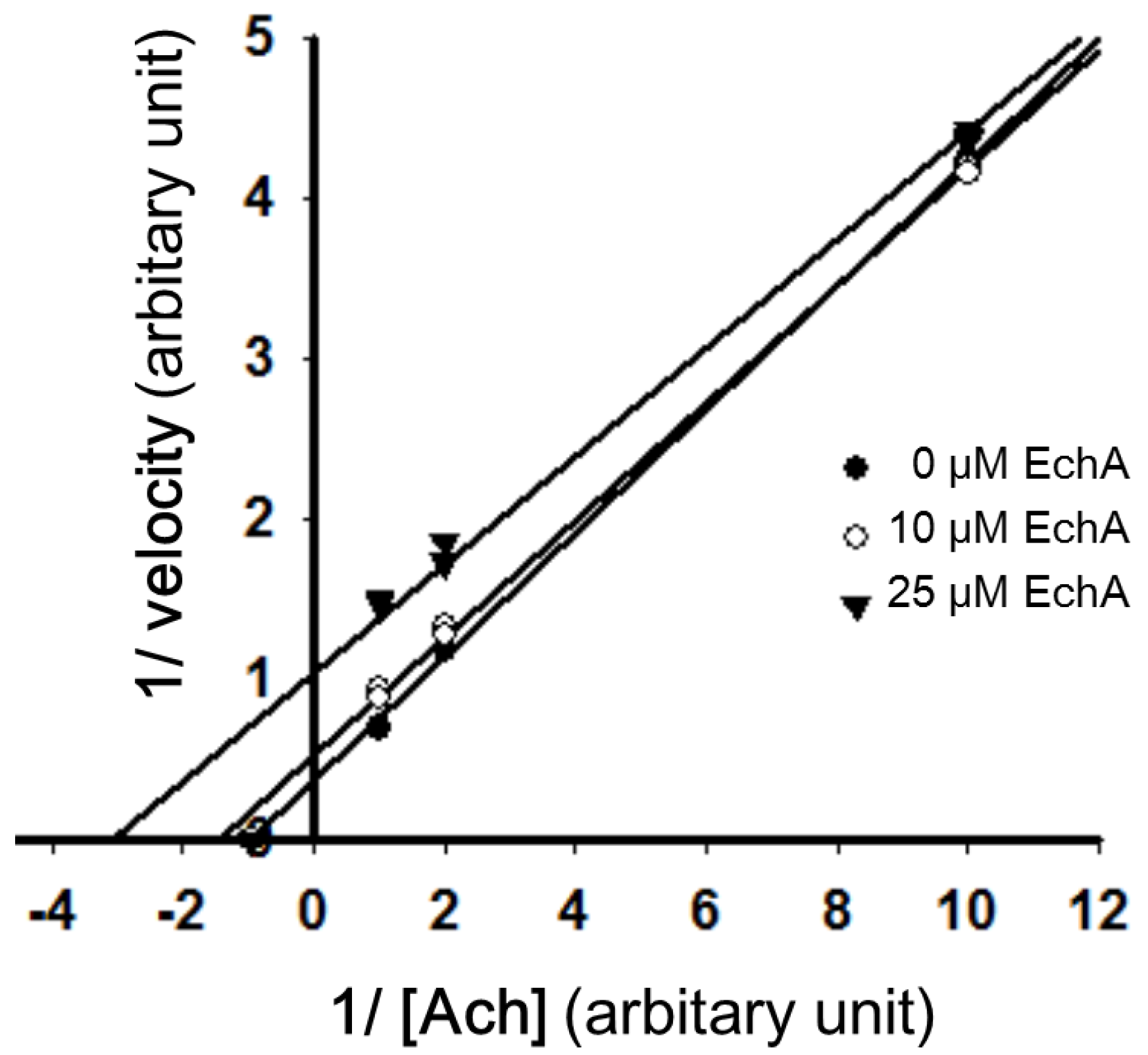
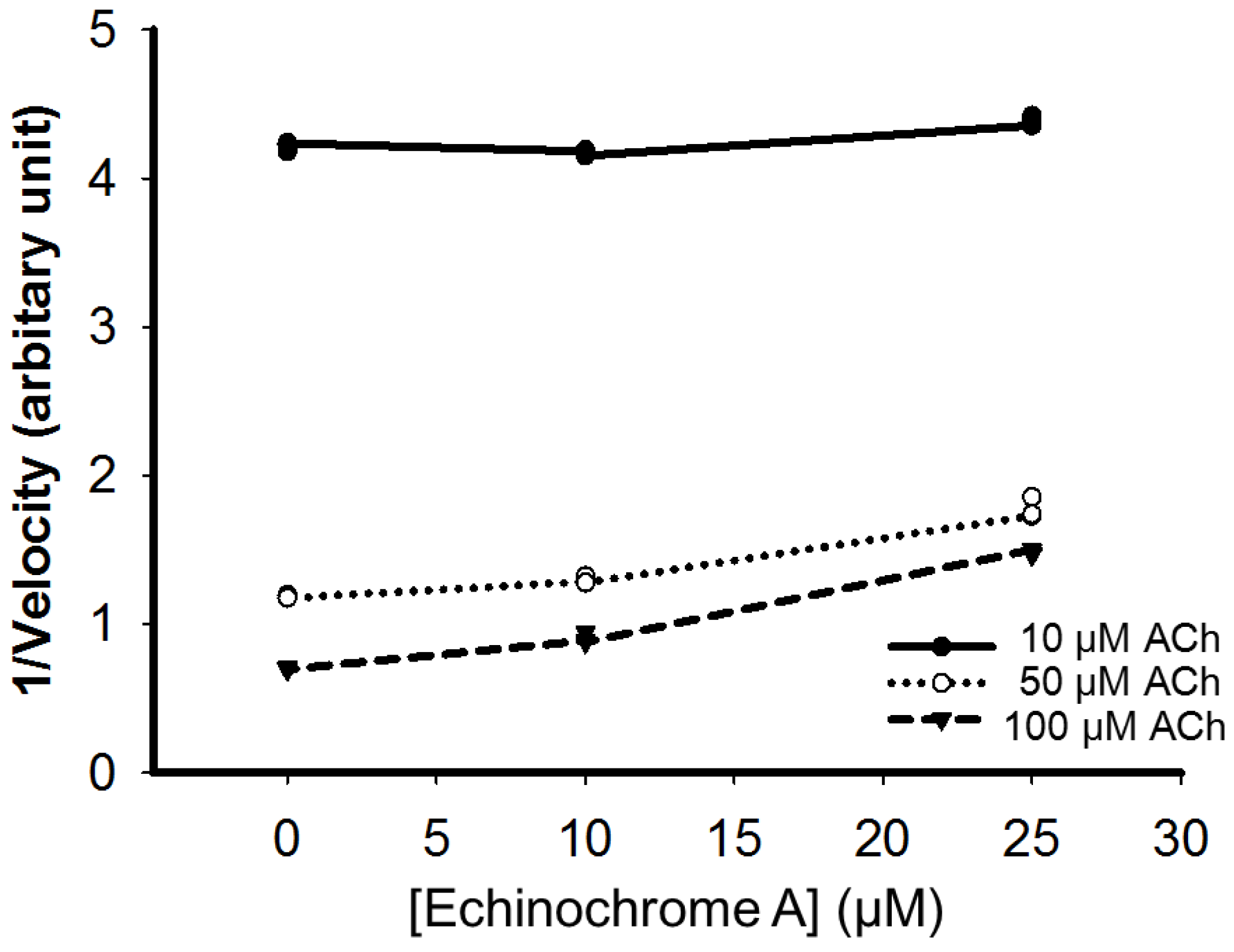
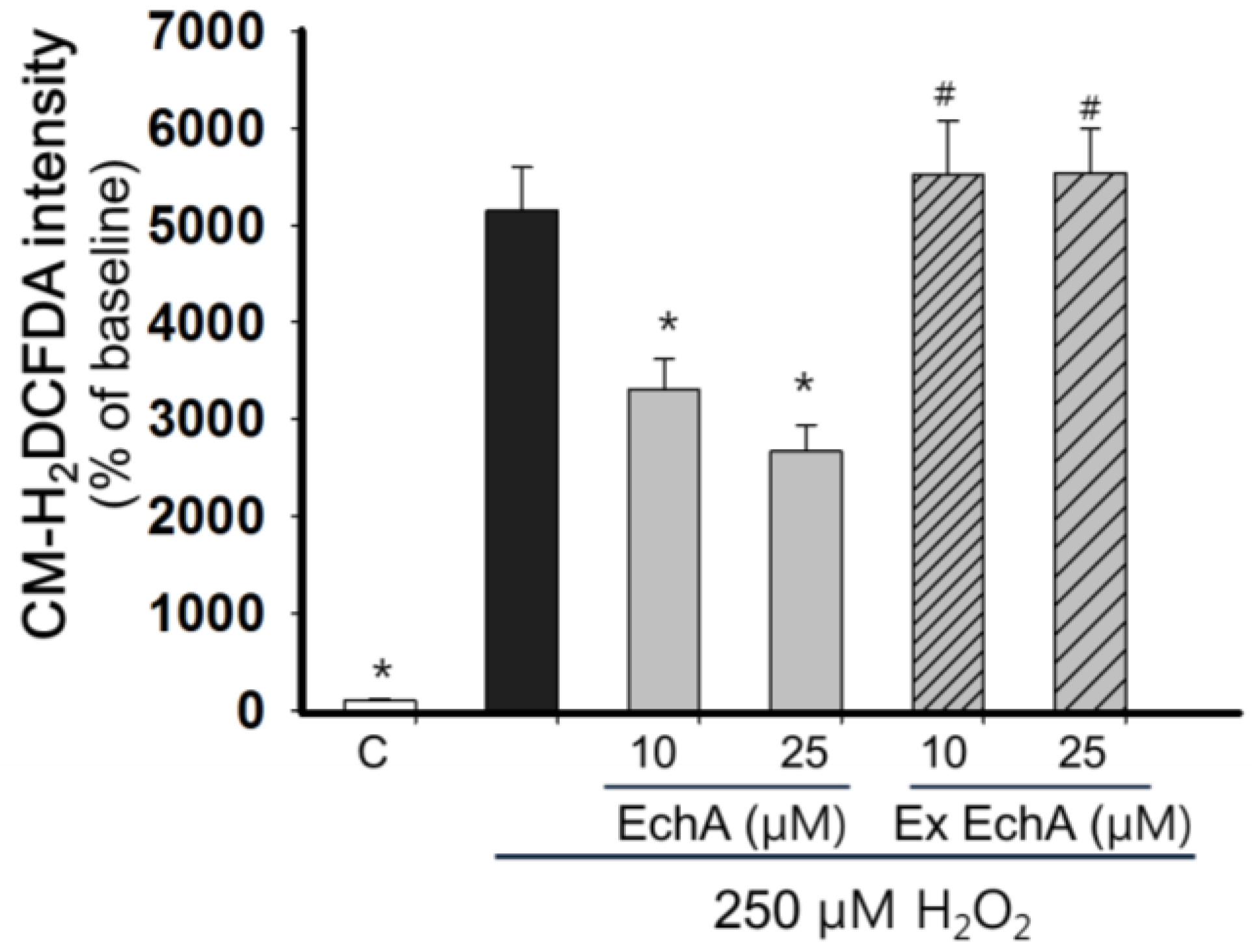
2.4. Nitric Oxide Scavenging Effect of Echinochrome A

3. Experimental Section
3.1. Experimental General
3.2. Cell Culture
3.3. Cell Cytotoxicity Test
3.4. Measurement of Reactive Oxygen Species Level
3.5. Anti-Acetylcholinesterase Activity Assay
3.6. Determination of Nitric Oxide Production
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Campbell, W.B.; Fleming, I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflug. Arch. 2010, 459, 881–895. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Yu, X.J.; Miao, Y.; Liu, J.J.; Wang, H.; Ren, J.; Zang, W.J. Acetylcholine mediates AMPK-dependent autophagic cytoprotection in H9c2 cells during hypoxia/reoxygenation injury. Cell. Physiol. Biochem. 2013, 32, 601–613. [Google Scholar] [CrossRef]
- Kakinuma, Y.; Ando, M.; Kuwabara, M.; Katare, R.G.; Okudela, K.; Kobayashi, M.; Sato, T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett. 2005, 579, 2111–2118. [Google Scholar] [CrossRef]
- Sung, S.H.; Kang, S.Y.; Lee, K.Y.; Park, M.J.; Kim, J.H.; Park, J.H.; Kim, Y.C.; Kim, J. (+)-Alpha-viniferin, a stilbene trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol. Pharm. Bull. 2002, 25, 125–127. [Google Scholar] [CrossRef]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing. Age Ageing 2006, 35, 336–338. [Google Scholar] [CrossRef]
- Virarkar, M.; Alappat, L.; Bradford, P.G.; Awad, A.B. l-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2013, 53, 1157–1167. [Google Scholar] [CrossRef]
- Pinho, B.R.; Ferreres, F.; Valentao, P.; Andrade, P.B. Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer’s disease treatment. J. Pharm. Pharmacol. 2013, 65, 1681–1700. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440. [Google Scholar] [CrossRef]
- Sangnoi, Y.; Sakulkeo, O.; Yuenyongsawad, S.; Kanjana-opas, A.; Ingkaninan, K.; Plubrukarn, A.; Suwanborirux, K. Acetylcholinesterase-inhibiting activity of pyrrole derivatives from a novel marine gliding bacterium, Rapidithrix thailandica. Mar. Drugs 2008, 6, 578–586. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef]
- Klegeris, A.; Korkina, L.G.; Greenfield, S.A. A possible interaction between acetylcholinesterase and dopamine molecules during autoxidation of the amine. Free Radic. Biol. Med. 1995, 18, 223–230. [Google Scholar]
- Prati, F.; Bartolini, M.; Simoni, E.; De Simone, A.; Pinto, A.; Andrisano, V.; Bolognesi, M.L. Quinones bearing non-steroidal anti-inflammatory fragments as multitarget ligands for Alzheimer's disease. Bioorg. Med. Chem. Lett. 2013, 23, 6254–6258. [Google Scholar] [CrossRef]
- Mischenko, N.P.; Fedoreev, S.A.; Zapara, T.A.; Ratushnyak, A.S. Effects of histochrom and emoxypin on biophysical properties of electroexitable cells. Bull. Exp. Biol. Med. 2009, 147, 196–200. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Iron chelators and free radical scavengers in naturally occurring polyhydroxylated 1,4-naphthoquinones. Hemoglobin 2008, 32, 165–179. [Google Scholar] [CrossRef]
- Elyakov, G.B.; Maximov, O.B.; Mischenko, N.P.; Koltsova, E.A.; Fedoreev, S.A.; Glebko, L.I.; Krasovskaya, N.P.; Artjukov, A.A. Histochrome® and Its Therapeutic Use in Acute Myocardial Infarction and Ischemic Heart Disease. U.S. Patent 6,410,601, 25 June 2002. [Google Scholar]
- Buimov, G.A.; Maksimov, I.V.; Perchatkin, V.A.; Repin, A.N.; Afanas’ev, S.A.; Markov, V.A.; Karpov, R.S. Effect of the bioantioxidant histochrome on myocardial injury in reperfusion therapy on patients with myocardial infarction. Ter. Arkh. 2002, 74, 12–16. [Google Scholar]
- Shvilkin, A.V.; Serebriakov, L.I.; Tskitishvili, O.V.; Sadretdinov, S.M.; Kol’tsova, E.A.; Maksimov, O.B.; Mishchenko, N.P.; Novikov, V.L.; Levitskii, D.O.; Ruda, M. Effect of echinochrom on experimental myocardial reperfusion injury. Kardiologiia 1991, 31, 79–81. [Google Scholar]
- Kuzuya, H.; Ikuta, K.; Nagatsu, T. Inhibition of dopamine-beta-hydroxylase by spinochrome A and echinochrome A, naphthoquinone pigments of echinoids. Biochem. Pharmacol. 1973, 22, 2722–2724. [Google Scholar]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreev, S.A.; Mishchenko, N.P.; Kol’tsova, E.A. Histochrome, a new antioxidant, in the treatment of ocular diseases. Vestn. Oftalmol. 1999, 115, 34–35. [Google Scholar]
- Elyakov, G.B.; Maximov, O.B.; Mischenko, N.P.; Koltsova, E.A.; Fedoreev, S.A.; Glebko, L.I.; Krasovskaya, N.P.; Artjukov, A.A. Histochrome and Its Therapeutic Use in Ophthalmology. U.S. Patent 6,384,084, 7 May 2002. [Google Scholar]
- Berg, R.M.; Moller, K.; Bailey, D.M. Neuro-oxidative-nitrosative stress in sepsis. J. Cereb. Blood Flow Metab. 2011, 31, 1532–1544. [Google Scholar] [CrossRef]
- Baek, J.G.; Jeong, H.L.; Park, J.S.; Seo, J.H.; Park, E.S.; Lim, J.Y.; Park, C.H.; Woo, H.O.; Youn, H.S.; Yeom, J.S. Successful treatment by exchange transfusion of a young infant with sodium nitroprusside poisoning. Korean J. Pediatr. 2010, 53, 805–808. [Google Scholar] [CrossRef]
- Shin, M.Y.; Kwun, I.S. Phosphate-induced rat vascular smooth muscle cell calcification and the implication of zinc deficiency in a7r5 cell viability. Prev. Nutr. Food Sci. 2013, 18, 92–97. [Google Scholar] [CrossRef]
- Pan, Z.; Guo, Y.; Qi, H.; Fan, K.; Wang, S.; Zhao, H.; Fan, Y.; Xie, J.; Guo, F.; Hou, Y.; et al. M3 subtype of muscarinic acetylcholine receptor promotes cardioprotection via the suppression of miR-376b-5p. PLoS One 2012, 7, e32571. [Google Scholar]
- Zakirova, A.N.; Lebedev, A.V.; Kukharchuk, V.V.; Mishchenko, N.P.; Fedoreev, S.A. The antioxidant histochrome: Its effect on lipid peroxidation and the blood rheological properties in patients with unstable stenocardia. Ter. Arkh. 1996, 68, 12–14. [Google Scholar]
- Magnotti, R.A., Jr.; Eberly, J.P.; Quarm, D.E.; McConnell, R.S. Measurement of acetylcholinesterase in erythrocytes in the field. Clin. Chem. 1987, 33, 1731–1735. [Google Scholar]
- Kasi, P.M. The use of intravenous neostigmine in palliation of severe ileus. Case Rep. Gastrointest. Med. 2013, 2013, 796739. [Google Scholar]
- Changwong, N.; Sabphon, C.; Ingkaninan, K.; Sawasdee, P. Acetyl- and butyryl-cholinesterase inhibitory activities of mansorins and mansonones. Phytother. Res. 2012, 26, 392–396. [Google Scholar]
- Kumar, P.; Singh, V.K.; Singh, D.K. Kinetics of enzyme inhibition by active molluscicidal agents ferulic acid, umbelliferone, eugenol and limonene in the nervous tissue of snail Lymnaea acuminata. Phytother. Res. 2009, 23, 172–177. [Google Scholar] [CrossRef]
- Lipton, S.A. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 2007, 8, 803–808. [Google Scholar] [CrossRef]
- Yan, Z.; Rafferty, B.; Caldwell, G.W.; Masucci, J.A. Rapidly distinguishing reversible and irreversible CYP450 inhibitors by using fluorometric kinetic analyses. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 281–287. [Google Scholar] [CrossRef]
- Kamal, M.A.; Greig, N.H.; Alhomida, A.S.; Al-Jafari, A.A. Kinetics of human acetylcholinesterase inhibition by the novel experimental Alzheimer therapeutic agent, tolserine. Biochem. Pharmacol. 2000, 60, 561–570. [Google Scholar] [CrossRef]
- Terpolilli, N.A.; Moskowitz, M.A.; Plesnila, N. Nitric oxide: Considerations for the treatment of ischemic stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1332–1346. [Google Scholar] [CrossRef]
- Kim, H.J.; Tsoy, I.; Park, M.K.; Lee, Y.S.; Lee, J.H.; Seo, H.G.; Chang, K.C. Iron released by sodium nitroprusside contributes to heme oxygenase-1 induction via the cAMP-protein kinase A-mitogen-activated protein kinase pathway in RAW 264.7 cells. Mol. Pharmacol. 2006, 69, 1633–1640. [Google Scholar]
- Schwendemann, J.; Sehringer, B.; Noethling, C.; Zahradnik, H.P.; Schaefer, W.R. Nitric oxide detection by DAF (diaminofluorescein) fluorescence in human myometrial tissue. Gynecol. Endocrinol. 2008, 24, 306–311. [Google Scholar] [CrossRef]
- Lee, S.R.; Kwak, J.H.; Park, D.S.; Pyo, S. Protective effect of kobophenol A on nitric oxide-induced cell apoptosis in human osteoblast-like MG-63 cells: Involvement of JNK, NF-kappaB and AP-1 pathways. Int. Immunopharmacol. 2011, 11, 1251–1259. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, S.R.; Pronto, J.R.D.; Sarankhuu, B.-E.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A from Sea Urchin Scaphechinus mirabilis. Mar. Drugs 2014, 12, 3560-3573. https://doi.org/10.3390/md12063560
Lee SR, Pronto JRD, Sarankhuu B-E, Ko KS, Rhee BD, Kim N, Mishchenko NP, Fedoreyev SA, Stonik VA, Han J. Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A from Sea Urchin Scaphechinus mirabilis. Marine Drugs. 2014; 12(6):3560-3573. https://doi.org/10.3390/md12063560
Chicago/Turabian StyleLee, Sung Ryul, Julius Ryan D. Pronto, Bolor-Erdene Sarankhuu, Kyung Soo Ko, Byoung Doo Rhee, Nari Kim, Natalia P. Mishchenko, Sergey A. Fedoreyev, Valentin A. Stonik, and Jin Han. 2014. "Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A from Sea Urchin Scaphechinus mirabilis" Marine Drugs 12, no. 6: 3560-3573. https://doi.org/10.3390/md12063560
APA StyleLee, S. R., Pronto, J. R. D., Sarankhuu, B.-E., Ko, K. S., Rhee, B. D., Kim, N., Mishchenko, N. P., Fedoreyev, S. A., Stonik, V. A., & Han, J. (2014). Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A from Sea Urchin Scaphechinus mirabilis. Marine Drugs, 12(6), 3560-3573. https://doi.org/10.3390/md12063560