Klymollins T–X, Bioactive Eunicellin-Based Diterpenoids from the Soft Coral Klyxum molle
Abstract
:1. Introduction
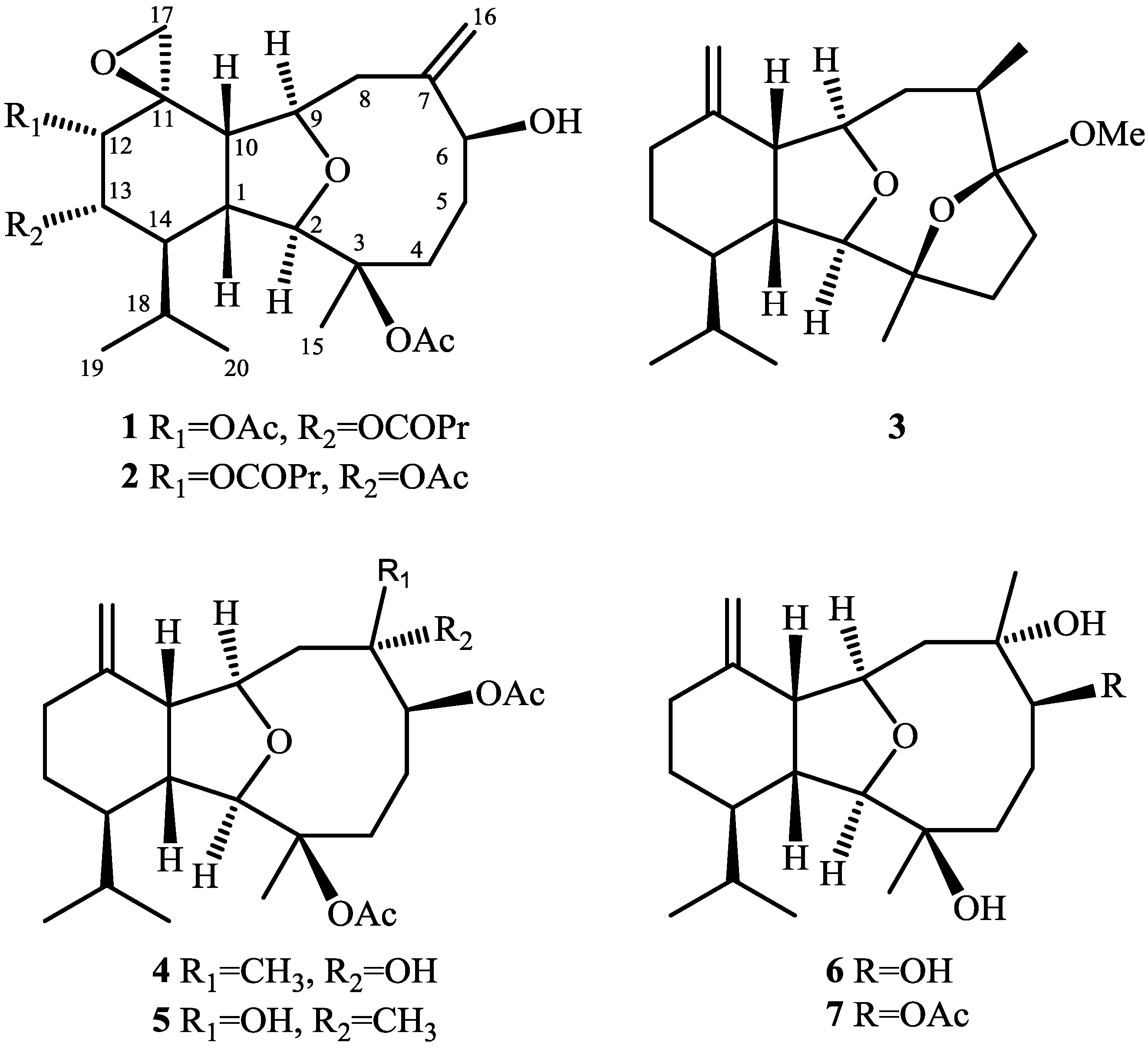
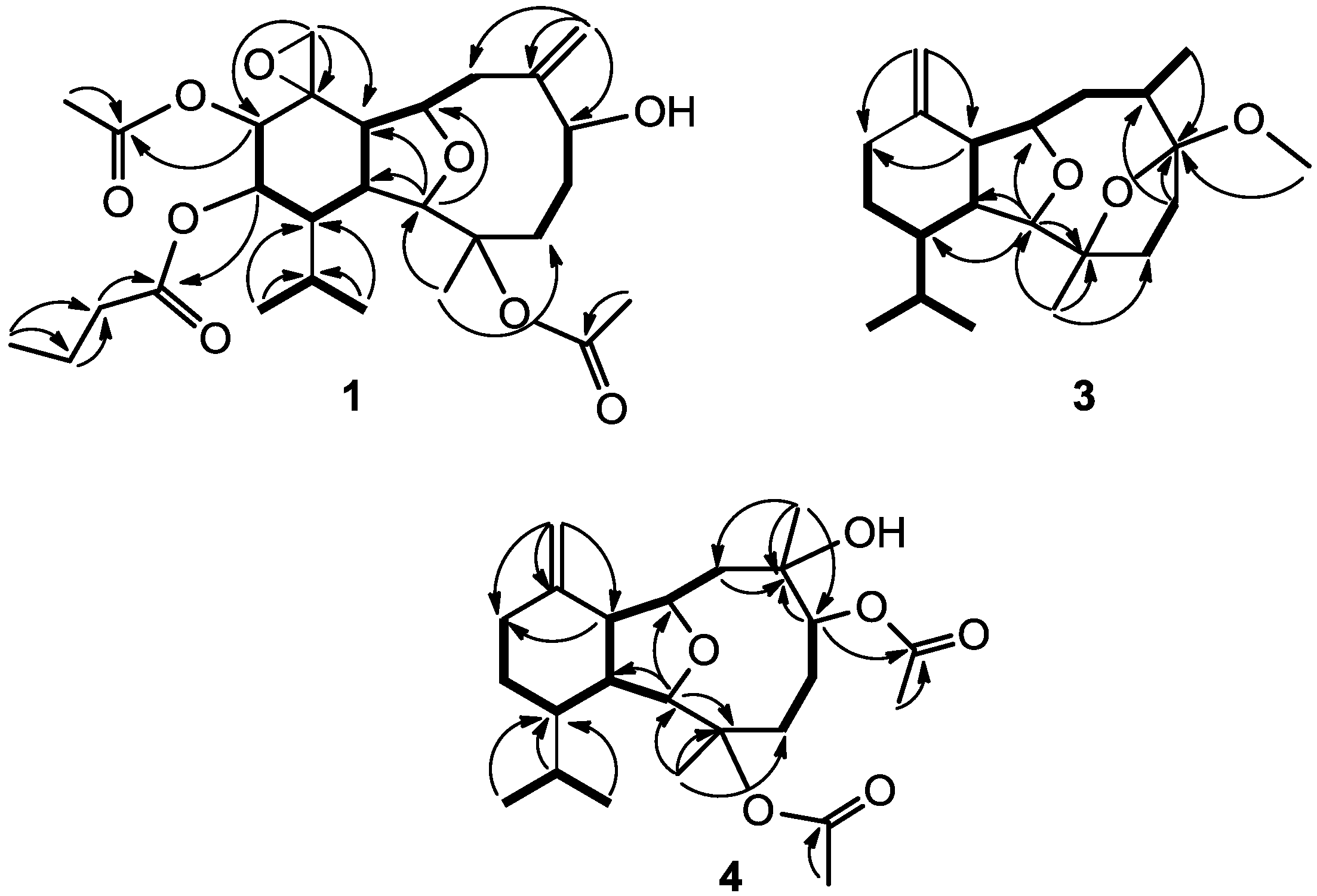
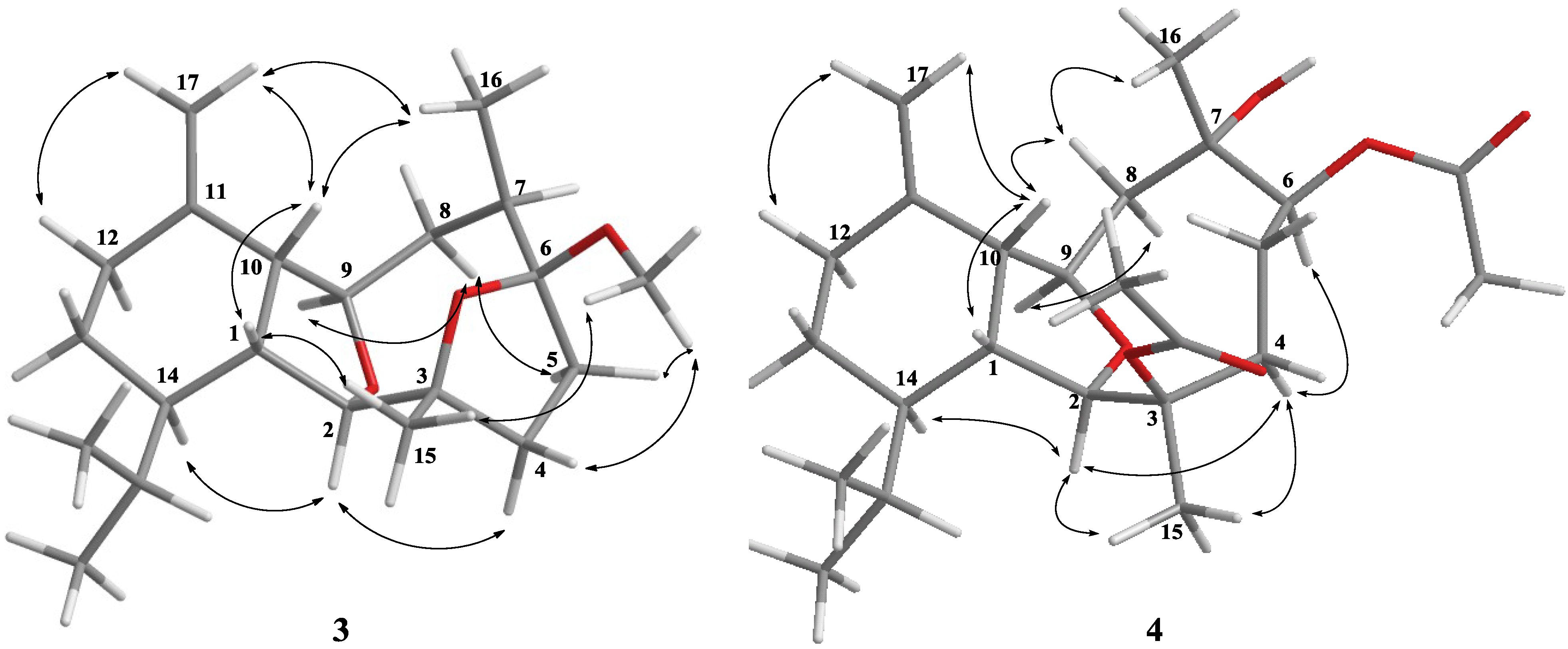
2. Results and Discussion

| 1 a | 2 b | 3 b | ||||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 2.33 m | 42.4 (CH) c | 2.38 t (7.2) d | 41.8 (CH) | 2.20 m | 46.0 (CH) |
| 2 | 3.97 brs e | 91.6 (CH) | 3.79 brs | 90.8 (CH) | 3.58 brs | 90.9 (CH) |
| 3 | 84.9 (C) | 84.6 (C) | 86.7 (C) | |||
| 4 | 1.49 m | 28.5 (CH2) | 1.53 m | 27.8 (CH2) | 2.10 m | 37.6 (CH2) |
| 5 | 1.62 m | 34.4 (CH2) | 1.69 m | 34.3 (CH2) | 2.76 m | 32.8 (CH2) |
| 6 | 4.24 dd (9.5, 4.0) d | 73.0 (CH) | 4.29 d (8.0) | 72.5 (CH) | 113.4 (C) | |
| 7 | 150.5 (C) | 150.0 (C) | 2.16 m | 38.8 (CH) | ||
| 8 | 2.57 d (13.5) | 41.4 (CH2) | 2.48 d (13.6) | 40.7 (CH2) | 2.41 ddd (16.0, 6.8, 3.2) | 36.0 (CH2) |
| 9 | 5.00 d (5.5) | 80.6 (CH) | 4.90 m | 80.1 (CH) | 4.05 dt (8.8, 3.2) | 82.1 (CH) |
| 10 | 2.17 t (8.5) | 43.5 (CH) | 2.26 t (10.4) | 42.9 (CH) | 3.92 t (8.0) | 46.6 (CH) |
| 11 | 55.7 (C) | 55.2 (C) | 148.1 (C) | |||
| 12 | 5.24 d (2.0) | 74.2 (CH) | 4.92 brs | 73.6 (CH) | 2.02 m | 31.2 (CH2) |
| 13 | 5.11 dd (11.0, 2.0) | 72.9 (CH) | 4.91 d (10.4) | 72.2 (CH) | 0.99 m1.70 m | 24.9 (CH2) |
| 14 | 2.11 m | 41.8 (CH) | 2.01 m | 40.7 (CH) | 1.26 m | 43.2 (CH) |
| 15 | 1.75 s | 22.9 (CH3) | 1.65 s | 22.1 (CH3) | 1.31 s | 23.2 (CH3) |
| 16 | 5.24 d (2.0) | 116.9 (CH2) | 4.98 brs | 116.8 (CH2) | 1.36 d (7.6) | 19.1 (CH3) |
| 17 | 1.99 d (5.0) | 53.0 (CH2) | 2.64 d (4.8) | 53.6 (CH2) | 4.77 brs | 109.8 (CH2) |
| 18 | 2.06 m | 28.3 (CH) | 2.03 m | 27.3 (CH) | 1.70 m | 29.1 (CH) |
| 19 | 0.89 d (7.0) | 16.3 (CH3) | 0.84 d (7.2) | 15.3 (CH3) | 0.95 d (6.8) | 21.9 (CH3) |
| 20 | 1.12 d (7.0) | 24.5 (CH3) | 1.09 d (7.2) | 24.0 (CH3) | 0.76 d (6.8) | 15.4 (CH3) |
| 3-OAc | 170.1 (C) | 169.4 (C) | ||||
| 6-OMe | 3.26 brs | 48.5 (CH3) | ||||
| 12-OAc | 170.1 (C) | |||||
| 12-OCOPr | 172.6 (C) | |||||
| 13-OAc | 170.0 (C) | |||||
| 13-OCOPr | 172.0 (C) | |||||
| 4 | 5 | |||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 2.18 m | 45.7 (CH) a | 2.19 m | 45.9 (CH) |
| 2 | 3.63 brs b | 92.1 (CH) | 3.59 brs | 92.3 (CH) |
| 3 | 86.7 (C) | 86.4 (C) | ||
| 4 | 2.04 m | 35.7 (CH2) | 1.94 m | 35.6 (CH2) |
| 5 | 1.26 m | 29.2 (CH2) | 1.37 m | 27.4 (CH2) |
| 6 | 5.63 d (6.0) | 84.7 (CH) | 5.22 d (6.0) | 83.4 (CH) |
| 7 | 75.6 (C) | 72.9 (C) | ||
| 8 | 1.86 m | 45.9 (CH2) | 1.81 dd (14.0, 3.6) | 45.2 (CH2) |
| 9 | 4.17 q (7.2) | 78.1 (CH) | 3.85 ddd (3.6, 7.6, 11.2) | 78.6 (CH) |
| 10 | 2.98 t (7.2) | 53.8 (CH) | 3.06 t (7.6) | 53.8 (CH) |
| 11 | 147.6 (C) | 147.4 (C) | ||
| 12 | 2.04 m | 31.5 (CH2) | 2.05 m | 31.6 (CH2) |
| 13 | 1.02 m | 24.6 (CH2) | 1.02 m | 24.6 (CH2) |
| 14 | 1.29 m | 43.9 (CH) | 1.23 m | 43.9 (CH) |
| 15 | 1.39 s | 22.9 (CH3) | 1.38 s | 23.1 (CH3) |
| 16 | 1.20 s | 23.7 (CH3) | 1.27 s | 25.4 (CH3) |
| 17 | 4.62 brs | 109.5 (CH2) | 4.64 brs | 109.9 (CH2) |
| 18 | 1.72 m | 29.0 (CH) | 1.74 m | 29.0 (CH) |
| 19 | 0.97 d (7.2) | 21.9 (CH3) | 0.98 d (7.2) | 21.9 (CH3) |
| 20 | 0.79 d (7.2) | 15.4 (CH3) | 0.79 d (7.2) | 15.4 (CH3) |
| 3-OAc | 169.6 (C) | 169.6 (C) | ||
| 6-OAc | 171.9 (C) | 170.1 (C) | ||
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
 −67 (c 0.15, CHCl3); IR (neat) νmax 3481, 2956, 2922, 2874, 2852, 1746, 1456, 1372, 1240, 1098 and 1043 cm−1; 13C and 1H NMR data (500 MHz; C6D6), see Table 1; ESIMS m/z 545 [M + Na]+; HRESIMS m/z 545.2722 [M + Na]+ (calcd for C27H40O11Na, 525.2726).
−67 (c 0.15, CHCl3); IR (neat) νmax 3481, 2956, 2922, 2874, 2852, 1746, 1456, 1372, 1240, 1098 and 1043 cm−1; 13C and 1H NMR data (500 MHz; C6D6), see Table 1; ESIMS m/z 545 [M + Na]+; HRESIMS m/z 545.2722 [M + Na]+ (calcd for C27H40O11Na, 525.2726). −57 (c 0.17, CHCl3); IR (neat) νmax 3481, 2958, 2927, 2875, 2854, 1735, 1456, 1370, 1243, 1098 and 1043 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; ESIMS m/z 545 [M + Na]+; HRESIMS m/z 545.2723 [M + Na]+ (calcd for C27H40O11Na, 545.2726).
−57 (c 0.17, CHCl3); IR (neat) νmax 3481, 2958, 2927, 2875, 2854, 1735, 1456, 1370, 1243, 1098 and 1043 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; ESIMS m/z 545 [M + Na]+; HRESIMS m/z 545.2723 [M + Na]+ (calcd for C27H40O11Na, 545.2726). −18 (c 1.14, CHCl3); IR (neat) νmax 2953, 2931, 2877, 1735, 1636, 1467, 1373, 1227, 1183, 1082 and 1038 cm–1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; LRESIMS m/z 357 [M + Na]+; HRESIMS m/z 357.2405 [M + Na]+ (calcd for C21H34O3Na, 357.2406).
−18 (c 1.14, CHCl3); IR (neat) νmax 2953, 2931, 2877, 1735, 1636, 1467, 1373, 1227, 1183, 1082 and 1038 cm–1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; LRESIMS m/z 357 [M + Na]+; HRESIMS m/z 357.2405 [M + Na]+ (calcd for C21H34O3Na, 357.2406). +14 (c 2.11, CHCl3); IR (neat) νmax 3466, 2959, 2935, 2872, 1732, 1644, 1448, 1370, 1250, 1103, 1049 and 1023 cm–1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 2; LRESIMS m/z 445 [M + Na]+; HRESIMS m/z 445.2563 [M + Na]+ (calcd for C24H38O6Na, 445.2566).
+14 (c 2.11, CHCl3); IR (neat) νmax 3466, 2959, 2935, 2872, 1732, 1644, 1448, 1370, 1250, 1103, 1049 and 1023 cm–1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 2; LRESIMS m/z 445 [M + Na]+; HRESIMS m/z 445.2563 [M + Na]+ (calcd for C24H38O6Na, 445.2566). +18 (c 1.34, CHCl3); IR (neat) νmax 3478, 2959, 2932, 2871, 1735, 1645, 1431, 1371, 1115 and 1021 cm–1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 2; LRESIMS m/z 445 [M + Na]+; HRESIMS m/z 445.2568 [M + Na]+ (calcd for C24H38O6Na, 445.2566).
+18 (c 1.34, CHCl3); IR (neat) νmax 3478, 2959, 2932, 2871, 1735, 1645, 1431, 1371, 1115 and 1021 cm–1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 2; LRESIMS m/z 445 [M + Na]+; HRESIMS m/z 445.2568 [M + Na]+ (calcd for C24H38O6Na, 445.2566).3.4. Cytotoxicity Testing
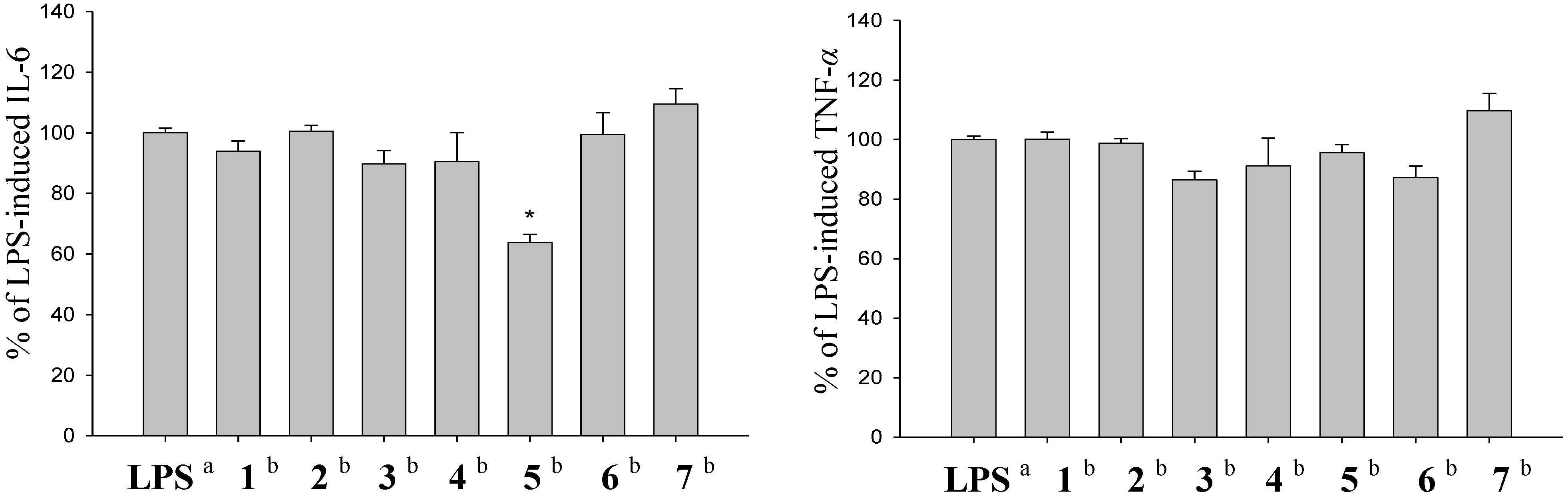
3.5. In Vitro Anti-Inflammatory Assay
4. Conclusions
Supplementary Files
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Chen, B.-W.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Simplexins A–I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wu, Y.-C.; Chiang, M.Y.; Su, J.-H.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron 2009, 65, 7016–7022. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Hwang, T.-L.; Weng, C.-F.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-J. Cladielloides A and B: New eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. Drugs 2010, 8, 2936–2945. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins A–H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef]
- Hassan, H.M.; Khanfar, M.A.; Elnagar, A.Y.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Pachycladins A–E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010, 73, 848–853. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Sung, P.-J.; Sheu, J.-H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010, 8, 2363–2366. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Lu, Y.; Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Kuo, Y.-H.; Sheu, J.-H. Simplexins J–O, eunicellin-based diterpenoids from a Dongsha Atoll soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011, 84, 626–632. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Su, Y.-D.; Chang, Y.-C.; Lu, M.-C.; Weng, C.-F.; Su, J.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs 2011, 9, 934–943. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2011, 9, 2036–2045. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Tai, C.-J.; Hwang, T.-L.; Sheu, J.-H. Krempfielins J–M, new eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 2741–2750. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, C.-Y.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Cytotoxic and anti-inflammatory eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 788–799. [Google Scholar] [CrossRef]
- Cai, Y.-S.; Yao, L.-G.; Di Pascale, A.; Irace, C.; Mollo, E.; Taglialatela-Scafati, O.; Guo, Y.-W. Polyoxygenated diterpenoids of the eunicellin-type from the Chinese soft coral Cladiella krempfi. Tetrahedron 2013, 69, 2214–2219. [Google Scholar] [CrossRef]
- Chen, T.-H.; Lu, M.-C.; Chang, Y.-C.; Su, Y.-D.; Chen, Y.-H.; Lin, N.-C.; Fang, L.-S.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellin-based diterpenoids from a Formosan soft coral Cladiella sp. Mar. Drugs 2013, 11, 4585–4593. [Google Scholar] [CrossRef]
- Shih, F.-Y.; Chen, T.-H.; Lu, M.-C.; Chen, W.-F.; Wen, Z.-H.; Kuo, Y.-H.; Sung, P.-J. Cladieunicellins K and L, new eunicellin-based diterpenoids from an octocoral Cladiella sp. Int. J. Mol. Sci. 2013, 14, 21781–21789. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wang, S.-Y.; Huang, C.-Y.; Chen, S.-L.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins I–M, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. Tetrahedron 2013, 69, 2296–2301. [Google Scholar] [CrossRef]
- Li, T.-T.; Tang, X.-L.; Chen, C.-L.; Zhang, X.-W.; Wu, R.-C.; Zhu, H.-Y.; Li, P.-L.; Li, G.-Q. New eunicellin diterpenes and 9,10-secosteroids from the gorgonian Muricella sibogae. Helv. Chim. Acta 2013, 96, 21781–21789. [Google Scholar]
- Lai, D.; Liu, D.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Antifouling eunicellin-type diterpenoids from the gorgonian Astrogorgia sp. J. Nat. Prod. 2012, 75, 1595–1602. [Google Scholar] [CrossRef]
- Hsu, F.-J.; Chen, B.-W.; Wen, Z.-H.; Huang, C.-Y.; Dai, C.-F.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Klymollins A–H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011, 74, 2467–2471. [Google Scholar] [CrossRef]
- Lin, M.-C.; Chen, B.-W.; Huang, C.-Y.; Dai, C.-F.; Hwang, T.-L.; Sheu, J.-H. Eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2013, 76, 1661–1667. [Google Scholar] [CrossRef]
- Sharma, P.; Alam, M. Sclerophytins A and B. Isolation and structures of novel cytotoxic diterpenes from the marine coral Sclerophytum capitalis. J. Chem. Soc. Perkin Trans. I 1988, 2537–2540. [Google Scholar] [CrossRef]
- Wei, W.-C.; Sung, P.-J.; Duh, C.-Y.; Chen, B.-W.; Sheu, J.-H.; Yang, N.-S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Wei, W.-C.; Lin, S.-Y.; Chen, Y.-J.; Wen, C.-C.; Huang, C.-Y.; Palanisamy, A.; Yang, N.-S.; Sheu, J.-H. Topical application of marine briarane-type diterpenes effectively inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and dermatitis in murine skin. J. Biomed. Sci. 2011, 18, 94. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, F.-Y.; Hsu, F.-J.; Tai, C.-J.; Wei, W.-C.; Yang, N.-S.; Sheu, J.-H. Klymollins T–X, Bioactive Eunicellin-Based Diterpenoids from the Soft Coral Klyxum molle. Mar. Drugs 2014, 12, 3060-3071. https://doi.org/10.3390/md12053060
Chang F-Y, Hsu F-J, Tai C-J, Wei W-C, Yang N-S, Sheu J-H. Klymollins T–X, Bioactive Eunicellin-Based Diterpenoids from the Soft Coral Klyxum molle. Marine Drugs. 2014; 12(5):3060-3071. https://doi.org/10.3390/md12053060
Chicago/Turabian StyleChang, Fang-Yu, Fang-Jung Hsu, Chi-Jen Tai, Wen-Chi Wei, Ning-Sun Yang, and Jyh-Horng Sheu. 2014. "Klymollins T–X, Bioactive Eunicellin-Based Diterpenoids from the Soft Coral Klyxum molle" Marine Drugs 12, no. 5: 3060-3071. https://doi.org/10.3390/md12053060
APA StyleChang, F.-Y., Hsu, F.-J., Tai, C.-J., Wei, W.-C., Yang, N.-S., & Sheu, J.-H. (2014). Klymollins T–X, Bioactive Eunicellin-Based Diterpenoids from the Soft Coral Klyxum molle. Marine Drugs, 12(5), 3060-3071. https://doi.org/10.3390/md12053060




