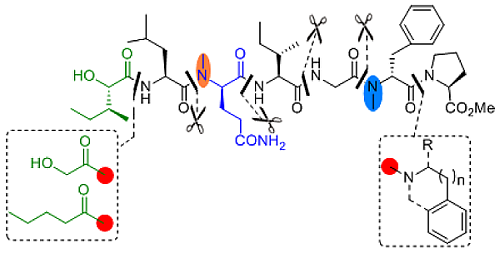
Design, Synthesis and Biological Evaluation of Tasiamide Analogues as Tumor Inhibitors
Abstract
:1. Introduction
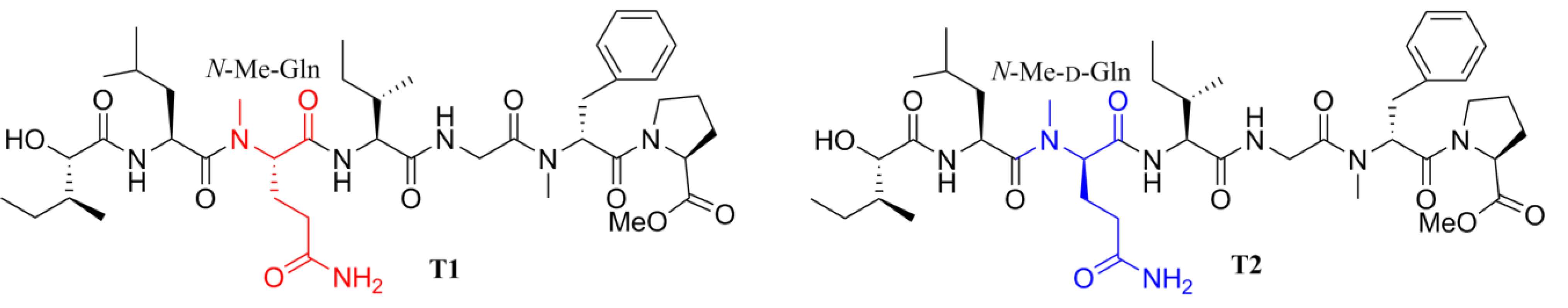
2. Results and Discussion
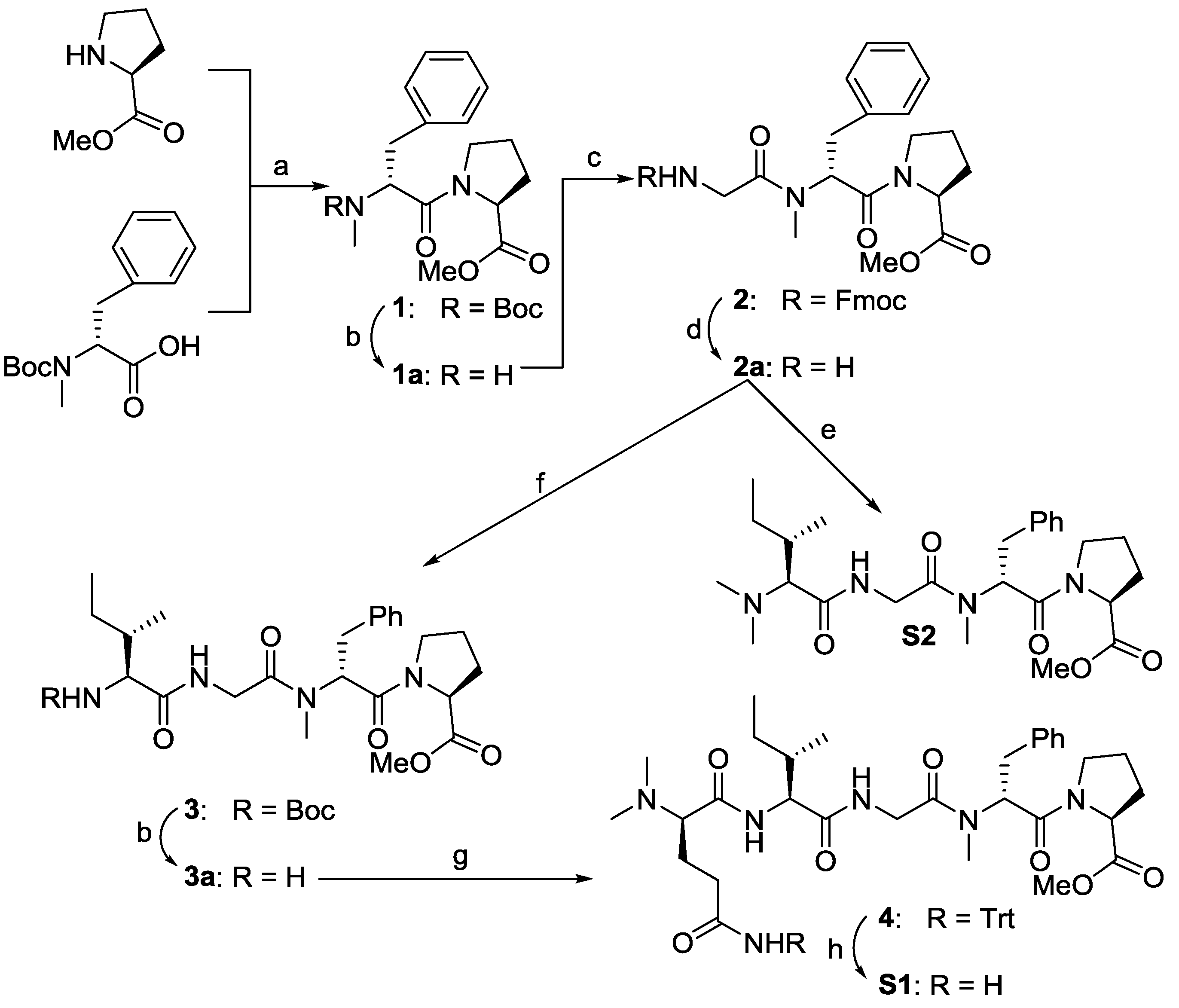
2.1. Chemistry
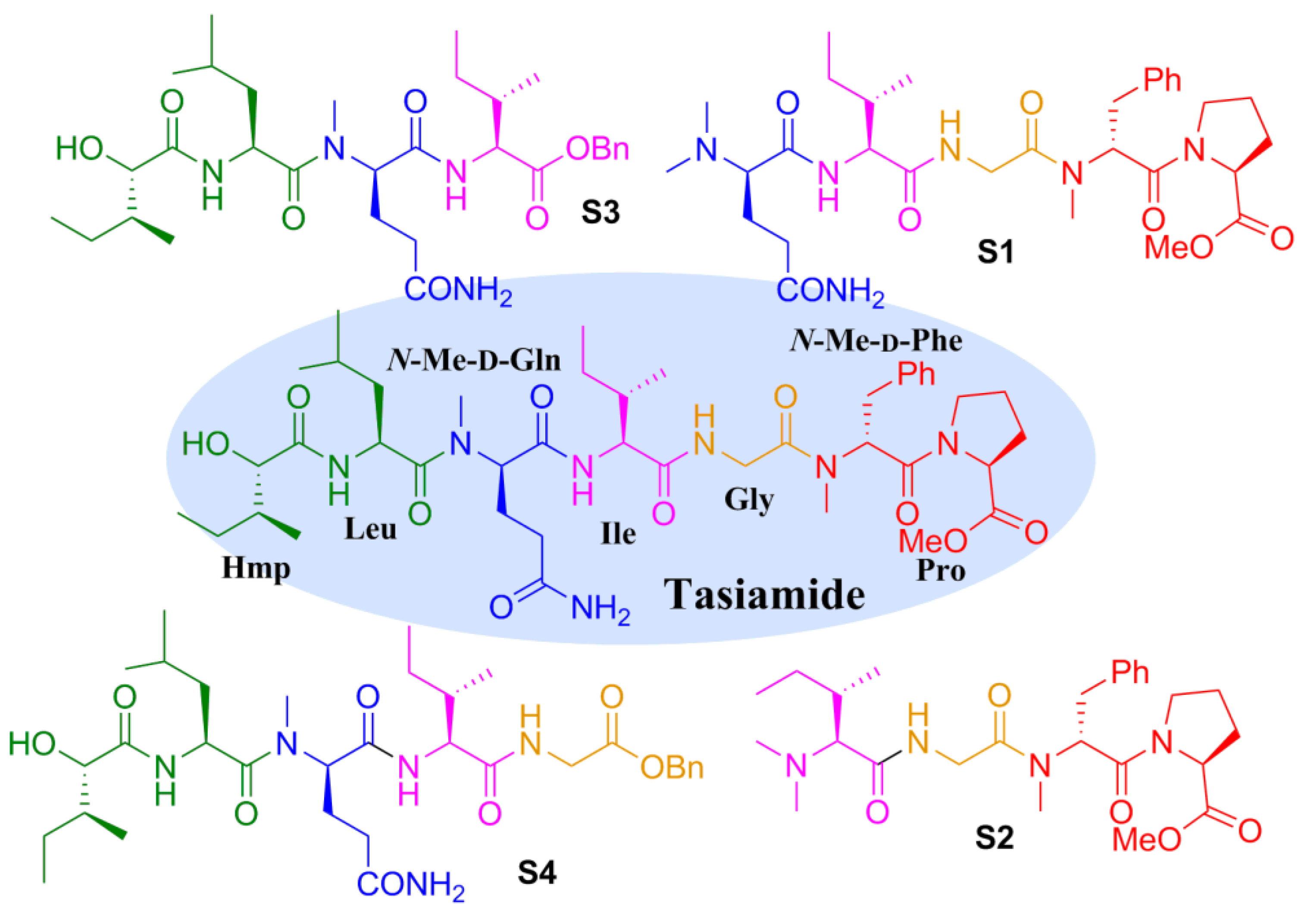
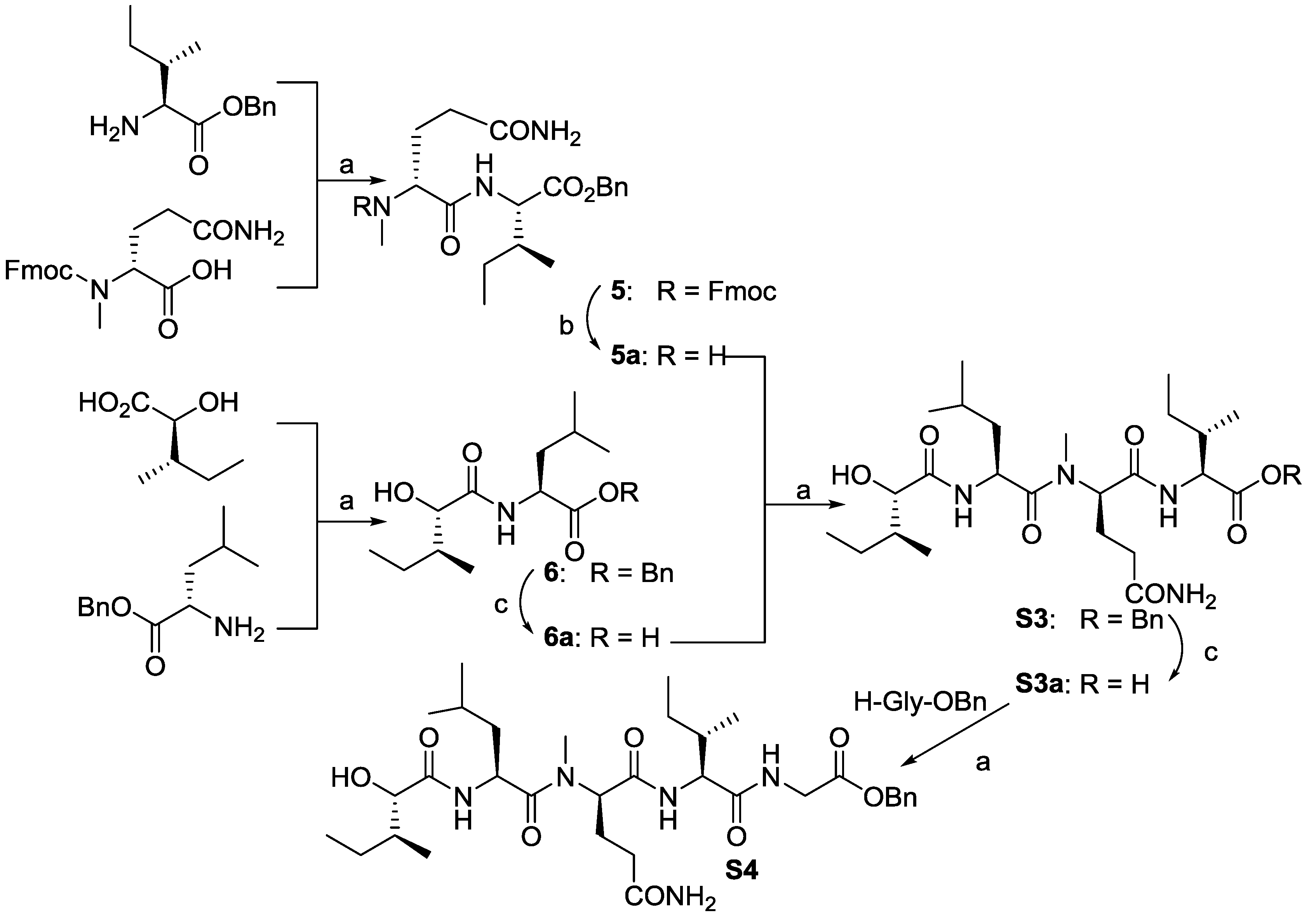
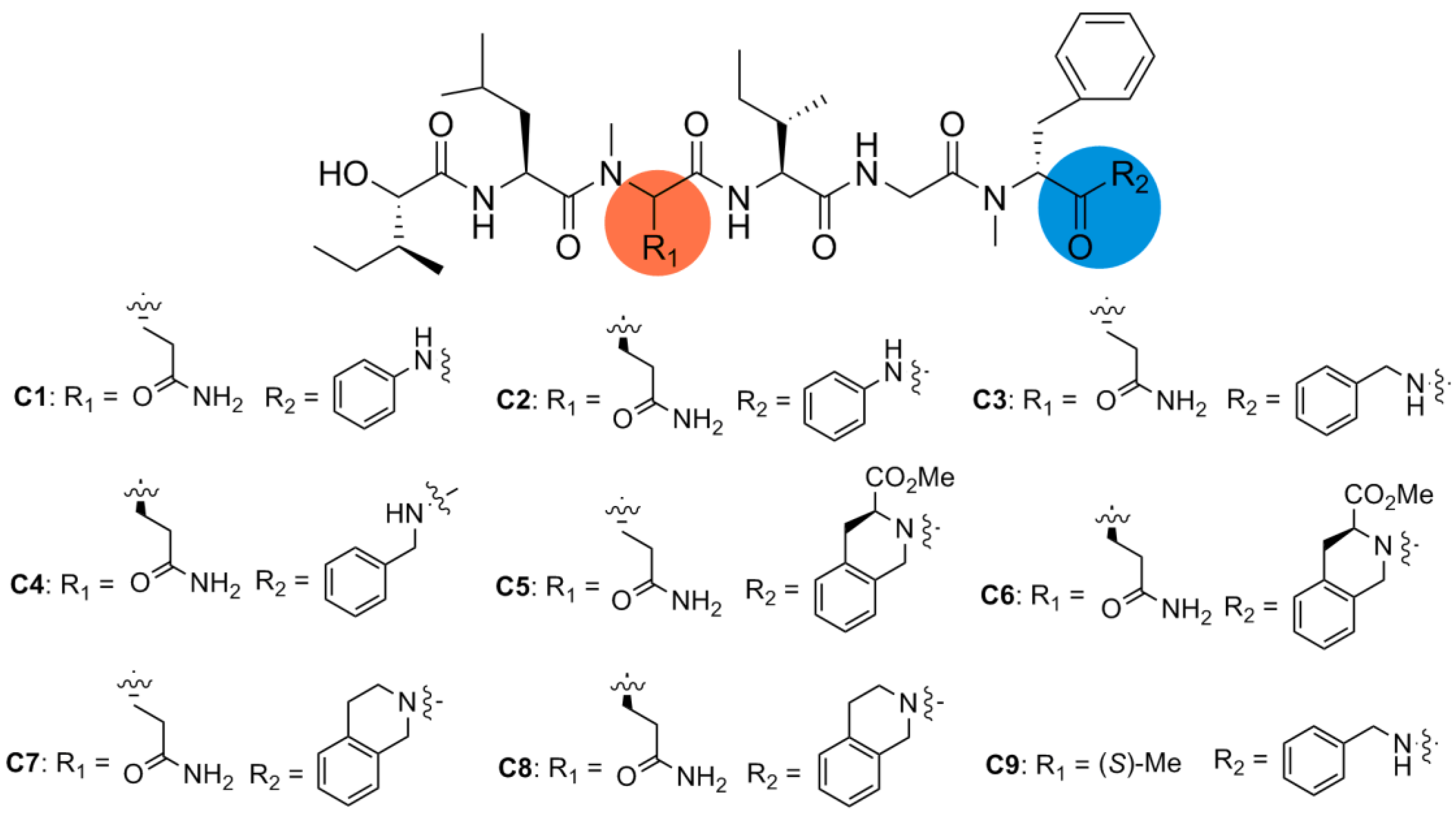
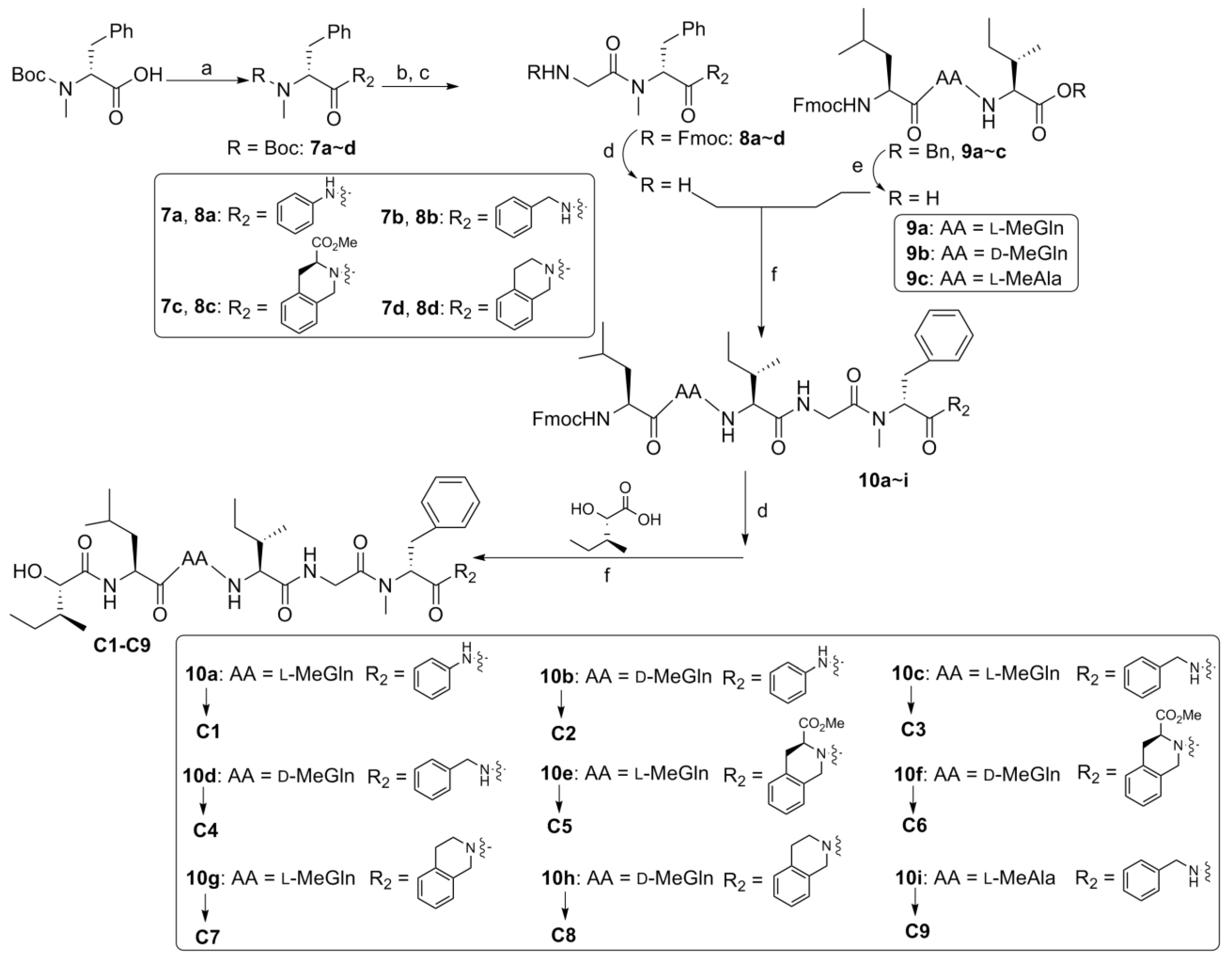
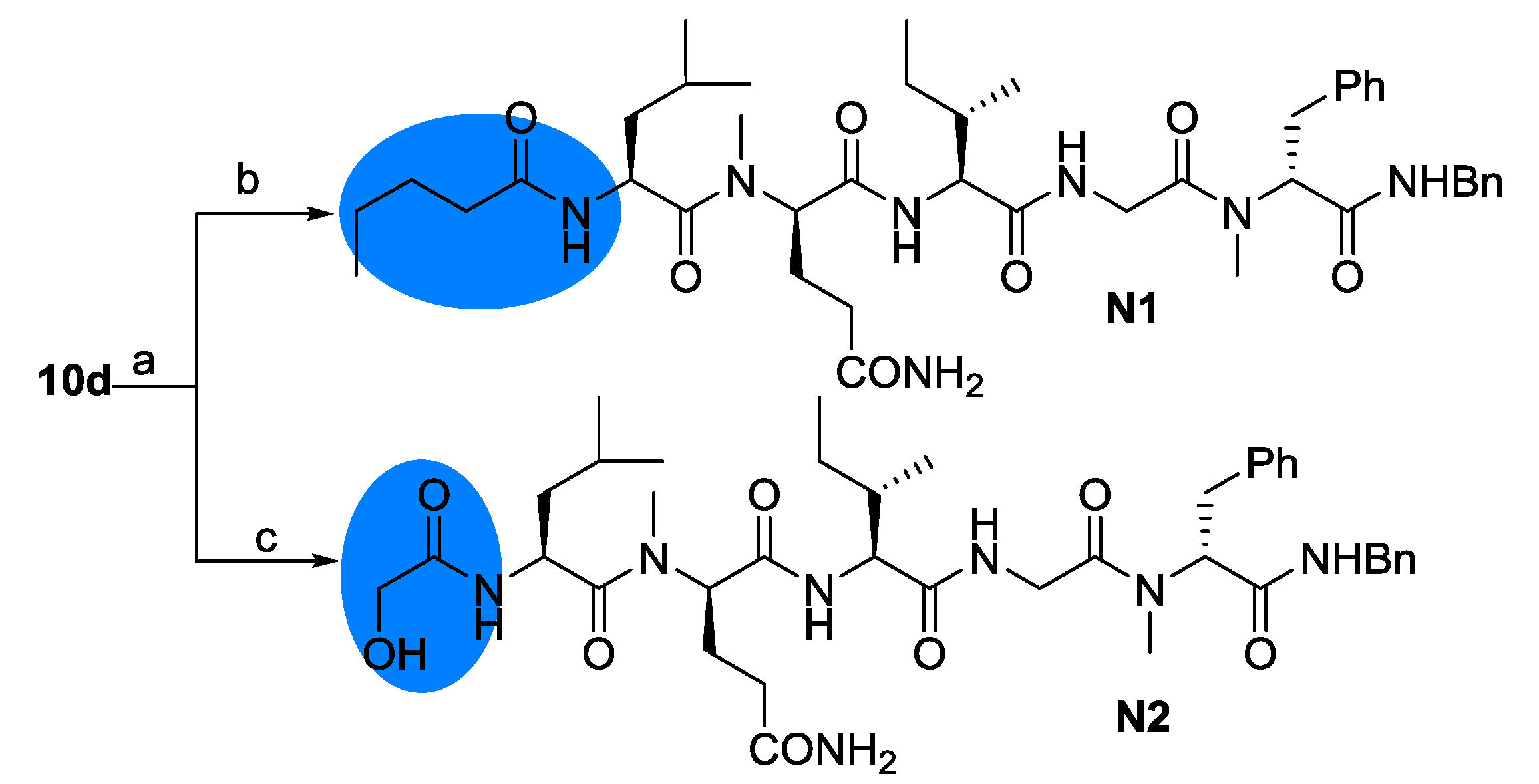
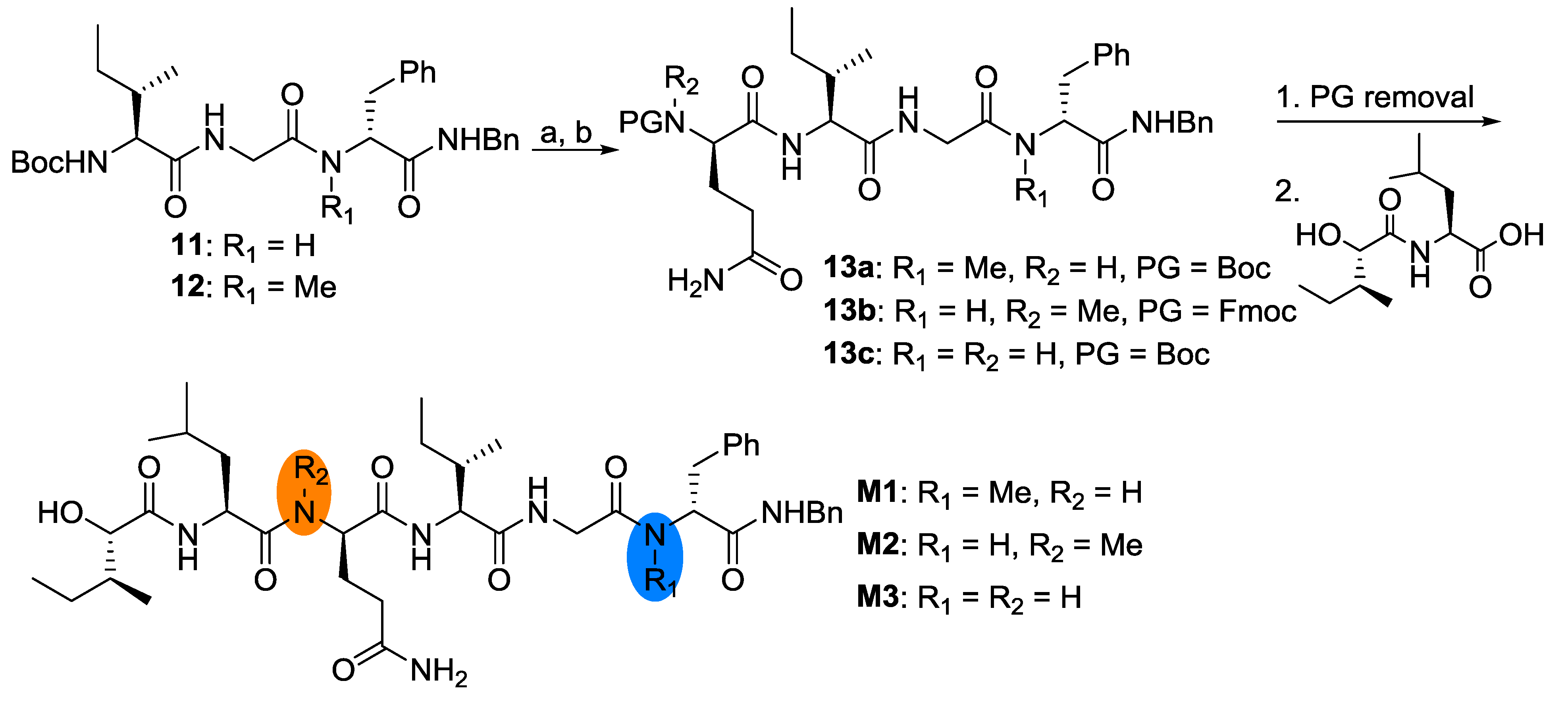
2.2. Biological Results and Discussion
| Compounds | KB b | A549 b | Compounds | KB b | A549 b |
|---|---|---|---|---|---|
| Etoposide c | 0.85 ± 0.08 | 0.99 ± 0.09 | C5 | 2.45 ± 0.41 | 5.24 ± 1.12 |
| T2 | 0.58 ± 0.07 | >50 | C6 | 8.52 ± 1.41 | 12.88 ± 1.32 |
| S1 | >50 | >50 | C7 | 3.80 ± 0.46 | 3.67 ± 0.66 |
| S2 | >50 | >50 | C8 | 3.64 ± 0.55 | >50 |
| S3 | >50 | >50 | C9 | >50 | >50 |
| S4 | >50 | >50 | N1 | >50 | >50 |
| C1 | 3.21 ± 0.6 | 4.17 ± 0.51 | N2 | >50 | >50 |
| C2 | 8.26 ± 1.22 | >50 | M1 | >50 | >50 |
| C3 | 2.08 ± 0.33 | 2.24 ± 0.44 | M2 | >50 | >50 |
| C4 | 1.29 ± 0.31 | 8.48 ± 1.39 | M3 | >50 | >50 |
3. Experimental Section
3.1. Chemistry
3.1.1. Materials and Methods
3.1.2. Synthesis of Boc-Ile-Gly-N-Me-d-Phe-Pro-OMe (3)
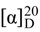 = 19.1 (c 0.05, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.31–7.08 (m, 5H), 6.82 (brs, 1H), 5.58 (t, J = 7.0 Hz, 1H), 5.03–4.99 (m, 1H), 4.42 (dd, J = 8.3, 5.5 Hz, 1H), 4.07–4.00 (m, 1H), 3.92–3.84 (m, 1H), 3.72 (s, 3H), 3.39–3.35 (m, 2H), 3.28 (dd, J = 13.7, 8.3 Hz, 1H), 2.99 (s, 3H), 2.84 (dd, J = 13.7, 7.1 Hz, 1H), 2.16–2.10 (m, 1H), 1.93–1.87 (m, 4H), 1.44–1.42 (m, 10H), 1.10–0.96 (m, 1H), 0.93–0.84 (m, 6H); ESI-MS m/z: 583.3 [M + Na]+; HRESIMS calcd. for C29H44N4O7Na [M + Na]+ 583.3108, found 583.3120.
= 19.1 (c 0.05, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.31–7.08 (m, 5H), 6.82 (brs, 1H), 5.58 (t, J = 7.0 Hz, 1H), 5.03–4.99 (m, 1H), 4.42 (dd, J = 8.3, 5.5 Hz, 1H), 4.07–4.00 (m, 1H), 3.92–3.84 (m, 1H), 3.72 (s, 3H), 3.39–3.35 (m, 2H), 3.28 (dd, J = 13.7, 8.3 Hz, 1H), 2.99 (s, 3H), 2.84 (dd, J = 13.7, 7.1 Hz, 1H), 2.16–2.10 (m, 1H), 1.93–1.87 (m, 4H), 1.44–1.42 (m, 10H), 1.10–0.96 (m, 1H), 0.93–0.84 (m, 6H); ESI-MS m/z: 583.3 [M + Na]+; HRESIMS calcd. for C29H44N4O7Na [M + Na]+ 583.3108, found 583.3120.3.1.3. Synthesis of N,N-Me2-d-Gln-Ile-Gly-N-Me-d-Phe-Pro-OMe (S1)
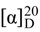 = 28.1 (c 0.14, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 9.30–9.10 (m, 1H), 8.79–8.71 (m, 1H), 8.00 (t, J = 7.8 Hz, 1H), 7.36–7.30 (m, 1H), 7.22–7.12 (m, 5H), 5.42 (t, J = 7.3 Hz, 1H), 4.51–4.49 (m, 1H), 4.35–4.31 (m, 2H), 4.10–3.99 (m, 1H), 3.82–3.79 (m, 1H), 3.70 (s, 3H), 3.26–3.24 (m, 1H), 3.23–3.19 (m, 1H), 3.00–2.95 (m, 4H), 2.85 (brs, 6H), 2.76–2.74 (m, 1H), 2.65–2.59 (m, 2H), 2.45–2.30 (m, 1H), 2.14–2.03 (m, 2H), 1.88–1.85 (m, 2H), 1.79–1.77 (m, 1H), 1.73–1.69 (m, 1H), 1.42–1.39 (m, 1H), 1.20–1.18 (m, 1H), 0.88–0.76 (m, 6H); 13C-NMR (CDCl3, 150 MHz) δ 172.6, 167.8, 136.9, 129.4, 128.5, 126.9, 59.5, 59.2, 59.1, 56.7, 52.4, 46.8, 41.2, 36.4, 35.1, 30.2, 29.8, 28.9, 25.2, 25.1, 24.9, 18.5, 15.6, 11.1; HRESIMS calcd. for C31H48N6O7Na [M + Na]+ 639.3482, found 639.3472.
= 28.1 (c 0.14, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 9.30–9.10 (m, 1H), 8.79–8.71 (m, 1H), 8.00 (t, J = 7.8 Hz, 1H), 7.36–7.30 (m, 1H), 7.22–7.12 (m, 5H), 5.42 (t, J = 7.3 Hz, 1H), 4.51–4.49 (m, 1H), 4.35–4.31 (m, 2H), 4.10–3.99 (m, 1H), 3.82–3.79 (m, 1H), 3.70 (s, 3H), 3.26–3.24 (m, 1H), 3.23–3.19 (m, 1H), 3.00–2.95 (m, 4H), 2.85 (brs, 6H), 2.76–2.74 (m, 1H), 2.65–2.59 (m, 2H), 2.45–2.30 (m, 1H), 2.14–2.03 (m, 2H), 1.88–1.85 (m, 2H), 1.79–1.77 (m, 1H), 1.73–1.69 (m, 1H), 1.42–1.39 (m, 1H), 1.20–1.18 (m, 1H), 0.88–0.76 (m, 6H); 13C-NMR (CDCl3, 150 MHz) δ 172.6, 167.8, 136.9, 129.4, 128.5, 126.9, 59.5, 59.2, 59.1, 56.7, 52.4, 46.8, 41.2, 36.4, 35.1, 30.2, 29.8, 28.9, 25.2, 25.1, 24.9, 18.5, 15.6, 11.1; HRESIMS calcd. for C31H48N6O7Na [M + Na]+ 639.3482, found 639.3472.3.1.4. Synthesis of N,N-Me2-Ile-Gly-N-Me-d-Phe-Pro-OMe (S2)
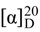 = 26.5 (c 0.13, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.32–7.19 (m, 5H), 6.80–6.78 (m, 1H), 5.50 (t, J = 7.8 Hz, 1H), 4.50–4.44 (m, 1H), 4.41 (m, 1H), 4.10 (d, J = 4.1 Hz, 1H), 3.90 (d, J = 3.2 Hz, 1H), 3.70 (s, 3H), 3.37 (t, J = 4.6 Hz, 2H), 3.28 (dd, J = 13.7, 8.2 Hz, 1H), 3.00 (s, 3H), 2.85 (dd, J = 13.7, 7.3 Hz, 1H), 2.26–2.00 (brs, 6H), 1.97–1.81 (m, 4H), 1.72–1.70 (m, 1H), 1.55–1.42 (m, 2H), 0.99–0.81 (m, 6H); ESI-MS m/z: 511.2 [M + Na]+; HRESIMS calcd. for C26H40N4O5Na [M + Na]+ 511.2896, found 511.2903.
= 26.5 (c 0.13, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.32–7.19 (m, 5H), 6.80–6.78 (m, 1H), 5.50 (t, J = 7.8 Hz, 1H), 4.50–4.44 (m, 1H), 4.41 (m, 1H), 4.10 (d, J = 4.1 Hz, 1H), 3.90 (d, J = 3.2 Hz, 1H), 3.70 (s, 3H), 3.37 (t, J = 4.6 Hz, 2H), 3.28 (dd, J = 13.7, 8.2 Hz, 1H), 3.00 (s, 3H), 2.85 (dd, J = 13.7, 7.3 Hz, 1H), 2.26–2.00 (brs, 6H), 1.97–1.81 (m, 4H), 1.72–1.70 (m, 1H), 1.55–1.42 (m, 2H), 0.99–0.81 (m, 6H); ESI-MS m/z: 511.2 [M + Na]+; HRESIMS calcd. for C26H40N4O5Na [M + Na]+ 511.2896, found 511.2903.3.1.5. Synthesis of HO-Hmp-Leu-N-Me-d-Gln-Ile-OBn (S3)
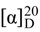 = −48.5 (c 0.29, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.35–7.28 (m, 5H), 7.20–7.18 (m, 1H), 7.12–7.09 (m, 1H), 6.94 (brs, 1H), 6.64 (brs, 1H), 5.20 (d, J = 12.1 Hz, 1H), 5.10 (d, J = 12.1 Hz, 1H), 4.76–4.73 (m, 1H), 4.50–4.47 (m, 1H), 3.95–3.91 (m, 1H), 3.11–2.82 (m, 4H), 2.30–2.12 (m, 3H), 2.05–1.83 (m, 3H), 1.71–1.65 (m, 3H), 1.44–1.11 (m, 4H), 0.98–0.73 (m, 18H); 13C-NMR (CDCl3, 150 MHz) δ 175.9, 175.2, 174.5, 171.9, 170.4, 135.4, 128.7, 128.6, 128.5, 76.3, 67.2, 56.8, 54.6, 48.1, 40.7, 40.1, 38.6, 37.4, 32.0, 30.7, 29.8, 25.0, 23.7, 23.4, 21.3, 15.7, 14.2, 11.9, 11.6; HRESIMS calcd. for C31H50N4O7Na [M + Na]+ 613.3577, found 613.3589.
= −48.5 (c 0.29, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.35–7.28 (m, 5H), 7.20–7.18 (m, 1H), 7.12–7.09 (m, 1H), 6.94 (brs, 1H), 6.64 (brs, 1H), 5.20 (d, J = 12.1 Hz, 1H), 5.10 (d, J = 12.1 Hz, 1H), 4.76–4.73 (m, 1H), 4.50–4.47 (m, 1H), 3.95–3.91 (m, 1H), 3.11–2.82 (m, 4H), 2.30–2.12 (m, 3H), 2.05–1.83 (m, 3H), 1.71–1.65 (m, 3H), 1.44–1.11 (m, 4H), 0.98–0.73 (m, 18H); 13C-NMR (CDCl3, 150 MHz) δ 175.9, 175.2, 174.5, 171.9, 170.4, 135.4, 128.7, 128.6, 128.5, 76.3, 67.2, 56.8, 54.6, 48.1, 40.7, 40.1, 38.6, 37.4, 32.0, 30.7, 29.8, 25.0, 23.7, 23.4, 21.3, 15.7, 14.2, 11.9, 11.6; HRESIMS calcd. for C31H50N4O7Na [M + Na]+ 613.3577, found 613.3589.3.1.6. Synthesis of HO-Hmp-Leu-N-Me-d-Gln-Ile-Gly-OBn (S4)
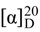 = −37 (c 0.07, MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.37–7.32 (m, 5H), 7.25 (d, J = 7.7 Hz, 1H), 7.09 (d, J = 8.8 Hz, 1H), 6.25 (brs, 1H), 5.98 (brs, 1H), 5.15 (d, J = 13.3 Hz, 2H), 5.15 (t, J = 7.7 Hz, 1H), 4.84–4.78 (m, 1H), 4.53 (brs, 1H), 4.32 (t, J = 7.7 Hz, 1H), 4.07 (m, 1H), 3.94–3.93 (m, 1H), 3.92 (d, J = 4.7 Hz, 1H), 3.08 (s, 3H), 2.34–2.27 (m, 1H), 2.26–2.20 (m, 1H), 2.19–2.14 (m, 1H), 2.01–1.95 (m, 1H), 1.87–1.82 (m, 1H), 1.80–1.77 (m, 1H), 1.68–1.60 (m, 2H), 1.58–1.52 (m, 1H), 1.51–1.45 (m, 1H), 1.44–1.37 (m, 1H), 1.24–1.19 (m, 1H), 1.12–1.06 (m, 1H), 0.97 (d, J = 6.6 Hz, 3H), 0.94 (d, J = 7.0 Hz, 3H), 0.90–0.83 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ: 174.7, 174.6, 172.0, 169.9, 169.5, 135.0, 128.6, 128.5, 128.4, 76.3, 67.2, 58.2, 56.4, 47.7, 41.3, 40.6, 38.4, 36.8, 32.3, 31.3, 25.0, 24.9, 23.7, 23.2, 23.1, 21.9, 15.6, 15.4, 11.8, 11.2; ESI-MS m/z: 670.4 [M + Na]+; HRESIMS calcd. for C33H53N5O8Na [M + Na]+ 670.3792, found 670.3808.
= −37 (c 0.07, MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.37–7.32 (m, 5H), 7.25 (d, J = 7.7 Hz, 1H), 7.09 (d, J = 8.8 Hz, 1H), 6.25 (brs, 1H), 5.98 (brs, 1H), 5.15 (d, J = 13.3 Hz, 2H), 5.15 (t, J = 7.7 Hz, 1H), 4.84–4.78 (m, 1H), 4.53 (brs, 1H), 4.32 (t, J = 7.7 Hz, 1H), 4.07 (m, 1H), 3.94–3.93 (m, 1H), 3.92 (d, J = 4.7 Hz, 1H), 3.08 (s, 3H), 2.34–2.27 (m, 1H), 2.26–2.20 (m, 1H), 2.19–2.14 (m, 1H), 2.01–1.95 (m, 1H), 1.87–1.82 (m, 1H), 1.80–1.77 (m, 1H), 1.68–1.60 (m, 2H), 1.58–1.52 (m, 1H), 1.51–1.45 (m, 1H), 1.44–1.37 (m, 1H), 1.24–1.19 (m, 1H), 1.12–1.06 (m, 1H), 0.97 (d, J = 6.6 Hz, 3H), 0.94 (d, J = 7.0 Hz, 3H), 0.90–0.83 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ: 174.7, 174.6, 172.0, 169.9, 169.5, 135.0, 128.6, 128.5, 128.4, 76.3, 67.2, 58.2, 56.4, 47.7, 41.3, 40.6, 38.4, 36.8, 32.3, 31.3, 25.0, 24.9, 23.7, 23.2, 23.1, 21.9, 15.6, 15.4, 11.8, 11.2; ESI-MS m/z: 670.4 [M + Na]+; HRESIMS calcd. for C33H53N5O8Na [M + Na]+ 670.3792, found 670.3808.3.1.7. General Procedure for the Preparation of Compounds 7a–d
3.1.8. General Procedure for the Preparation of Compounds 8a–d
3.1.9. General Procedure for the Preparation of Compounds C1–C9
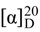 = −17.0 (c 0.01 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.83 (d, J = 7.7 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.51–7.06 (m, 11H), 5.34 (t, J = 7.7 Hz, 1H), 5.07 (dd, J = 10.4, 3.3 Hz, 1H), 4.80 (brs, 1H), 4.69–4.66 (m, 1H), 4.42 (dd, J = 8.1, 3.7 Hz, 1H), 4.12–4.07 (m, 1H), 3.93 (dd, J = 13.2, 4.0 Hz, 1H), 3.88 (d, J = 3.3 Hz, 1H), 3.43 (dd, J = 13.9, 8.0 Hz, 1H), 3.15 (s, 3H), 2.99 (s, 3H), 2.99–2.93 (m, 1H), 2.33–2.15 (m, 3H), 2.10–1.93 (m, 3H), 1.83–1.67 (m, 3H), 1.46–1.39 (m, 1H), 1.26–1.09 (m, 3H), 0.96–0.79 (m, 18H); 13C-NMR (CDCl3, 150 MHz) δ 177.5, 173.2, 171.3, 170.4, 169.6, 167.3, 138.5, 137.4, 129.1, 129.0, 128.8, 128.7, 126.9, 124.1, 120.2, 119.7, 63.8, 57.9, 54.1, 49.7, 41.4, 38.6, 38.5, 38.0, 36.7, 36.4, 34.4, 31.9, 31.1, 30.4, 30.3, 30.2, 30.0, 29.9, 29.7, 29.4, 28.8, 27.1, 25.1, 24.5, 24.1, 23.9, 23.8, 23.7, 23.5, 23.4, 22.7, 20.9, 20.5, 15.7, 15.6, 15.4, 15.3, 14.1, 12.2, 12.0, 11.9, 11.5; ESI-MS m/z: 794.4 [M + H]+, 816.4 [M + Na]+; HRESIMS calcd. for C42H63N7O8Na [M + Na]+ 816.4636, found 816.4668.
= −17.0 (c 0.01 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.83 (d, J = 7.7 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.51–7.06 (m, 11H), 5.34 (t, J = 7.7 Hz, 1H), 5.07 (dd, J = 10.4, 3.3 Hz, 1H), 4.80 (brs, 1H), 4.69–4.66 (m, 1H), 4.42 (dd, J = 8.1, 3.7 Hz, 1H), 4.12–4.07 (m, 1H), 3.93 (dd, J = 13.2, 4.0 Hz, 1H), 3.88 (d, J = 3.3 Hz, 1H), 3.43 (dd, J = 13.9, 8.0 Hz, 1H), 3.15 (s, 3H), 2.99 (s, 3H), 2.99–2.93 (m, 1H), 2.33–2.15 (m, 3H), 2.10–1.93 (m, 3H), 1.83–1.67 (m, 3H), 1.46–1.39 (m, 1H), 1.26–1.09 (m, 3H), 0.96–0.79 (m, 18H); 13C-NMR (CDCl3, 150 MHz) δ 177.5, 173.2, 171.3, 170.4, 169.6, 167.3, 138.5, 137.4, 129.1, 129.0, 128.8, 128.7, 126.9, 124.1, 120.2, 119.7, 63.8, 57.9, 54.1, 49.7, 41.4, 38.6, 38.5, 38.0, 36.7, 36.4, 34.4, 31.9, 31.1, 30.4, 30.3, 30.2, 30.0, 29.9, 29.7, 29.4, 28.8, 27.1, 25.1, 24.5, 24.1, 23.9, 23.8, 23.7, 23.5, 23.4, 22.7, 20.9, 20.5, 15.7, 15.6, 15.4, 15.3, 14.1, 12.2, 12.0, 11.9, 11.5; ESI-MS m/z: 794.4 [M + H]+, 816.4 [M + Na]+; HRESIMS calcd. for C42H63N7O8Na [M + Na]+ 816.4636, found 816.4668.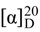 = −27.5 (c 0.02 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.30–7.06 (m, 13H), 6.15 (brs, 1H), 5.89 (brs, 1H), 5.28–5.22 (m, 1H), 5.05 (t, J = 9.1 Hz, 1H), 4.95–4.90 (m, 1H), 4.82 (d, J = 4.8 Hz, 1H), 4.31–4.29 (m, 1H), 4.04–4.00 (m, 1H), 3.91 (d, J = 3.7 Hz, 1H), 3.78–3.73 (m, 1H), 3.37 (dd, J = 17.4, 6.9 Hz, 1H), 3.08–2.92 (m, 4H), 2.82 (s, 3H), 2.39 (brs, 1H), 2.33–2.28 (m, 1H), 2.23–2.14 (m, 1H), 1.99–1.97 (m, 1H), 1.84–1.82 (m, 2H), 1.64–1.57 (m, 3H), 1.45–1.41 (m, 2H), 1.24–1.17 (m, 1H), 1.13–1.04 (m, 1H), 0.99–0.93 (m, 9H), 0.89–0.84 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 174.4, 174.3, 174.0, 171.4, 170.3, 169.7, 168.2, 136.5, 128.7, 128.6, 127.0, 76.5, 58.8, 57.8, 47.2, 41.3, 41.1, 40.6, 38.5, 38.4, 38.3, 37.3, 34.7, 32.2, 31.6, 31.1, 29.7, 25.0, 24.8, 23.7, 23.1, 23.0, 22.9, 22.1, 21.7, 15.6, 14.2, 11.8, 11.3; ESI-MS m/z: 794.4 [M + H]+; HRESIMS calcd. for C42H63N7O8Na [M + Na]+ 816.4636, found 816.4670.
= −27.5 (c 0.02 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.30–7.06 (m, 13H), 6.15 (brs, 1H), 5.89 (brs, 1H), 5.28–5.22 (m, 1H), 5.05 (t, J = 9.1 Hz, 1H), 4.95–4.90 (m, 1H), 4.82 (d, J = 4.8 Hz, 1H), 4.31–4.29 (m, 1H), 4.04–4.00 (m, 1H), 3.91 (d, J = 3.7 Hz, 1H), 3.78–3.73 (m, 1H), 3.37 (dd, J = 17.4, 6.9 Hz, 1H), 3.08–2.92 (m, 4H), 2.82 (s, 3H), 2.39 (brs, 1H), 2.33–2.28 (m, 1H), 2.23–2.14 (m, 1H), 1.99–1.97 (m, 1H), 1.84–1.82 (m, 2H), 1.64–1.57 (m, 3H), 1.45–1.41 (m, 2H), 1.24–1.17 (m, 1H), 1.13–1.04 (m, 1H), 0.99–0.93 (m, 9H), 0.89–0.84 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 174.4, 174.3, 174.0, 171.4, 170.3, 169.7, 168.2, 136.5, 128.7, 128.6, 127.0, 76.5, 58.8, 57.8, 47.2, 41.3, 41.1, 40.6, 38.5, 38.4, 38.3, 37.3, 34.7, 32.2, 31.6, 31.1, 29.7, 25.0, 24.8, 23.7, 23.1, 23.0, 22.9, 22.1, 21.7, 15.6, 14.2, 11.8, 11.3; ESI-MS m/z: 794.4 [M + H]+; HRESIMS calcd. for C42H63N7O8Na [M + Na]+ 816.4636, found 816.4670.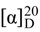 = −19.7 (c 0.01 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.99 (brs, 1H), 7.73 (brs, 1H), 7.54 (d, J = 8.5 Hz, 1H),7.27–7.16 (m, 5H), 7.11 (d, J = 7.0 Hz, 2H), 5.31 (t, J = 7.7 Hz, 1H), 5.13 (dd, J = 10.3, 4.4 Hz, 1H), 4.76–4.71 (m, 1H), 4.45 (dd, J = 14.6, 6.6 Hz, 1H), 4.31 (dd, J = 7.7, 5.5 Hz, 1H), 4.25 (dd, J = 9.0, 5.4 Hz, 1H), 3.94–3.80 (m, 3H), 3.47 (s, 3H), 3.38 (dd, J = 14.3, 8.0 Hz, 1H), 2.96 (s, 3H), 2.96–2.86 (m, 1H), 2.33–1.94 (m, 4H), 1.88–1.92 (m, 1H), 1.77–1.70 (m, 2H), 1.45–1.29 (m, 3H), 1.21–1.15 (m, 2H), 1.08–1.01 (m, 1H), 0.98–0.80 (m, 18H); 13C-NMR (CDCl3, 150 MHz) δ 176.8, 176.0, 175.7, 174.7, 171.3, 170.7, 170.3, 169.2, 138.4, 138.2, 137.5, 136.8, 129.2, 129.0, 128.9, 128.6, 128.5, 127.7, 127.5, 127.3, 127.2, 126.8, 76.6, 76.5, 60.4, 58.1, 58.0, 50.8, 49.0, 48.2, 48.1, 43.6, 43.3, 41.3, 39.5, 38.5, 38.4, 36.6, 34.5, 34.4, 31.3, 30.5, 25.0, 24.9, 24.8, 24.6, 24.0, 23.9, 23.5, 23.4, 21.6, 21.1, 20.8, 15.7, 15.5, 15.4, 14.2, 11.9, 11.8, 11.4; ESI-MS m/z: 808.4 [M + H]+; HRESIMS calcd. for C43H65N7O8Na [M + Na]+ 830.4792, found 830.4814.
= −19.7 (c 0.01 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.99 (brs, 1H), 7.73 (brs, 1H), 7.54 (d, J = 8.5 Hz, 1H),7.27–7.16 (m, 5H), 7.11 (d, J = 7.0 Hz, 2H), 5.31 (t, J = 7.7 Hz, 1H), 5.13 (dd, J = 10.3, 4.4 Hz, 1H), 4.76–4.71 (m, 1H), 4.45 (dd, J = 14.6, 6.6 Hz, 1H), 4.31 (dd, J = 7.7, 5.5 Hz, 1H), 4.25 (dd, J = 9.0, 5.4 Hz, 1H), 3.94–3.80 (m, 3H), 3.47 (s, 3H), 3.38 (dd, J = 14.3, 8.0 Hz, 1H), 2.96 (s, 3H), 2.96–2.86 (m, 1H), 2.33–1.94 (m, 4H), 1.88–1.92 (m, 1H), 1.77–1.70 (m, 2H), 1.45–1.29 (m, 3H), 1.21–1.15 (m, 2H), 1.08–1.01 (m, 1H), 0.98–0.80 (m, 18H); 13C-NMR (CDCl3, 150 MHz) δ 176.8, 176.0, 175.7, 174.7, 171.3, 170.7, 170.3, 169.2, 138.4, 138.2, 137.5, 136.8, 129.2, 129.0, 128.9, 128.6, 128.5, 127.7, 127.5, 127.3, 127.2, 126.8, 76.6, 76.5, 60.4, 58.1, 58.0, 50.8, 49.0, 48.2, 48.1, 43.6, 43.3, 41.3, 39.5, 38.5, 38.4, 36.6, 34.5, 34.4, 31.3, 30.5, 25.0, 24.9, 24.8, 24.6, 24.0, 23.9, 23.5, 23.4, 21.6, 21.1, 20.8, 15.7, 15.5, 15.4, 14.2, 11.9, 11.8, 11.4; ESI-MS m/z: 808.4 [M + H]+; HRESIMS calcd. for C43H65N7O8Na [M + Na]+ 830.4792, found 830.4814.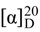 = −7.3 (c 0.05 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.27–7.06 (m, 12H), 5.95 (brs, 1H), 5.69 (brs, 1H), 5.31 (t, J = 8.0 Hz, 1H), 4.96 (t, J = 7.3 Hz, 1H), 4.94–4.91 (m, 1H), 4.43 (dd, J = 14.9, 6.0 Hz, 1H), 4.37 (dd, J = 13.7, 6.1 Hz, 1H), 4.26 (dd, J = 14.8, 5.3 Hz, 1H), 4.03 (dd, J = 17.6, 4.7 Hz, 1H), 3.89 (d, J = 4.2 Hz, 1H), 3.78 (d, J = 17.2 Hz, 1H), 3.33 (dd, J = 14.1, 8.3 Hz, 1H), 3.00 (s, 3H), 2.99–2.92 (m, 1H), 2.96 (s, 3H), 2.25–2.19 (m, 1H), 2.18–2.07 (m, 2H), 1.97–1.91 (m, 1H), 1.86–1.84 (m, 1H), 1.80–1.76 (m, 1H), 1.64–1.58 (m, 1H), 1.58–1.55 (m, 1H), 1.47–1.39 (m, 3H), 1.21–1.14 (m, 1H), 1.12–1.04 (m, 1H), 0.95–0.93 (m, 6H), 0.92 (d, J = 7.0 Hz, 3H), 0.89–0.84 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 175.1, 174.7, 174.5, 174.3, 174.1, 171.6, 171.5, 169.9, 169.4, 168.8, 168.3, 168.2, 138.3, 138.0, 137.1, 136.7, 129.1, 128.9, 128.5, 127.6, 127.5, 127.3, 127.2, 127.1, 126.8, 77.1, 62.0, 58.0, 57.9, 57.7, 56.7, 53.4, 47.4, 47.2, 43.5, 43.3, 41.3, 41.2, 40.4, 38.5, 38.4, 36.9, 36.1, 35.1, 34.8, 32.0, 31.9, 31.3, 30.1, 30.4, 29.7, 29.4, 24.9, 24.7, 24.2, 23.8, 23.2, 23.1, 22.7, 22.0, 21.7, 15.7, 15.5, 15.2, 14.1, 11.8, 11.7, 11.3, 11.0; ESI-MS m/z: 808.5 [M + H]+, 830.5 [M + Na]+; HRESIMS calcd. for C43H65N7O8Na [M + Na]+ 830.4792, found 830.4828.
= −7.3 (c 0.05 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.27–7.06 (m, 12H), 5.95 (brs, 1H), 5.69 (brs, 1H), 5.31 (t, J = 8.0 Hz, 1H), 4.96 (t, J = 7.3 Hz, 1H), 4.94–4.91 (m, 1H), 4.43 (dd, J = 14.9, 6.0 Hz, 1H), 4.37 (dd, J = 13.7, 6.1 Hz, 1H), 4.26 (dd, J = 14.8, 5.3 Hz, 1H), 4.03 (dd, J = 17.6, 4.7 Hz, 1H), 3.89 (d, J = 4.2 Hz, 1H), 3.78 (d, J = 17.2 Hz, 1H), 3.33 (dd, J = 14.1, 8.3 Hz, 1H), 3.00 (s, 3H), 2.99–2.92 (m, 1H), 2.96 (s, 3H), 2.25–2.19 (m, 1H), 2.18–2.07 (m, 2H), 1.97–1.91 (m, 1H), 1.86–1.84 (m, 1H), 1.80–1.76 (m, 1H), 1.64–1.58 (m, 1H), 1.58–1.55 (m, 1H), 1.47–1.39 (m, 3H), 1.21–1.14 (m, 1H), 1.12–1.04 (m, 1H), 0.95–0.93 (m, 6H), 0.92 (d, J = 7.0 Hz, 3H), 0.89–0.84 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 175.1, 174.7, 174.5, 174.3, 174.1, 171.6, 171.5, 169.9, 169.4, 168.8, 168.3, 168.2, 138.3, 138.0, 137.1, 136.7, 129.1, 128.9, 128.5, 127.6, 127.5, 127.3, 127.2, 127.1, 126.8, 77.1, 62.0, 58.0, 57.9, 57.7, 56.7, 53.4, 47.4, 47.2, 43.5, 43.3, 41.3, 41.2, 40.4, 38.5, 38.4, 36.9, 36.1, 35.1, 34.8, 32.0, 31.9, 31.3, 30.1, 30.4, 29.7, 29.4, 24.9, 24.7, 24.2, 23.8, 23.2, 23.1, 22.7, 22.0, 21.7, 15.7, 15.5, 15.2, 14.1, 11.8, 11.7, 11.3, 11.0; ESI-MS m/z: 808.5 [M + H]+, 830.5 [M + Na]+; HRESIMS calcd. for C43H65N7O8Na [M + Na]+ 830.4792, found 830.4828.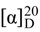 = −9.9 (c 0.05 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.36 (d, J = 8.0 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 7.28–6.99 (m, 9H), 6.96 (t, J = 4.4 Hz, 1H), 6.73 (brs, 1H), 6.18 (brs, 1H), 5.78 (t, J = 7.5 Hz, 1H), 5.21 (t, J = 5.5 Hz, 1H), 5.13 (t, J = 7.7 Hz, 1H), 4.85–4.81 (m, 1H), 4.53 (d, J = 15.8 Hz, 1H), 4.43 (d, J = 15.4 Hz, 1H), 4.29 (t, J = 7.3 Hz, 1H), 4.17 (dd, J = 17.4, 5.0 Hz, 1H), 3.98 (brs, 1H), 3.84 (dd, J = 17.6, 3.7 Hz, 1H), 3.64 (s, 3H), 3.27 (dd, J = 13.7, 7.5 Hz, 1H), 3.21–3.15 (m, 1H), 3.14 (s, 3H), 3.11–3.04 (m, 1H), 3.01 (s, 3H), 2.93 (dd, J = 13.7, 7.5 Hz, 1H), 2.36–2.32 (m, 1H), 2.23–2.18 (m, 2H), 2.07–2.02 (m, 1H), 1.96–1.93 (m, 1H), 1.86–1.82 (m, 1H), 1.73–1.68 (m, 1H), 1.66–1.61 (m, 1H), 1.47–1.38 (m, 3H), 1.25–1.20 (m, 1H), 1.16–1.09 (m, 1H), 0.99–0.94 (m, 9H), 0.91–0.85 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 175.0, 174.1, 171.1, 170.5, 169.2, 168.1, 136.7, 132.2, 132.1, 129.3, 128.5, 128.1, 127.5, 127.1, 126.9, 126.1, 76.2, 58.0, 55.4, 55.2, 52.6, 52.4, 47.9, 45.1, 41.2, 40.5, 38.5, 36.6, 35.2, 31.5, 30.7, 30.5, 29.7, 24.9, 24.7, 23.9, 23.6, 23.4, 21.3, 15.7, 15.6, 11.9, 11.3; ESI-MS m/z: 892.4 [M + H]+, 914.4 [M + Na]+; HRESIMS calcd. for C47H69N7O10Na [M + Na]+ 914.5004, found 914.5018.
= −9.9 (c 0.05 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.36 (d, J = 8.0 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 7.28–6.99 (m, 9H), 6.96 (t, J = 4.4 Hz, 1H), 6.73 (brs, 1H), 6.18 (brs, 1H), 5.78 (t, J = 7.5 Hz, 1H), 5.21 (t, J = 5.5 Hz, 1H), 5.13 (t, J = 7.7 Hz, 1H), 4.85–4.81 (m, 1H), 4.53 (d, J = 15.8 Hz, 1H), 4.43 (d, J = 15.4 Hz, 1H), 4.29 (t, J = 7.3 Hz, 1H), 4.17 (dd, J = 17.4, 5.0 Hz, 1H), 3.98 (brs, 1H), 3.84 (dd, J = 17.6, 3.7 Hz, 1H), 3.64 (s, 3H), 3.27 (dd, J = 13.7, 7.5 Hz, 1H), 3.21–3.15 (m, 1H), 3.14 (s, 3H), 3.11–3.04 (m, 1H), 3.01 (s, 3H), 2.93 (dd, J = 13.7, 7.5 Hz, 1H), 2.36–2.32 (m, 1H), 2.23–2.18 (m, 2H), 2.07–2.02 (m, 1H), 1.96–1.93 (m, 1H), 1.86–1.82 (m, 1H), 1.73–1.68 (m, 1H), 1.66–1.61 (m, 1H), 1.47–1.38 (m, 3H), 1.25–1.20 (m, 1H), 1.16–1.09 (m, 1H), 0.99–0.94 (m, 9H), 0.91–0.85 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 175.0, 174.1, 171.1, 170.5, 169.2, 168.1, 136.7, 132.2, 132.1, 129.3, 128.5, 128.1, 127.5, 127.1, 126.9, 126.1, 76.2, 58.0, 55.4, 55.2, 52.6, 52.4, 47.9, 45.1, 41.2, 40.5, 38.5, 36.6, 35.2, 31.5, 30.7, 30.5, 29.7, 24.9, 24.7, 23.9, 23.6, 23.4, 21.3, 15.7, 15.6, 11.9, 11.3; ESI-MS m/z: 892.4 [M + H]+, 914.4 [M + Na]+; HRESIMS calcd. for C47H69N7O10Na [M + Na]+ 914.5004, found 914.5018.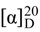 = −29.1 (c 0.01 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.29–6.97 (m, 12H), 5.81 (t, J = 7.7 Hz, 2H), 5.57 (brs, 1H), 5.20 (dd, J = 6.2, 4.8 Hz, 1H), 5.07 (t, J = 7.5 Hz, 1H), 4.98 (dd, J = 15.4, 6.9 Hz, 1H), 4.53 (d, J = 15.4 Hz, 1H), 4.45 (d, J = 15.4 Hz, 1H), 4.33 (dd, J = 9.2, 6.6 Hz, 1H), 4.10 (dd, J = 17.2, 4.7 Hz, 1H), 3.93 (dd, J = 4.2 Hz, 1H), 3.80 (dd, J = 17.4, 3.5 Hz, 1H), 3.64 (s, 3H), 3.28 (dd, J = 13.9, 7.7 Hz, 1H), 3.17 (dd, J = 15.8, 6.4 Hz, 1H), 3.06 (dd, J = 15.8, 6.2 Hz, 1H), 3.02 (s, 3H), 3.00 (s, 3H), 2.92 (dd, J = 13.7, 7.5 Hz, 1H), 2.33 (td, J = 13.9, 7.4 Hz, 1H), 2.28–2.22 (m, 1H), 2.15–2.12 (m, 1H), 2.05–1.97 (m, 1H), 1.95 (brs, 1H), 1.88–1.80 (m, 1H), 1.65–1.60 (m, 2H), 1.51–1.39 (m, 3H), 1.23–1.19 (m, 1H), 1.17–1.10 (m, 1H), 0.97–0.95 (m, 9H), 0.93–0.86 (m, 9H); ESI-MS m/z: 892.4 [M + H]+; HRESIMS calcd. For C47H69N7O10Na [M + Na]+ 914.5004, found 914.5032.
= −29.1 (c 0.01 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.29–6.97 (m, 12H), 5.81 (t, J = 7.7 Hz, 2H), 5.57 (brs, 1H), 5.20 (dd, J = 6.2, 4.8 Hz, 1H), 5.07 (t, J = 7.5 Hz, 1H), 4.98 (dd, J = 15.4, 6.9 Hz, 1H), 4.53 (d, J = 15.4 Hz, 1H), 4.45 (d, J = 15.4 Hz, 1H), 4.33 (dd, J = 9.2, 6.6 Hz, 1H), 4.10 (dd, J = 17.2, 4.7 Hz, 1H), 3.93 (dd, J = 4.2 Hz, 1H), 3.80 (dd, J = 17.4, 3.5 Hz, 1H), 3.64 (s, 3H), 3.28 (dd, J = 13.9, 7.7 Hz, 1H), 3.17 (dd, J = 15.8, 6.4 Hz, 1H), 3.06 (dd, J = 15.8, 6.2 Hz, 1H), 3.02 (s, 3H), 3.00 (s, 3H), 2.92 (dd, J = 13.7, 7.5 Hz, 1H), 2.33 (td, J = 13.9, 7.4 Hz, 1H), 2.28–2.22 (m, 1H), 2.15–2.12 (m, 1H), 2.05–1.97 (m, 1H), 1.95 (brs, 1H), 1.88–1.80 (m, 1H), 1.65–1.60 (m, 2H), 1.51–1.39 (m, 3H), 1.23–1.19 (m, 1H), 1.17–1.10 (m, 1H), 0.97–0.95 (m, 9H), 0.93–0.86 (m, 9H); ESI-MS m/z: 892.4 [M + H]+; HRESIMS calcd. For C47H69N7O10Na [M + Na]+ 914.5004, found 914.5032.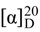 = −3.3 (c 0.02 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.57 (d, J = 8.8 Hz, 1H), 7.40 (d, J = 8.8 Hz, 1H), 7.24–7.05 (m, 9H), 7.00 (t, J = 4.2 Hz, 1H), 6.80 (brs, 1H), 6.53 (brs, 1H), 5.76 (t, J = 7.5 Hz, 1H), 5.17 (dd, J = 9.8, 5.9 Hz, 1H), 4.81–4.76 (m, 1H), 4.72 (d, J = 16.5 Hz, 1H), 4.58 (d, J = 16.5 Hz, 1H), 4.26 (t, J = 7.3 Hz, 1H), 4.06 (dd, J = 17.6, 4.7 Hz, 1H), 4.00 (dt, J = 12.8, 5.9 Hz, 1H), 3.96 (d, J = 2.9 Hz, 1H), 3.88 (dd, J = 17.6, 3.7 Hz, 1H), 3.72 (t, J = 3.8 Hz, 1H), 3.64 (dd, J = 7.3, 4.7 Hz, 1H), 3.57 (dd, J = 8.4, 4.7 Hz, 1H), 3.51 (dd, J = 7.7, 4.7 Hz, 1H), 3.30 (dd, J = 13.6, 8.0 Hz, 1H), 3.15 (s, 3H), 2.94–2.88 (m, 1H), 2.86–2.82 (m, 1H), 2.79 (s, 3H), 2.79–2.76 (m, 1H), 2.26–2.19 (m, 1H), 2.18–2.13 (m, 2H), 2.08–2.01 (m, 1H), 1.93–1.88 (m, 1H), 1.84–1.81 (m, 1H), 1.75–1.70 (m, 1H), 1.69–1.64 (m, 1H), 1.45–1.38 (m, 3H), 1.25–1.19 (m, 1H), 1.12–1.07 (m, 1H), 0.98–0.93 (m, 9H), 0.83–0.91 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 175.5, 175.4, 174.2, 171.2, 171.0, 170.6, 168.6, 168.2, 167.9, 136.7, 134.2, 134.0, 132.8, 132.7, 129.2, 128.6, 128.5, 128.4, 127.1, 126.9, 126.6, 126.5, 126.4, 76.4, 57.9, 54.8, 54.7, 54.0, 48.0, 47.1, 44.7, 43.1, 41.1, 41.3, 41.2, 40.1, 40.0, 38.5, 36.6, 36.5, 35.7, .35.4, 31.4, 30.6, 30.0, 29.7, 29.5, 29.3, 24.9, 24.7, 24.0, 23.7, 23.4, 21.2, 15.6, 15.5, 11.8, 11.3; ESI-MS m/z: 834.3 [M + H]+, 856.3 [M + Na]+; HRESIMS calcd. For C45H67N7O8Na [M + Na]+ 856.4949, found 856.4991.
= −3.3 (c 0.02 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.57 (d, J = 8.8 Hz, 1H), 7.40 (d, J = 8.8 Hz, 1H), 7.24–7.05 (m, 9H), 7.00 (t, J = 4.2 Hz, 1H), 6.80 (brs, 1H), 6.53 (brs, 1H), 5.76 (t, J = 7.5 Hz, 1H), 5.17 (dd, J = 9.8, 5.9 Hz, 1H), 4.81–4.76 (m, 1H), 4.72 (d, J = 16.5 Hz, 1H), 4.58 (d, J = 16.5 Hz, 1H), 4.26 (t, J = 7.3 Hz, 1H), 4.06 (dd, J = 17.6, 4.7 Hz, 1H), 4.00 (dt, J = 12.8, 5.9 Hz, 1H), 3.96 (d, J = 2.9 Hz, 1H), 3.88 (dd, J = 17.6, 3.7 Hz, 1H), 3.72 (t, J = 3.8 Hz, 1H), 3.64 (dd, J = 7.3, 4.7 Hz, 1H), 3.57 (dd, J = 8.4, 4.7 Hz, 1H), 3.51 (dd, J = 7.7, 4.7 Hz, 1H), 3.30 (dd, J = 13.6, 8.0 Hz, 1H), 3.15 (s, 3H), 2.94–2.88 (m, 1H), 2.86–2.82 (m, 1H), 2.79 (s, 3H), 2.79–2.76 (m, 1H), 2.26–2.19 (m, 1H), 2.18–2.13 (m, 2H), 2.08–2.01 (m, 1H), 1.93–1.88 (m, 1H), 1.84–1.81 (m, 1H), 1.75–1.70 (m, 1H), 1.69–1.64 (m, 1H), 1.45–1.38 (m, 3H), 1.25–1.19 (m, 1H), 1.12–1.07 (m, 1H), 0.98–0.93 (m, 9H), 0.83–0.91 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 175.5, 175.4, 174.2, 171.2, 171.0, 170.6, 168.6, 168.2, 167.9, 136.7, 134.2, 134.0, 132.8, 132.7, 129.2, 128.6, 128.5, 128.4, 127.1, 126.9, 126.6, 126.5, 126.4, 76.4, 57.9, 54.8, 54.7, 54.0, 48.0, 47.1, 44.7, 43.1, 41.1, 41.3, 41.2, 40.1, 40.0, 38.5, 36.6, 36.5, 35.7, .35.4, 31.4, 30.6, 30.0, 29.7, 29.5, 29.3, 24.9, 24.7, 24.0, 23.7, 23.4, 21.2, 15.6, 15.5, 11.8, 11.3; ESI-MS m/z: 834.3 [M + H]+, 856.3 [M + Na]+; HRESIMS calcd. For C45H67N7O8Na [M + Na]+ 856.4949, found 856.4991.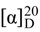 = −15.1 (c 0.04 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.24–7.04 (m, 11H), 5.95 (brs, 1H), 5.76 (dd, J = 15.4, 7.7 Hz, 1H), 5.70 (brs, 1H), 5.07 (t, J = 7.5 Hz, 1H), 4.95 (dd, J = 14.5, 7.1 Hz, 1H), 4.71 (d, J = 17.2 Hz, 1H), 4.62 (d, J = 16.8 Hz, 1H), 4.58 (s, 1H), 4.30 (t, J = 7.0 Hz, 1H), 4.04 (dd, J = 17.4, 4.9 Hz, 1H), 4.01–3.96 (m, 1H), 3.92 (dd, J = 4.0 Hz, 1H), 3.80 (dd, J = 17.6, 3.7 Hz, 1H), 3.59–3.54 (m, 1H), 3.29 (dd, J = 13.7, 8.6 Hz, 1H), 3.03 (s, 3H), 3.00 (s, 3H), 2.97–2.89 (m, 1H), 2.87–2.81 (m, 1H), 2.79–2.74 (m, 1H), 2.32 (td, J = 13.9, 7.0 Hz, 1H), 2.26-2.20 (m, 1H), 2.19–2.14 (m, 1H), 1.99 (td, J = 14.3, 7.7 Hz, 1H), 1.84–1.80 (m, 2H), 1.63–1.57 (m, 3H), 1.48–1.41 (m, 2H), 1.26–1.19 (m, 1H), 1.17–1.09 (m, 1H), 0.98–0.94 (m, 9H), 0.91–0.85 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 174.5, 174.3, 174.1, 171.5, 171.3, 169.7, 168.7, 168.3, 167.7, 167.4, 136.6, 134.3, 134.0, 132.8, 132.7, 129.1, 128.9, 128.6, 128.5, 128.4, 127.1, 126.9, 126.6, 126.5, 126.3, 125.6, 76.5, 57.8, 56.3, 54.6, 53.9, 47.9, 44.7, 43.1, 41.3, 41.2, 41.1, 41.0, 38.3, 37.1, 35.8, 35.5, 32.2, 31.0, 29.9, 29.7, 29.5, 29.3, 28.1, 24.9, 24.8, 23.8, 23.1, 23.0, 22.1, 15.6, 11.8, 11.3; ESI-MS m/z: 834.3 [M + H]+, 856.3 [M + Na]+; HRESIMS calcd. for C45H67N7O8Na [M + Na]+ 856.4949, found 856.4977.
= −15.1 (c 0.04 MeOH); 1H-NMR (CDCl3, 600 MHz) δ 7.24–7.04 (m, 11H), 5.95 (brs, 1H), 5.76 (dd, J = 15.4, 7.7 Hz, 1H), 5.70 (brs, 1H), 5.07 (t, J = 7.5 Hz, 1H), 4.95 (dd, J = 14.5, 7.1 Hz, 1H), 4.71 (d, J = 17.2 Hz, 1H), 4.62 (d, J = 16.8 Hz, 1H), 4.58 (s, 1H), 4.30 (t, J = 7.0 Hz, 1H), 4.04 (dd, J = 17.4, 4.9 Hz, 1H), 4.01–3.96 (m, 1H), 3.92 (dd, J = 4.0 Hz, 1H), 3.80 (dd, J = 17.6, 3.7 Hz, 1H), 3.59–3.54 (m, 1H), 3.29 (dd, J = 13.7, 8.6 Hz, 1H), 3.03 (s, 3H), 3.00 (s, 3H), 2.97–2.89 (m, 1H), 2.87–2.81 (m, 1H), 2.79–2.74 (m, 1H), 2.32 (td, J = 13.9, 7.0 Hz, 1H), 2.26-2.20 (m, 1H), 2.19–2.14 (m, 1H), 1.99 (td, J = 14.3, 7.7 Hz, 1H), 1.84–1.80 (m, 2H), 1.63–1.57 (m, 3H), 1.48–1.41 (m, 2H), 1.26–1.19 (m, 1H), 1.17–1.09 (m, 1H), 0.98–0.94 (m, 9H), 0.91–0.85 (m, 9H); 13C-NMR (CDCl3, 150 MHz) δ 174.5, 174.3, 174.1, 171.5, 171.3, 169.7, 168.7, 168.3, 167.7, 167.4, 136.6, 134.3, 134.0, 132.8, 132.7, 129.1, 128.9, 128.6, 128.5, 128.4, 127.1, 126.9, 126.6, 126.5, 126.3, 125.6, 76.5, 57.8, 56.3, 54.6, 53.9, 47.9, 44.7, 43.1, 41.3, 41.2, 41.1, 41.0, 38.3, 37.1, 35.8, 35.5, 32.2, 31.0, 29.9, 29.7, 29.5, 29.3, 28.1, 24.9, 24.8, 23.8, 23.1, 23.0, 22.1, 15.6, 11.8, 11.3; ESI-MS m/z: 834.3 [M + H]+, 856.3 [M + Na]+; HRESIMS calcd. for C45H67N7O8Na [M + Na]+ 856.4949, found 856.4977.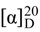 = −20.2 (c 0.1 MeOH); 1H-NMR (dimethyl sulfoxide-d6 (DMSO-d6), 600 MHz) δ 8.69 (t, J = 5.9 Hz, 1H), 7.91 (br, 1H), 7.67 (br, 1H), 7.50 (br, 1H), 7.30–7.10 (m, 10H), 5.20–5.18 (m, 1H), 4.98–4.96 (m, 1H), 4.80–4.77 (m, 1H), 4.30–4.27 (m, 2H), 4.20–4.16 (m, 1H), 4.00–3.96 (m, 1H), 3.70–3.67 (m, 2H), 3.25–3.18 (m, 1H), 2.92–2.88 (m, 1H), 2.90 (s, 3H), 2.87 (s, 3H), 1.70–1.66 (m, 2H), 1.57–1.55 (m, 1H), 1.45–1.42 (m, 1H), 1.36–1.31 (m, 2H), 1.27–1.23 (m, 1H), 1.20–1.18 (m, 3H), 1.14–0.97 (m, 2H), 0.90–0.74 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 173.7, 173.6, 171.9, 171.1, 170.8, 169.3, 138.1, 136.8, 129.0, 128.7, 128.6, 127.6, 127.4, 126.9, 76.4, 58.6, 58.1, 57.6, 47.5, 43.4, 41.6, 41.5, 38.9, 37.2, 37.0, 34.8, 29.8, 24.9, 24.7, 23.6, 23.5, 22.0, 21.5, 15.7, 15.6, 11.9, 11.5; HRESIMS calcd. for C41H62N6O7Na [M + Na]+ 773.4578, found 773.4577.
= −20.2 (c 0.1 MeOH); 1H-NMR (dimethyl sulfoxide-d6 (DMSO-d6), 600 MHz) δ 8.69 (t, J = 5.9 Hz, 1H), 7.91 (br, 1H), 7.67 (br, 1H), 7.50 (br, 1H), 7.30–7.10 (m, 10H), 5.20–5.18 (m, 1H), 4.98–4.96 (m, 1H), 4.80–4.77 (m, 1H), 4.30–4.27 (m, 2H), 4.20–4.16 (m, 1H), 4.00–3.96 (m, 1H), 3.70–3.67 (m, 2H), 3.25–3.18 (m, 1H), 2.92–2.88 (m, 1H), 2.90 (s, 3H), 2.87 (s, 3H), 1.70–1.66 (m, 2H), 1.57–1.55 (m, 1H), 1.45–1.42 (m, 1H), 1.36–1.31 (m, 2H), 1.27–1.23 (m, 1H), 1.20–1.18 (m, 3H), 1.14–0.97 (m, 2H), 0.90–0.74 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 173.7, 173.6, 171.9, 171.1, 170.8, 169.3, 138.1, 136.8, 129.0, 128.7, 128.6, 127.6, 127.4, 126.9, 76.4, 58.6, 58.1, 57.6, 47.5, 43.4, 41.6, 41.5, 38.9, 37.2, 37.0, 34.8, 29.8, 24.9, 24.7, 23.6, 23.5, 22.0, 21.5, 15.7, 15.6, 11.9, 11.5; HRESIMS calcd. for C41H62N6O7Na [M + Na]+ 773.4578, found 773.4577.3.1.10. General Procedure for the Preparation of Compounds N1–N2
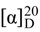 = −21.8 (c 0.10, CHCl3); 1H-NMR (DMSO-d6, 600 MHz) δ 8.63 (br, 1H), 8.10 (br, 1H), 7.95 (br, 1H), 7.80 (br, 1H), 7.28–7.17 (m, 11H), 6.75–6.72 (m, 1H), 5.22–5.18 (m, 1H), 4.90–4.85 (m, 1H), 4.29–4.17 (m, 3H), 4.04–4.00 (m, 1H), 3.70–3.66 (m, 1H), 3.50–3.47 (m, 1H), 3.20–3.17 (m, 1H), 2.93–2.71 (m, 6H), 2.58–2.55 (m, 1H), 2.31–1.88 (m, 6H), 1.77–1.73 (m, 2H), 1.50–1.23 (m, 8H), 0.93–0.76 (m, 15H); 13C-NMR (DMSO-d6, 150 MHz) δ 174.4, 173.1, 172.9, 170.1, 169.4, 169.2, 169.0, 139.7, 138.4, 129.3, 128.7, 128.6, 127.7, 127.5, 127.1, 58.7, 47.9, 42.9, 42.8, 41.4, 41.0, 35.4, 34.6, 31.9, 31.4, 29.6, 29.3, 28.0, 27.9, 24.8, 23.5, 22.3, 22.1, 21.9, 15.8, 14.5, 14.2, 11.5; HRESIMS calcd. for C42H64N7O7 [M + H]+ 778.4867, found 778.4860.
= −21.8 (c 0.10, CHCl3); 1H-NMR (DMSO-d6, 600 MHz) δ 8.63 (br, 1H), 8.10 (br, 1H), 7.95 (br, 1H), 7.80 (br, 1H), 7.28–7.17 (m, 11H), 6.75–6.72 (m, 1H), 5.22–5.18 (m, 1H), 4.90–4.85 (m, 1H), 4.29–4.17 (m, 3H), 4.04–4.00 (m, 1H), 3.70–3.66 (m, 1H), 3.50–3.47 (m, 1H), 3.20–3.17 (m, 1H), 2.93–2.71 (m, 6H), 2.58–2.55 (m, 1H), 2.31–1.88 (m, 6H), 1.77–1.73 (m, 2H), 1.50–1.23 (m, 8H), 0.93–0.76 (m, 15H); 13C-NMR (DMSO-d6, 150 MHz) δ 174.4, 173.1, 172.9, 170.1, 169.4, 169.2, 169.0, 139.7, 138.4, 129.3, 128.7, 128.6, 127.7, 127.5, 127.1, 58.7, 47.9, 42.9, 42.8, 41.4, 41.0, 35.4, 34.6, 31.9, 31.4, 29.6, 29.3, 28.0, 27.9, 24.8, 23.5, 22.3, 22.1, 21.9, 15.8, 14.5, 14.2, 11.5; HRESIMS calcd. for C42H64N7O7 [M + H]+ 778.4867, found 778.4860.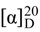 = −9.3 (c 0.11, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.64 (br, 1H), 7.45 (br, 2H), 7.36 (br, 1H), 7.16–7.03 (m, 10H), 6.92–6.88 (m, 2H), 5.16–5.12 (m, 1H), 4.80–4.76 (m, 2H), 4.49–4.37 (m, 2H), 4.21–4.17 (m, 1H), 4.08–3.90 (m, 2H), 3.76–3.58 (m, 2H), 3.30–3.26 (m, 1H), 2.97–2.95 (m, 1H), 2.88 (s, 3H), 2.81 (s, 3H), 2.25–2.10 (m, 3H), 1.66–1.34 (m, 3H), 1.29–1.03 (m, 4H), 0.90–0.63 (m, 12H); HRESIMS calcd. for C39H58N7O8 [M + H]+ 752.4347, found 752.4344.
= −9.3 (c 0.11, CHCl3); 1H-NMR (CDCl3, 600 MHz) δ 7.64 (br, 1H), 7.45 (br, 2H), 7.36 (br, 1H), 7.16–7.03 (m, 10H), 6.92–6.88 (m, 2H), 5.16–5.12 (m, 1H), 4.80–4.76 (m, 2H), 4.49–4.37 (m, 2H), 4.21–4.17 (m, 1H), 4.08–3.90 (m, 2H), 3.76–3.58 (m, 2H), 3.30–3.26 (m, 1H), 2.97–2.95 (m, 1H), 2.88 (s, 3H), 2.81 (s, 3H), 2.25–2.10 (m, 3H), 1.66–1.34 (m, 3H), 1.29–1.03 (m, 4H), 0.90–0.63 (m, 12H); HRESIMS calcd. for C39H58N7O8 [M + H]+ 752.4347, found 752.4344.3.1.11. General Procedure for the Preparation of Compounds 13a–c
3.1.12. General Procedure for the Preparation of Compounds M1–M3
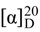 = −3.7 (c 0.11, MeOH); 1H-NMR (DMSO-d6, 600 MHz) δ 8.70 (t, J = 5.9 Hz, 1H), 8.22 (br, 1H), 7.93 (t, J = 5.0 Hz, 1H), 7.71 (d, J = 9.4 Hz, 1H), 7.60 (d, J = 8.7 Hz, 1H), 7.30–7.14 (m, 11H), 6.75–6.73 (m, 1H), 5.25–5.21 (m, 1H), 4.45–4.43 (m, 1H), 4.40–4.17 (m, 4H), 4.01–3.97 (m, 1H), 3.77–3.72 (m, 1H), 3.46–3.40 (m, 1H), 3.24 (dd, J = 14.6, 5.9 Hz, 1H), 2.95–2.90 (m, 1H), 2.87 (s, 3H), 2.12–2.09 (m, 2H), 1.84–1.80 (m, 1H), 1.70–1.64 (m, 3H), 1.55–1.51 (m, 1H), 1.49–1.33 (m, 3H), 1.25–1.21 (m, 1H), 1.16–1.01 (m, 3H), 0.88–0.76 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 174.4, 173.4, 172.5, 171.4, 170.2, 169.5, 169.0, 139.9, 138.5, 129.7, 128.9, 128.8, 128.7, 127.8, 127.5, 127.2, 126.8, 75.6, 58.7, 57.2, 52.6, 50.8, 42.7, 41.4, 38.7, 37.5, 34.7, 32.0, 31.5, 28.1, 24.7, 24.6, 23.8, 23.6, 22.2, 16.1, 15.8, 12.3, 11.7; HRESIMS calcd. For C42H64N7O8 [M + H]+ 794.4816, found 794.4801.
= −3.7 (c 0.11, MeOH); 1H-NMR (DMSO-d6, 600 MHz) δ 8.70 (t, J = 5.9 Hz, 1H), 8.22 (br, 1H), 7.93 (t, J = 5.0 Hz, 1H), 7.71 (d, J = 9.4 Hz, 1H), 7.60 (d, J = 8.7 Hz, 1H), 7.30–7.14 (m, 11H), 6.75–6.73 (m, 1H), 5.25–5.21 (m, 1H), 4.45–4.43 (m, 1H), 4.40–4.17 (m, 4H), 4.01–3.97 (m, 1H), 3.77–3.72 (m, 1H), 3.46–3.40 (m, 1H), 3.24 (dd, J = 14.6, 5.9 Hz, 1H), 2.95–2.90 (m, 1H), 2.87 (s, 3H), 2.12–2.09 (m, 2H), 1.84–1.80 (m, 1H), 1.70–1.64 (m, 3H), 1.55–1.51 (m, 1H), 1.49–1.33 (m, 3H), 1.25–1.21 (m, 1H), 1.16–1.01 (m, 3H), 0.88–0.76 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 174.4, 173.4, 172.5, 171.4, 170.2, 169.5, 169.0, 139.9, 138.5, 129.7, 128.9, 128.8, 128.7, 127.8, 127.5, 127.2, 126.8, 75.6, 58.7, 57.2, 52.6, 50.8, 42.7, 41.4, 38.7, 37.5, 34.7, 32.0, 31.5, 28.1, 24.7, 24.6, 23.8, 23.6, 22.2, 16.1, 15.8, 12.3, 11.7; HRESIMS calcd. For C42H64N7O8 [M + H]+ 794.4816, found 794.4801.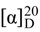 = −34.7 (c 0.11, MeOH); 1H-NMR (DMSO-d6, 600 MHz) δ 8.43 (t, J = 5.8 Hz, 1H), 8.27 (t, J = 6.1 Hz, 1H), 8.13 (br, 1H), 7.68 (d, J = 8.2 Hz, 1H), 7.62 (d, J = 8.3 Hz, 1H), 7.28–7.18 (m, 10H), 7.13–7.09 (m, 2H), 4.94–4.90 (m, 1H), 4.77–4.72 (m, 1H), 4.51–4.44 (m, 1H), 4.31–4.27 (m, 1H), 4.21–4.18 (m, 1H), 4.11–4.07 (m, 1H), 3.77–3.71 (m, 2H), 3.62–3.59 (m, 1H), 3.05–3.01 (m, 1H), 2.94 (s, 3H), 2.83–2.79 (m, 1H), 1.82–1.77 (m, 2H), 1.71–1.62 (m, 5H), 1.38–1.23 (m, 3H), 1.18–1.08 (m, 2H), 1.05–0.98 (m, 1H), 0.93–0.76 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 175.1, 174.1, 173.4, 171.9, 171.4, 170.6, 169.1, 139.6, 138.3, 129.7, 128.8, 128.7, 127.6, 127.2, 126.9, 73.0, 57.7, 56.0, 54.9, 47.3, 43.2, 42.6, 42.5, 41.7, 38.7, 38.2, 37.0, 32.1, 29.6, 24.8, 24.4, 23.8, 23.7, 21.9, 16.0, 15.8, 12.2, 11.5; HRESIMS calcd. for C42H64N7O8 [M + H]+ 794.4816, found 794.4782.
= −34.7 (c 0.11, MeOH); 1H-NMR (DMSO-d6, 600 MHz) δ 8.43 (t, J = 5.8 Hz, 1H), 8.27 (t, J = 6.1 Hz, 1H), 8.13 (br, 1H), 7.68 (d, J = 8.2 Hz, 1H), 7.62 (d, J = 8.3 Hz, 1H), 7.28–7.18 (m, 10H), 7.13–7.09 (m, 2H), 4.94–4.90 (m, 1H), 4.77–4.72 (m, 1H), 4.51–4.44 (m, 1H), 4.31–4.27 (m, 1H), 4.21–4.18 (m, 1H), 4.11–4.07 (m, 1H), 3.77–3.71 (m, 2H), 3.62–3.59 (m, 1H), 3.05–3.01 (m, 1H), 2.94 (s, 3H), 2.83–2.79 (m, 1H), 1.82–1.77 (m, 2H), 1.71–1.62 (m, 5H), 1.38–1.23 (m, 3H), 1.18–1.08 (m, 2H), 1.05–0.98 (m, 1H), 0.93–0.76 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 175.1, 174.1, 173.4, 171.9, 171.4, 170.6, 169.1, 139.6, 138.3, 129.7, 128.8, 128.7, 127.6, 127.2, 126.9, 73.0, 57.7, 56.0, 54.9, 47.3, 43.2, 42.6, 42.5, 41.7, 38.7, 38.2, 37.0, 32.1, 29.6, 24.8, 24.4, 23.8, 23.7, 21.9, 16.0, 15.8, 12.2, 11.5; HRESIMS calcd. for C42H64N7O8 [M + H]+ 794.4816, found 794.4782.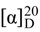 = 46.0 (c 0.1, MeOH); 1H-NMR (DMSO-d6, 600 MHz) δ 8.4 (t, J = 5.9 Hz, 1H), 8.28 (t, J = 5.5 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 8.15 (br, 1H), 7.85 (d, J = 6.6 Hz, 1H), 7.58 (d, J = 8.7 Hz, 1H), 7.28–7.17 (m, 10H), 7.13 (d, J = 7.3 Hz, 2H), 4.55–4.50 (m, 1H), 4.48–4.42 (m, 1H), 4.34–4.18 (m, 3H), 4.11–4.02 (m, 1H), 3.79–3.72 (m, 2H), 3.60–3.55 (m, 1H), 3.03–2.99 (m, 1H), 2.85–2.79 (m, 1H), 2.15–2.04 (m, 2H), 1.86–1.83 (m, 1H), 1.73–1.66 (m, 3H), 1.53–1.49 (m, 1H), 1.48–1.41 (m, 3H), 1.34–1.32 (m, 1H), 1.14–1.02 (m, 2H), 0.86–0.75 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 174.5, 173.4, 172.6, 172.2, 171.9, 171.4, 169.2, 139.6, 138.4, 129.7, 128.8, 127.5, 127.2, 126.9, 75.5, 57.7, 55.4, 55.1, 52.7, 50.7, 42.6, 42.2, 38.7, 38.1, 37.0, 32.1, 27.9, 24.8, 24.7, 23.8, 23.6, 22.1, 16.0, 15.7, 12.3, 11.6; HRESIMS calcd. for C41H62N7O8 [M + H]+ 780.4660, found 780.4648.
= 46.0 (c 0.1, MeOH); 1H-NMR (DMSO-d6, 600 MHz) δ 8.4 (t, J = 5.9 Hz, 1H), 8.28 (t, J = 5.5 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 8.15 (br, 1H), 7.85 (d, J = 6.6 Hz, 1H), 7.58 (d, J = 8.7 Hz, 1H), 7.28–7.17 (m, 10H), 7.13 (d, J = 7.3 Hz, 2H), 4.55–4.50 (m, 1H), 4.48–4.42 (m, 1H), 4.34–4.18 (m, 3H), 4.11–4.02 (m, 1H), 3.79–3.72 (m, 2H), 3.60–3.55 (m, 1H), 3.03–2.99 (m, 1H), 2.85–2.79 (m, 1H), 2.15–2.04 (m, 2H), 1.86–1.83 (m, 1H), 1.73–1.66 (m, 3H), 1.53–1.49 (m, 1H), 1.48–1.41 (m, 3H), 1.34–1.32 (m, 1H), 1.14–1.02 (m, 2H), 0.86–0.75 (m, 18H); 13C-NMR (DMSO-d6, 150 MHz) δ 174.5, 173.4, 172.6, 172.2, 171.9, 171.4, 169.2, 139.6, 138.4, 129.7, 128.8, 127.5, 127.2, 126.9, 75.5, 57.7, 55.4, 55.1, 52.7, 50.7, 42.6, 42.2, 38.7, 38.1, 37.0, 32.1, 27.9, 24.8, 24.7, 23.8, 23.6, 22.1, 16.0, 15.7, 12.3, 11.6; HRESIMS calcd. for C41H62N7O8 [M + H]+ 780.4660, found 780.4648.3.2. Cytotoxicity Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gademann, K.; Portmann, C. Secondary metabolites from cyanobacteria: Complex structures and powerful bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [CrossRef]
- Burjar, A.M.; Banaigs, B.; Abuo-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Tasiamide, a cytotoxic peptide from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2002, 65, 1336–1339. [Google Scholar] [CrossRef]
- Ma, Z.-H.; Song, N.; Li, C.-X.; Zhang, W.; Wang, P.; Li, Y.-X. Total synthesis and stereochemical reassignment of tasiamide. J. Pept. Sci. 2008, 14, 1139–1147. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Li, L.; Salvador, L.A.; Chen, T.; Chen, W.; Felsenstein, K.M.; Ladd, T.B.; Price, A.R.; Golde, T.E.; et al. Cyanobacterial peptides as a prototype for the design of potent β-secretase inhibitors and the development of selective chemical probes for other proteases. J. Med. Chem. 2012, 55, 10749–10765. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, W.; Ding, N.; Lo, J.; Liu, Y.; Clare-Salzler, M.J.; Luesch, H.; Li, Y. Total synthesis of grassystatin A, a probe for cathepsin E function. Bioorg. Med. Chem. 2012, 20, 4774–4780. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, W.; Zong, C.; Wang, P.; Li, Y. Total synthesis and stereochemical reassignment of tasiamide B. J. Pept. Sci. 2010, 16, 364–374. [Google Scholar]
- Aurelio, L.; Brownlee, R.T.C.; Hughes, A.B. Synthetic preparation of N-methyl-α-amino acids. Chem. Rev. 2004, 104, 5823–5846. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, W.; Sun, T.; Ma, Z.; Li, Y. Design, Synthesis and Biological Evaluation of Tasiamide Analogues as Tumor Inhibitors. Mar. Drugs 2014, 12, 2308-2325. https://doi.org/10.3390/md12042308
Zhang W, Sun T, Ma Z, Li Y. Design, Synthesis and Biological Evaluation of Tasiamide Analogues as Tumor Inhibitors. Marine Drugs. 2014; 12(4):2308-2325. https://doi.org/10.3390/md12042308
Chicago/Turabian StyleZhang, Wei, Tiantian Sun, Zhenhua Ma, and Yingxia Li. 2014. "Design, Synthesis and Biological Evaluation of Tasiamide Analogues as Tumor Inhibitors" Marine Drugs 12, no. 4: 2308-2325. https://doi.org/10.3390/md12042308
APA StyleZhang, W., Sun, T., Ma, Z., & Li, Y. (2014). Design, Synthesis and Biological Evaluation of Tasiamide Analogues as Tumor Inhibitors. Marine Drugs, 12(4), 2308-2325. https://doi.org/10.3390/md12042308