Overexpression and Characterization of a Novel Thermostable β-Agarase YM01-3, from Marine Bacterium Catenovulum agarivorans YM01T
Abstract
:1. Introduction
2. Results and Discussion
2.1. Agarolytic Activity of the Extracellular Proteins of YM01T
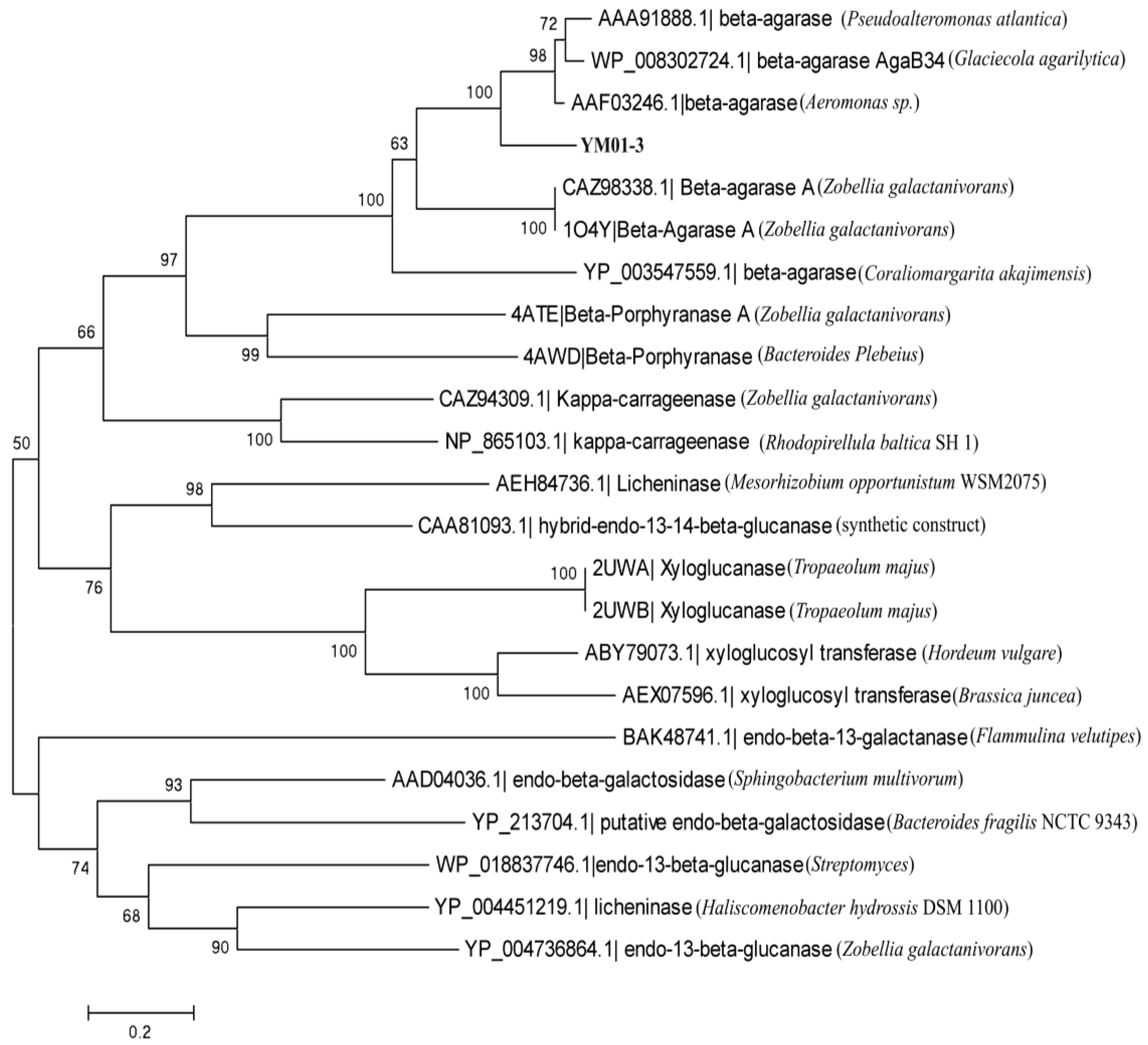
2.2. Cloning and Sequence Analysis of the YM01-3 Gene
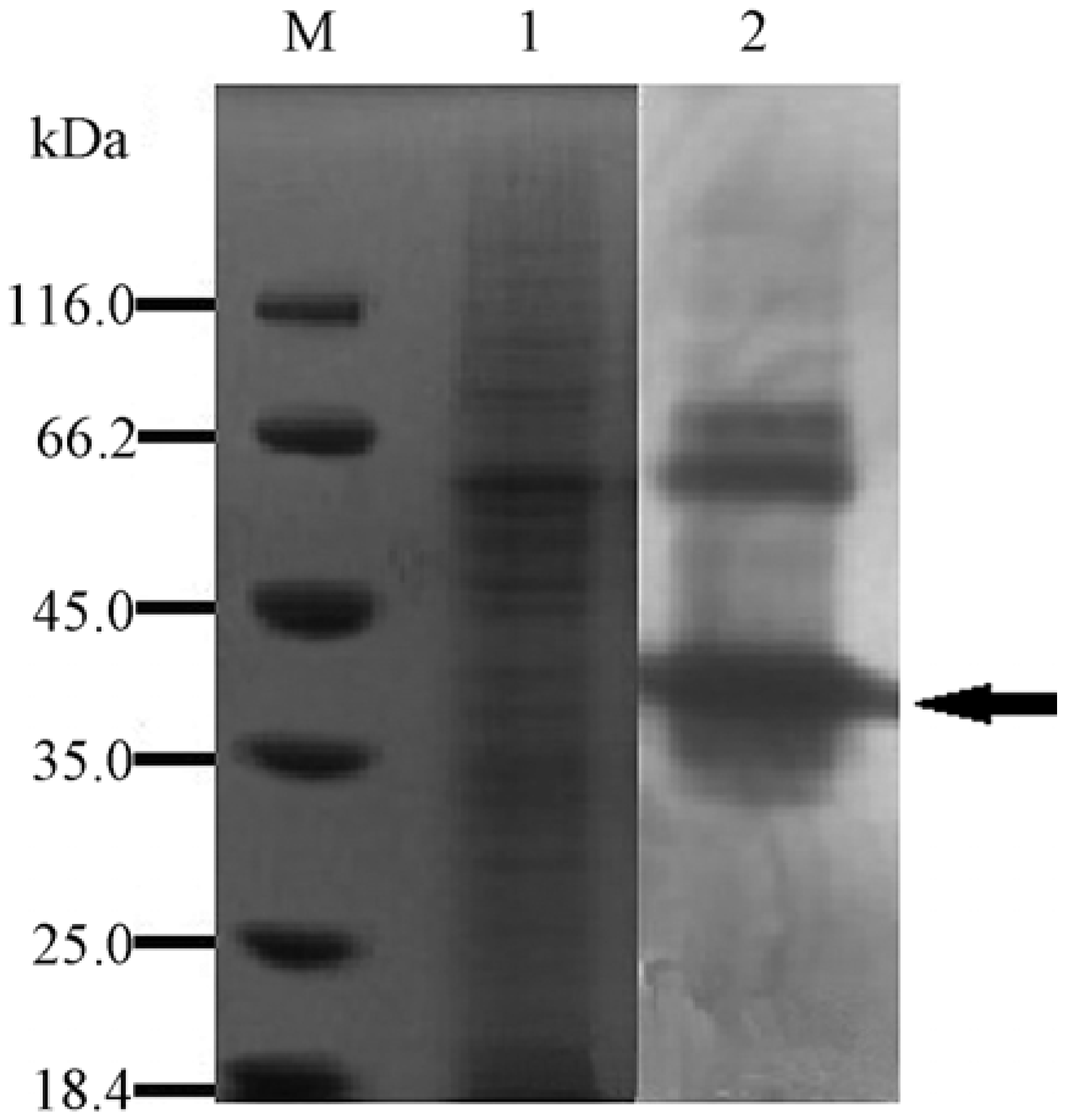
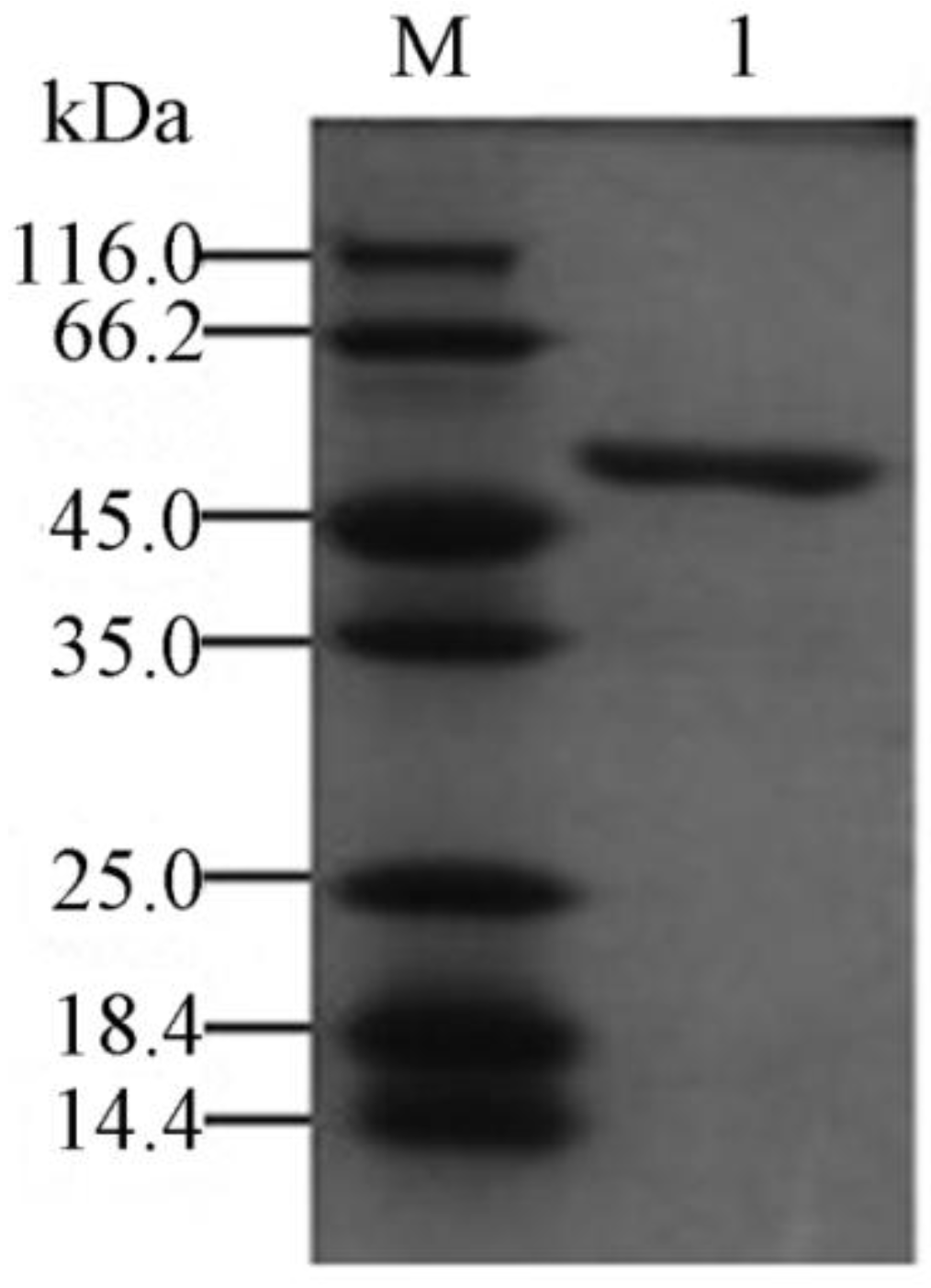
2.3. Expression and Purification of the Recombinant YM01-3


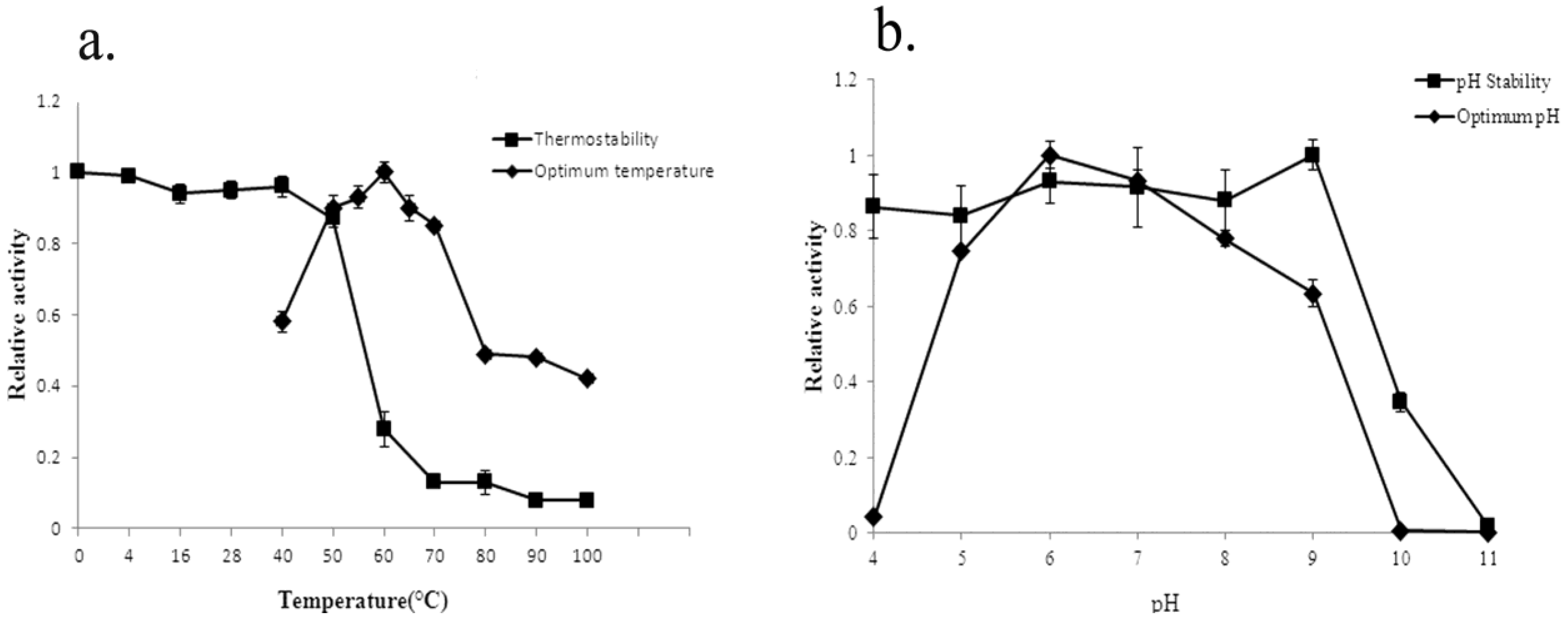
2.4. Biochemical Characterization of YM01-3
| Reagent | Concentration (mM) a | Relative Activity (%) b |
|---|---|---|
| None | 100 | |
| SDS | 1 | 109 ± 0.02 |
| Urea | 1 | 101 ± 0.056 |
| EDTA | 1 | 81 ± 0.016 |
| Na+ | 1 | 122 ± 0.13 ** |
| K+ | 1 | 122 ± 0.12 ** |
| Ca2+ | 1 | 113 ± 0.13 * |
| Mg2+ | 1 | 88 ± 0.07 |
| Mn2+ | 1 | 28 ± 0.04 ** |
| Fe3+ | 1 | 14 ± 0.03 ** |
| Cu2+ | 1 | 34 ± 0.08 ** |
| Ni2+ | 10 | 1 ± 0.01 ** |
| Family | Bacterial Species and Protein | Accession No. | Optimal Temperature | Thermostability | Vmax (μmol min−1mg−1) | Reference |
|---|---|---|---|---|---|---|
| GH16 (β-agarase) | Catenovulum agarivorans YM01T (YM01-3) | AGU13985 | 60 °C | Stable below 50 °C for 1 h. | 1.14 × 104 | This study |
| Agarivorans sp. LQ48 (AgaA) | ACM50513 | 40 °C | Stable below 40 °C for 1 h. | 909.1 | [27] | |
| Vibrio sp. strain PO-303 (AgaA) | BAF62129 | 40 °C | Stable under 37 °C for 10 min. | 22.5 | [28] | |
| Vibrio sp. strain PO-303 (AgaD) | BAF34350 | 40 °C | Stable under 45 °C for 10 min. | 101 | [29] | |
| Pseudoalteromonas sp. CY24 (AgaA) | AAN39119 | 40 °C | Stable below 30 °C for 1 h. | 482 | [30] | |
| Microbulbifer elongatus JAMB-A7 (RagaA7) | BAC99022 | 50 °C | Stable up to 50 °C. | 398 | [31] | |
| Microbulbifer sp. Strain CMC-5 ( β-agarase) | None a | 50 °C | 62% activity remained at 50 °C for 30 min. | 0.133 | [32] | |
| Microbulbifer thermotolerans JAMB-A94 (AgaA) | BAK08910 | 55 °C | Stable up to 60 °C. | 517 | [33] | |
| Vibrio sp. F-6 (AG-b) | None a | 55 °C | Stable below 60 °C for 30 min. | 307.4 | [16] | |
| Pseudoalteromonas sp. AG4 (AgaP) | ADD60418 | 55 °C | Stable below 55 °C for 1 h. | ND b | [12] | |
| GH50 (β-agarase) | Agarivorans sp. JA-1 (β-agarase) | ABK97391 | 40 °C | Stable up to 60 °C. | ND b | [34] |
| Vibrio sp. Strain CN41 (AgaACN41) | ADM25828 | 40 °C | Stable below 40 °C. | 3 | [35] | |
| Agarivorans sp. JAMB-AII (AgaAII) | BAD99519 | 40 °C | Stable up to 40 °C. | 371 | [36] | |
| Streptomyces coelicolor A3(2) (Sco3487) | CAB61811 | 40 °C | Stable below 50 °C for 1h. | 10.75 | [37] | |
| Saccharophagus degradans 2-40 (Aga50D) | ABD81904 | 30 °C | Stable up to 40 °C. | 17.9 | [38] | |
| Alteromonas sp. E-l (β-agarase) | BAE97587 | 40 °C | Stable up to 40 °C for 30 min. | ND b | [4] | |
| Agarivorans albusYKW-34 (AgaA34) | P85974 | 40 °C | Stable up to 50 °C for 1 h. | 529 | [39] | |
| Vibrio sp. F-6 (AG-a) | None a | 40 °C | Stable below 50 °C for 30 min. | 230 | [16] | |
| Agarivorans sp. HZ105 (HZ2) | ADY17919 | 40 °C | ND b | 235 | [40] | |
| GH86 (β-agarase) | Microbulbifer sp. JAMB-A94 (rAgaO) | BAK08903 | 45 °C | Stable up to 40 °C. | ND b | [41] |
| GH96 (α-agarase) | Alteromonas agarilytica GJB (AgaA) | AAF26838 | 42.5 °C | Stable up to 30 °C during for several days. | ND b | [3] |
| Thalassomonas sp. JAMB-A33 (AgaA33) | BAF44076 | 45 °C | Stable up to 40 °C for 30 min. | 40.7 | [42] | |
| GH118 (β-agarase) | Vibrio sp. Strain PO-303 (Agarase C) | BAF03590 | 35 °C | Stable under 37 °C. | 329 | [43] |
| Pseudoalteromonas sp. CY24 (AgaB) | AAQ56237 | 40 °C | Stable under 35 °C for 1 h. | ND b | [44] | |
| Catenovulum sp. X3 (AgaXa) | GU975829 | 52 °C | Stable below 42 °C. | 588.2 | [45] |
3. Experimental Section
3.1. Bacterial Strains and Growth Conditions
3.2. Analysis of the Agarolytic Activity of the Extracellular Proteins of YM01T
3.3. Cloning and Sequence Analysis of the YM01-3 Gene
3.4. Overexpression and Purification of the Recombinant YM01-3 Protein
3.5. Agarolytic Activity Assay of the Recombinant YM01-3
3.6. Biochemical Characterization of the Recombinant YM01-3
3.7. Identification of Hydrolysis Products by Purified YM01-3
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Araki, C.H. Acetylation of agar like substance of Gelidium amansii. J. Chem. Soc. 1937, 58, 1338–1350. [Google Scholar]
- Hamer, G.K.; Bhattacharjee, S.S.; Yaphe, W. Analysis of the enzymic hydrolysis products of agarose by 13C-NMR spectroscopy. Carbohydr. Res. 1977, 54, C7–C10. [Google Scholar] [CrossRef]
- Potin, P.; Richard, C.; Rochas, C.; Kloareg, B. Purification and characterization of the α-agarase from Alteromonas agarlyticus (Cataldi) comb. nov., strain GJ1B. Eur. J. Biochem. 1993, 214, 599–607. [Google Scholar] [CrossRef]
- Kirimura, K.; Masuda, N.; Iwasaki, Y.; Nakagawa, H.; Kobayashi, R.; Usami, S. Purification and characterization of a novel beta-agarase from an alkalophilic bacterium, Alteromonas sp. E-1. J. Biosci. Bioeng. 1999, 87, 436–441. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic. Acids. Res. 2009, 37, 233–238. [Google Scholar] [CrossRef]
- Carbohydrate-Active enZYmes Database. Available online: http://www.cazy.org (accessed on 1 October 2013).
- Michel, G.; Nyval, P.; Barbeyron, T.; Czjzek, M.; Helbert, W. Bioconversion of red seaweed galactans: A focus on bacterial agarases and carrageenases. Appl. Microbiol. Biotechnol. 2006, 71, 23–33. [Google Scholar] [CrossRef]
- Rhee, Y.J.; Han, C.R.; Kim, W.C.; Jun, D.Y.; Rhee, I.K.; Kim, Y.H. Isolation of a novel freshwater agarolytic Cellvibrio sp. KY-YJ-3 and characterization of its extracellular beta-agarase. J. Microbiol. Biotechnol. 2010, 20, 1378–1385. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, B.K.; Xu, Y.; Zhong, M.Q.; Liu, G.M. Production and purification of agarase from a marine agarolytic bacterium Agarivorans sp. HZ105. J. Appl. Microbiol. 2009, 106, 181–190. [Google Scholar] [CrossRef]
- Wang, J.X.; Mou, H.J.; Jiang, X.L.; Guan, H.S. Characterization of a novel β-agarase from marine Alteromonas sp. SY37-12 and its degrading products. Appl. Microbiol. Biotechnol. 2006, 71, 833–839. [Google Scholar] [CrossRef]
- Zhong, Z.; Toukdarian, A.; Helinski, D.; Knauf, V.; Sykes, S.; Wilkinson, J.E.; O’Bryne, C.; Shea, T.; DeLoughery, C.; Caspi, R. Sequence analysis of a 101-kilobase plasmid required for agar degradation by a Microscilla isolate. Appl. Environ. Microbiol. 2001, 67, 5771–5779. [Google Scholar] [CrossRef]
- Oh, C.; Nikapitiya, C.; Lee, Y.; Whang, I.; Kim, S.J.; Kang, D.H.; Lee, J. Cloning, purification and biochemical characterization of β agarase from the marine bacterium Pseudoalteromonas sp. AG4. J. Ind. Microbiol. Biotechnol. 2010, 37, 483–494. [Google Scholar] [CrossRef]
- Morrice, L.M.; McLean, M.W.; Williamson, F.B.; Long, W.F. Purifications and some properties of beta-agarases I and II from Pseudomonas atlantica. Eur. J. Biochem. 1983, 135, 553–558. [Google Scholar] [CrossRef]
- Nedashkovskaya, O.I.; Suzuki, M.; Lee, J.S.; Lee, K.C.; Shevchenko, L.S.; Mikhailov, V.V. Pseudozobellia thermophila gen. nov. sp. nov., a bacterium of the family Flavobacteriaceae, isolated from the green alga Ulva fenestrata. Int. J. Syst. Evol. Microbiol. 2009, 59, 806–810. [Google Scholar] [CrossRef]
- Ekborg, N.A.; Taylor, L.E.; Longmire, A.G.; Henrissat, B.; Weiner, R.M.; Hutcheson, S.W. Genomic and proteomic analyses of the agarolytic system expressed by Saccharophagus degradans 2-40. Appl. Environ. Microbiol. 2006, 72, 3396–3405. [Google Scholar] [CrossRef]
- Fu, W.; Han, B.; Duan, D.; Liu, W.; Wang, C. Purification and characterization of agarases from a marine bacterium Vibrio sp.F-6. J. Ind. Microbiol. Biotechnol. 2008, 35, 915–922. [Google Scholar] [CrossRef]
- Lakshmikanth, M.; Manohar, S.; Lalitha, J. Purification and characterization of β-agarase from agar-liquefying soil bacterium Acinetobacter sp., AG LSL-1. Process. Biochem. 2009, 44, 999–1003. [Google Scholar] [CrossRef]
- Suzuki, H.; Sawai, Y.; Suzuki, T.; Kawai, K. Purification and characterization of an extracellular beta-agarase from Bacillus sp. MK03. J. Biosci. Bioeng. 2003, 95, 328–334. [Google Scholar]
- Van der Meulen, H.J.; Harder, W. Production and characterization of the agarase of Cytoplaga flevensis. Antonie van Leeuwenhoek 1975, 41, 431–447. [Google Scholar] [CrossRef]
- De Ruiter, G.; Rudolph, B. Carrageenan biotechnology. Trends Food. Sci. Technol. 1997, 8, 389–395. [Google Scholar] [CrossRef]
- Yan, S.L.; Yu, M.; Wang, Y.; Shen, C.; Zhang, X.-H. Catenovulum agarivorans gen. nov. sp. nov., a peritrichously flagellated, chain-forming, agar-hydrolysing gammaproteobacterium isolated from seawater. Int. J. Syst. Evol. Microbiol. 2011, 61, 2866–2873. [Google Scholar]
- Shi, X.; Yu, M.; Yan, S.; Dong, S.; Zhang, X.-H. Genome sequence of the thermostable-agarase-producing marine bacterium Catenovulum agarivorans YM01T, which reveals the presence of a series of agarase-encoding genes. J. Bacteriol. 2012, 194, 54–84. [Google Scholar]
- Petersen, T.N.; Brunak, S.; Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Allouch, J.; Jam, M.; Helbert, W.; Barbeyron, T.; Kloareg, B.; Henrissat, B.; Czjzek, M. The three-dimensional structures of two β-agarase. J. Biol. Chem. 2003, 278, 47171–47180. [Google Scholar]
- Henrissat, B.; Davies, G.J. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Shi, X.; Cui, F.; Zhang, X.-H.; College of Marine Life Sciences, Ocean University of China, Qingdao, China. Unpublished work. 2014.
- Long, M.X.; Yu, Z.N.; Xu, X. A novel β-Agarase with high pH stability from marine Agarivorans sp. LQ48. Mar. Biotechnol. 2010, 12, 62–69. [Google Scholar] [CrossRef]
- Dong, J.; Tamaru, Y.; Araki, T. A unique beta-agarase, AgaA, from a marine bacterium, Vibrio sp. strain PO-303. Appl. Microbiol. Biotechnol. 2007, 74, 1248–1255. [Google Scholar]
- Dong, J.; Tamaru, Y.; Araki, T. Molecular cloning, expression, and characterization of a beta-agarase gene, agaD, from a marine bacterium, Vibrio sp. strain PO-303. Biosci. Biotechnol. Biochem. 2007, 71, 38–46. [Google Scholar] [CrossRef]
- Lu, X.Z.; Chu, Y.; Wu, Q.Q.; Gu, Y.C.; Han, F.; Yu, W.G. Cloning, expression and characterization of a new agarase-encoding gene from marine Pseudoalteromonas sp. Biotechnol. Lett. 2009, 31, 1565–1570. [Google Scholar] [CrossRef]
- Ohta, Y.; Hatada, Y.; Nogi, Y.; Miyazaki, M.; Li, Z.; Akita, M.; Hidaka, Y.; Goda, S.; Ito, S.; Horikoshi, K. Enzymatic properties and nucleotide and amino acid sequences of a thermostable beta-agarase from a novel species of deep-sea Microbulbifer. Appl. Microbiol. Biotechnol. 2004, 64, 505–514. [Google Scholar] [CrossRef]
- Jonnadula, R.; Ghadi, S.C. Purification and charaterization of β-agarase from seaweed decomposing bacterium Microbulbifer sp. strain CMC-5. Biotechnol. Bioproce 2011, 16, 513–519. [Google Scholar] [CrossRef]
- Ohta, Y.; Nogi, Y.; Miyazaki, M.; Li, Z.; Hatada, Y.; Ito, S.; Horikoshi, K. Enzymatic properties and nucleotide and amino acid sequences of a thermostable beta-agarase from the novel marine isolate, JAMB-A94. Biosci. Biotechnol. Biochem. 2004, 68, 1073–1081. [Google Scholar] [CrossRef]
- Lee, D.G.; Jang, M.K.; Lee, O.H.; Jang, M.K.; Kim, N.Y.; Ju, S.A.; Lee, S.H. Over-production of a glycoside hydrolase family 50 beta-agarase from Agarivorans sp. JA-1 in Bacillus subtilis and the whitening effect of its product. Biotechnol. Lett. 2008, 30, 911–918. [Google Scholar] [CrossRef]
- Liao, L.; Xu, X.W.; Jiang, X.W.; Cao, Y.; Yi, N.; Huo, Y.Y.; Wu, Y.H.; Zhu, X.F.; Zhang, X.Q.; Wu, M. Cloning, expression, and characterization of a new beta-agarase from Vibrio sp. strain CN41. Appl. Environ. Microbiol. 2011, 77, 7077–7079. [Google Scholar] [CrossRef]
- Ohta, Y.; Hatada, Y.; Ito, S.; Horikoshi, K. High-level expression of a neoagarobiose-producing β-agarase gene from Agarivorans sp. JAMB-AII in Bacillus subtilis and enzymic properties of the recombinant enzyme. Biotechnol. Appl. Biochem. 2005, 41, 183–191. [Google Scholar] [CrossRef]
- Temuujin, U.; Chi, W.J.; Chang, Y.K.; Hong, S.K. Identification and biochemical characterization of Sco3487 from Streptomyces coelicolor A3(2), an exo- and endo-type β-agarase-producing neoagarobiose. J. Bacteriol. 2012, 94, 142–149. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Lee, D.; Kim, H.S.; Bang, W.G.; Kim, K.; Choi, I.G. Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2-40: An exo-type β-agarase producing neoagarobiose. Appl. Microbiol. Biotechnol. 2010, 86, 227–234. [Google Scholar] [CrossRef]
- Fu, X.T.; Lin, H.; Kim, S.M. Purification and characterization of a novel β-agarase, AgaA34, from Agarivorans albus YKW-34. Appl. Microbiol. Biotechnol. 2008, 78, 265–273. [Google Scholar] [CrossRef]
- Lin, B.; Lu, G.; Zheng, Y.; Xie, W.; Li, S.; Hu, Z. Gene cloning, expression and characterization of a neoagarotetraose-producing β-agarase from the marine bacterium Agarivorans sp. HZ105. World J. Microbiol. Biotechnol. 2011, 28, 1691–1697. [Google Scholar]
- Ohta, H.; Hatada, Y.; Nogi, Y.; Li, Z.; Ito, S.; Horikoshi, K. Cloning, expression, and characterization of a glycoside hydrolase family 86 β-agarase from a deap-sea Microbulbifer-like isolate. Appl. Microbiol. Biotechnol. 2004, 66, 266–275. [Google Scholar] [CrossRef]
- Ohta, Y.; Hatada, Y.; Miyazaki, M.; Nogi, Y.; Ito, S.; Horikoshi, K. Purification and characterization of a novel α-agarase from a Thalassomonas sp. Curr. Microbiol. 2005, 50, 212–216. [Google Scholar] [CrossRef]
- Dong, J.; Hashikawa, S.; Konishi, T.; Tamaru, Y.; Araki, T. Cloning of the novel gene encoding beta-agarase C from a marine bacterium, Vibrio sp. strain PO-303, and characterization of the gene product. Appl. Environ. Microbiol. 2006, 72, 6399–6401. [Google Scholar] [CrossRef]
- Ma, C.P.; Lu, X.Z.; Shi, C.; Li, J.B.; Gu, Y.C.; Ma, Y.M.; Chu, Y.; Han, F.; Gong, Q.H.; Yu, W.G. Molecular cloning and characterization of a novel β-Agarase, AgaB, from marine Pseudoalteromonas sp.CY24. J. Biol. Chem. 2007, 282, 3747–3754. [Google Scholar]
- Xie, W.; Lin, B.; Zhou, Z.; Lu, G.; Lun, J.; Xia, C.; Li, S.; Hu, Z. Characterization of a novel β-agarase from an agar-degrading bacterium Catenovulum sp. X3. Appl. Microbiol. Biotechnol. 2013, 97, 4907–4915. [Google Scholar] [CrossRef]
- Fu, X.T.; Kim, S.M. Agarase: Review of major sources, categories, purification method, enzyme characteristics and applications. Mar. Drugs 2010, 8, 200–218. [Google Scholar] [CrossRef]
- Bannikova, G.E.; Lopatin, S.A.; Varlamov, V.P.; Kuznetsov, B.B.; Kozina, I.V.; Miroshnichenko, M.L.; Chernykh, N.A.; Turova, T.P.; Bonch-Osmolovskaya, E.A. The thermophilic bacteria hydrolyzing agar: Characterization of thermostable agarase. Appl. Microbiol. Biotechnol. 2008, 45, 366–371. [Google Scholar]
- Lin, L.L.; Chyau, C.C.; Hsu, W.H. Production and properties of a raw starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol. Appl. Biochem. 1998, 28, 61–68. [Google Scholar]
- Fitter, J.; Herrmann, R.; Hauss, T.; Lechner, R.E.; Dencher, N.A. Dynamical properties of alpha-amylase in the folded and unfolded state: The role of thermal equilibrium fluctuations for conformational entropy and protein stabilization. Phys. B 2001, 301, 1–7. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic. Acids Res. 1997, 25, 3389–3402. [Google Scholar]
- BLAST Assembled RefSeq Genomes. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 December 2012).
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7. [Google Scholar] [CrossRef]
- Clustal Omega, Multipal Sequence Aligment. Available online: http://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 15 October 2012).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, F.; Dong, S.; Shi, X.; Zhao, X.; Zhang, X.-H. Overexpression and Characterization of a Novel Thermostable β-Agarase YM01-3, from Marine Bacterium Catenovulum agarivorans YM01T. Mar. Drugs 2014, 12, 2731-2747. https://doi.org/10.3390/md12052731
Cui F, Dong S, Shi X, Zhao X, Zhang X-H. Overexpression and Characterization of a Novel Thermostable β-Agarase YM01-3, from Marine Bacterium Catenovulum agarivorans YM01T. Marine Drugs. 2014; 12(5):2731-2747. https://doi.org/10.3390/md12052731
Chicago/Turabian StyleCui, Fangyuan, Sujie Dong, Xiaochong Shi, Xia Zhao, and Xiao-Hua Zhang. 2014. "Overexpression and Characterization of a Novel Thermostable β-Agarase YM01-3, from Marine Bacterium Catenovulum agarivorans YM01T" Marine Drugs 12, no. 5: 2731-2747. https://doi.org/10.3390/md12052731
APA StyleCui, F., Dong, S., Shi, X., Zhao, X., & Zhang, X.-H. (2014). Overexpression and Characterization of a Novel Thermostable β-Agarase YM01-3, from Marine Bacterium Catenovulum agarivorans YM01T. Marine Drugs, 12(5), 2731-2747. https://doi.org/10.3390/md12052731




