Cladieunicellins M–Q, New Eunicellins from Cladiella sp.
Abstract
:1. Introduction
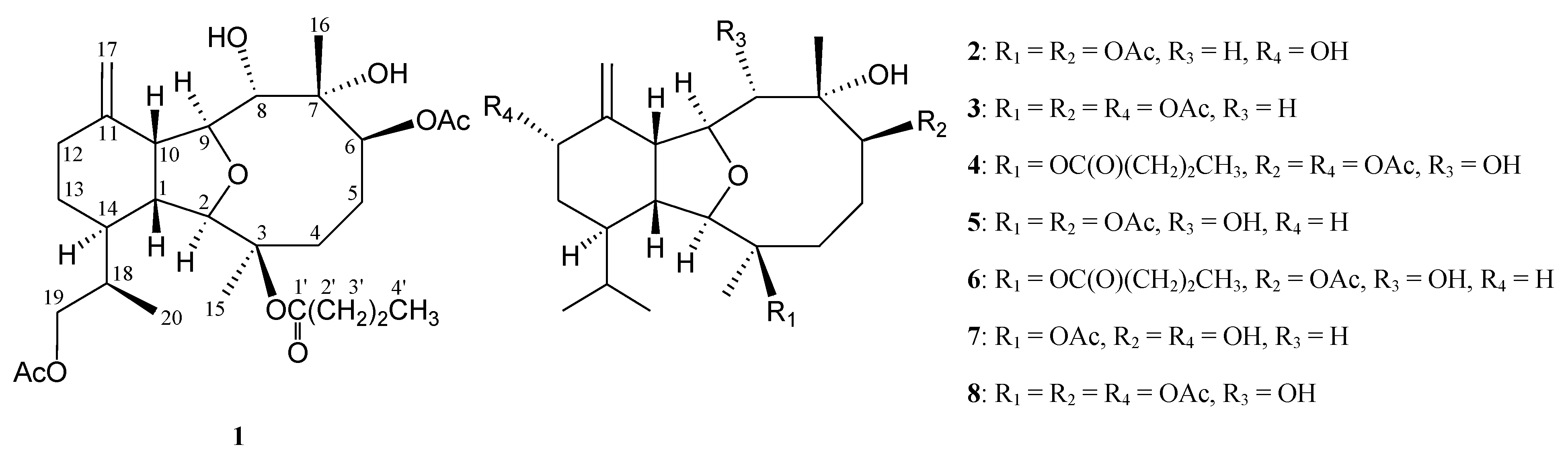
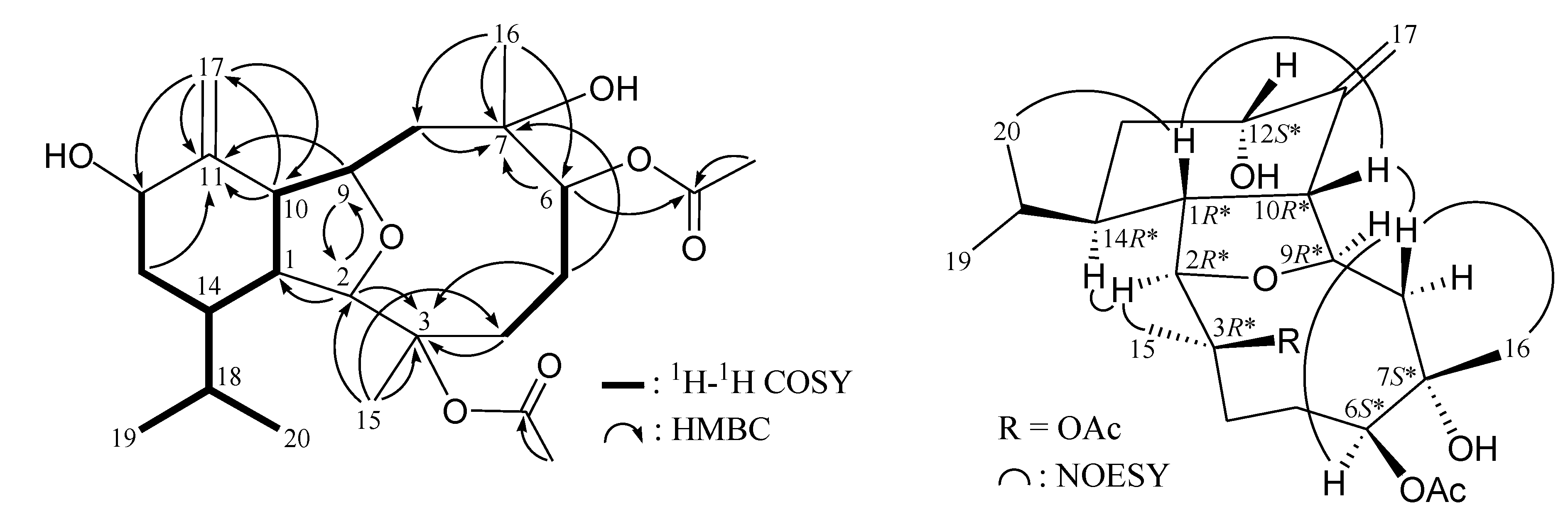
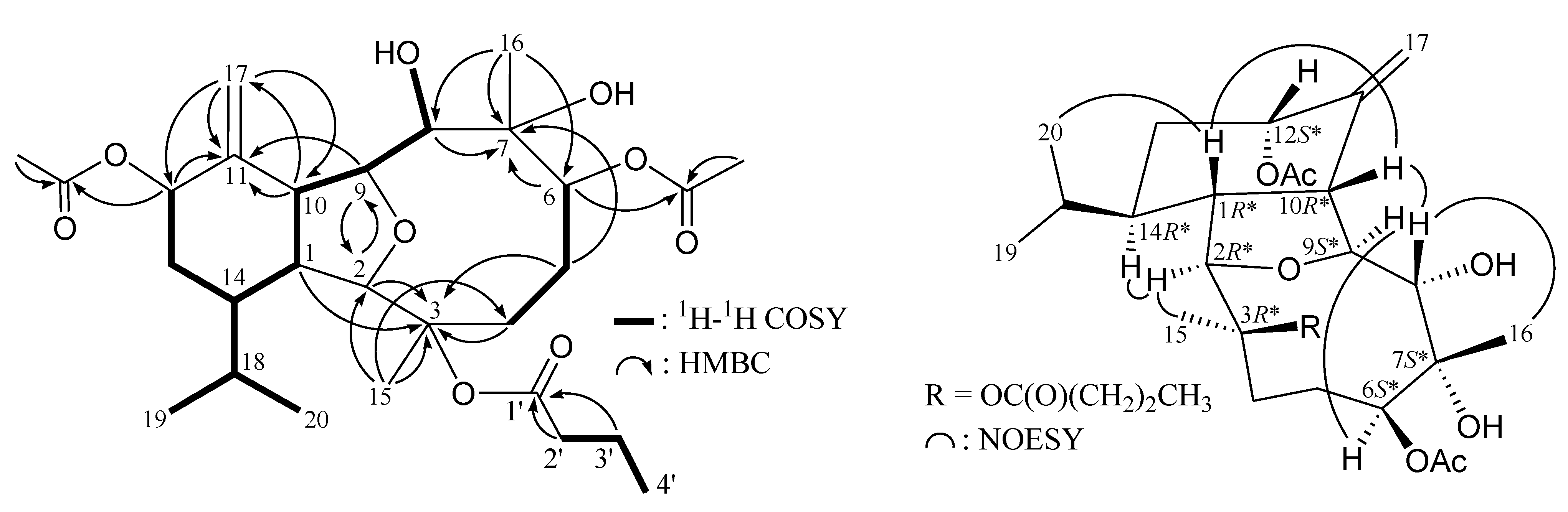
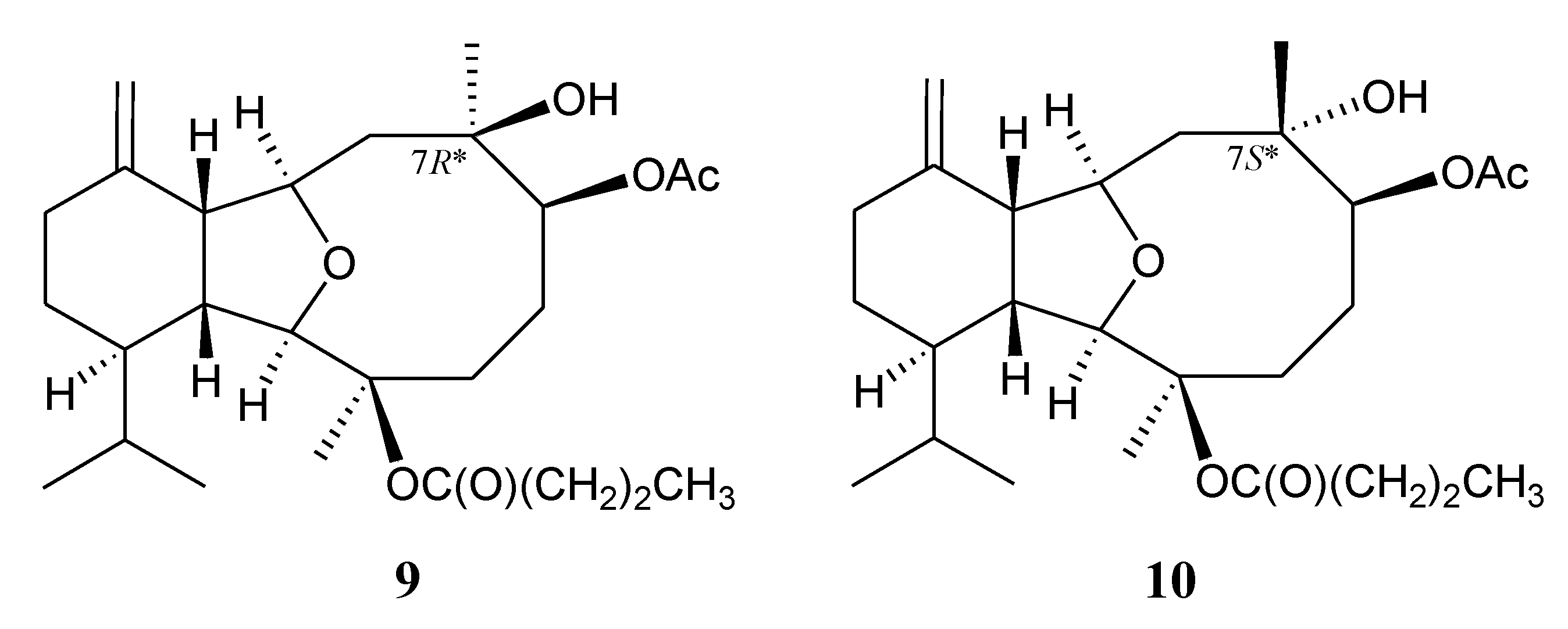
2. Results and Discussion
| Position | δH ( J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.23 dd (10.8, 7.2) | 45.1, CH | H-10, H-14 | C-3, -9, -10, -14, -18 |
| 2 | 3.57 s | 92.7, CH | n.o. a | C-1, -3, -10, -14, -15 |
| 3 | 86.0, C | |||
| 4 | 2.59 dd (13.6, 7.2) | 35.4, CH2 | H2-5 | C-2, -3, -6, -15 |
| 2.00 m | ||||
| 5 | 1.55–1.40 m | 28.5, CH2 | H2-4, H-6 | C-3, -6, -7 |
| 6 | 5.72 d (4.8) | 82.2, CH | H2-5 | C-4, -5, -7, -16, acetate carbonyl |
| 7 | 78.3, C | |||
| 8 | 3.58 dd (9.6, 8.8) | 80.0, CH | H-9, OH-8 | C-9, -10 |
| 9 | 3.84 dd (8.8, 6.8) | 81.5, CH | H-8, H-10 | C-2, -8, -11 |
| 10 | 3.38 dd (7.2, 6.8) | 53.5, CH | H-1, H-9 | C-1, -2, -8, -9, -11, -12, -14, -17 |
| 11 | 147.8, C | |||
| 12 | 2.28 m; 2.03 m | 31.5, CH2 | H2-13 | n.o. |
| 13 | 1.69 m; 1.10 m | 25.4, CH2 | H2-12, H-14 | n.o. |
| 14 | 1.48 m | 39.0, CH | H-1, H2-13, H-18 | C-18 |
| 15 | 1.38 s | 22.9, CH3 | C-2, -3, -4 | |
| 16 | 1.29 s | 18.4, CH3 | C-6, -7, -8 | |
| 17 | 4.91 br s | 111.1, CH2 | C-10, -11, -12 | |
| 4.79 dd (2.0, 1.6) | ||||
| 18 | 1.92 m | 34.0, CH | H-14, H2-19, H3-20 | C-19 |
| 19 | 3.95 d (6.4) | 67.5, CH2 | H-18 | C-14, -18, -20, acetate carbonyl |
| 20 | 0.84 d (7.2) | 10.7, CH3 | H-18 | C-14, -18, -19 |
| 3-n-butyrate | 172.3, C | |||
| 2.32 m | 37.3, CH2 | H2-3′ | C-1′, -3′, -4′ | |
| 1.66 m | 18.4, CH2 | H2-2′, H3-4′ | C-1′, -2′, -4′ | |
| 0.99 t (7.2) | 13.7, CH3 | H2-3′ | C-2′, -3′ | |
| 6-OAc | 171.9, C | |||
| 2.08 s | 21.4, CH3 | Acetate carbonyl | ||
| 19-OAc | 171.2, C | |||
| 2.09 s | 21.1, CH3 | Acetate carbonyl | ||
| OH-7 | 2.36 s | C-6, -7, -16 | ||
| OH-8 | 1.82 d (9.6) | H-8 | C-7, -8 |
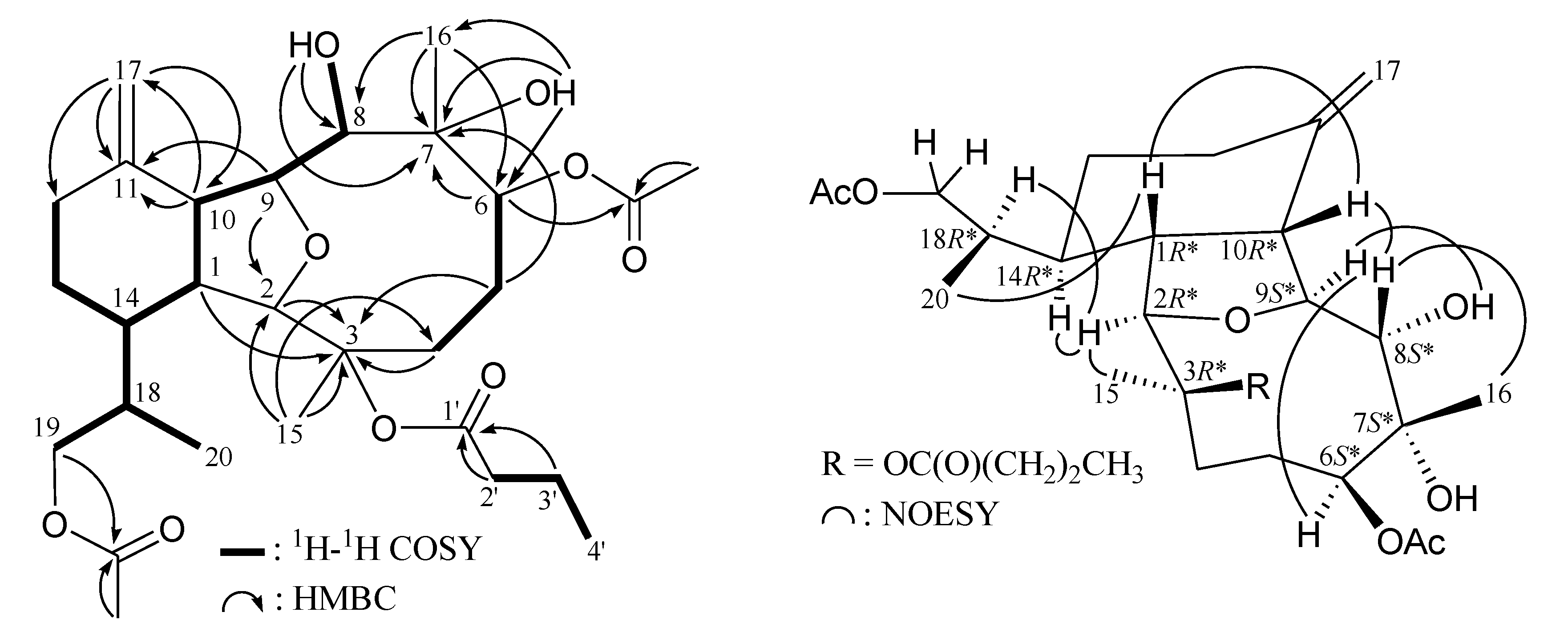
| 2 | 3 | 4 | 5 | |
|---|---|---|---|---|
| δH a | δH a | δH a | δH a | |
| 1 | 2.22 dd (10.0, 7.2) b | 2.24 dd (11.2, 7.6) | 2.23 dd (11.6, 6.8) | 2.23 dd (10.8, 7.2) |
| 2 | 3.71 s | 3.71 s | 3.67 s | 3.62 s |
| 4 | 2.55 dd (14.8, 8.8) | 2.58 dd (14.8, 8.4) | 2.52 dd (14.8, 8.4) | 2.54 dd (14.8, 8.4) |
| 2.03 m | 2.01 m | 2.00 m | 1.98 m | |
| 5 | 1.52 m | 1.56 m | 1.53 m | 1.52 m |
| 1.45 m | 1.46 dd (10.0, 6.0) | 1.43 dd (9.6, 6.8) | 1.47 dd (9.2, 6.4) | |
| 6 | 5.61 d (5.6) | 5.63 d (6.0) | 5.64 d (5.6) | 5.84 dd (6.0, 1.2) |
| 8 | 1.88 m; 1.82 m | 1.88 m; 1.82 m | 3.43 dd (10.8, 9.2) | 3.55 dd (9.2, 9.2) |
| 9 | 4.49 ddd (7.2, 6.4, 6.4) | 4.37 ddd (10.0, 7.2, 5.2) | 3.95 dd (9.2, 6.8) | 3.83 dd (9.2, 6.8) |
| 10 | 2.94 dd (7.2, 7.2) | 3.00 dd (7.6, 7.2) | 3.34 dd (6.8, 6.8) | 3.31 dd (7.2, 6.8) |
| 12 | 4.40 dd (4.0, 2.4) | 5.48 dd (4.0, 2.8) | 5.41 dd (4.0, 2.8) | 2.29 ddd (14.0, 3.6, 3.6) |
| 2.05 m | ||||
| 13 | 1.89 m | 1.93 ddd (14.0, 4.0, 4.0) | 1.90 ddd (14.0, 4.0, 4.0) | 1.75 m |
| 1.30 dd (12.8, 11.6) | 1.30 ddd (14.0, 14.0, 2.8) | 1.31 m | 1.06 m | |
| 14 | 1.86 m | 1.71 m | 1.64 m | 1.27 m |
| 15 | 1.41 s | 1.42 s | 1.38 s | 1.38 s |
| 16 | 1.19 s | 1.20 s | 1.25 s | 1.28 s |
| 17 | 5.00 d (1.2) | 5.14 d (1.6) | 5.22 d (2.0) | 4.87 br s |
| 4.81 d (1.2) | 4.93 br s | 5.17 d (2.0) | 4.77 br s | |
| 18 | 1.80 m | 1.83 m | 1.77 m | 1.73 m |
| 19 | 0.98 d (6.4) | 0.95 d (6.8) | 0.93 d (7.2) | 0.96 d (6.8) |
| 20 | 0.80 d (6.8) | 0.79 d (7.2) | 0.77 d (6.4) | 0.78 d (6.8) |
| 3-n-butyrate | 2.29 t (6.8) | |||
| 1.62 sext (6.8) | ||||
| 0.94 t (6.8) | ||||
| 3-OAc | 2.08 s | 2.09 s | 2.10 s | |
| 6-OAc | 2.06 s | 2.07 s | 2.06 s | 2.07 s |
| 12-OAc | 2.04 s | 2.03 s | ||
| 6-OH | ||||
| 7-OH | 2.32 br s | 2.57 br s | 2.43 br s | |
| 8-OH | 2.80 d (10.8) | 1.93 d (9.2) |
| 2 | 3 | 4 | 5 | |
|---|---|---|---|---|
| δC a | δC a | δC a | δC a | |
| 1 | 44.8, CH b | 44.7, CH | 44.3, CH | 45.7, CH |
| 2 | 91.2, CH | 91.1, CH | 91.8, CH | 92.4, CH |
| 3 | 86.7, C | 86.7, C | 86.1, C | 86.3, C |
| 4 | 35.2, CH2 | 35.4, CH2 | 34.9, CH2 | 35.2, CH2 |
| 5 | 29.2, CH2 | 29.1, CH2 | 28.6, CH2 | 28.5, CH2 |
| 6 | 83.9, CH | 84.3, CH | 81.8, CH | 82.1, CH |
| 7 | 75.4, C | 75.4, C | 78.3, C | 78.2, C |
| 8 | 46.2, CH2 | 46.1, CH2 | 79.6, CH | 80.0, CH |
| 9 | 79.9, CH | 79.2, CH | 82.5, CH | 81.4, CH |
| 10 | 51.5, CH | 51.8, CH | 51.1, CH | 53.4, CH |
| 11 | 147.8, C | 142.8, C | 143.2, C | 148.5, C |
| 12 | 71.1, CH | 72.8, CH | 73.6, CH | 31.8, CH2 |
| 13 | 30.6, CH2 | 28.5, CH2 | 28.6, CH2 | 24.9, CH2 |
| 14 | 35.6, CH | 36.4, CH | 37.1, CH | 44.2, CH |
| 15 | 23.0, CH3 | 23.0, CH3 | 23.0, CH3 | 22.8, CH3 |
| 16 | 23.5, CH3 | 23.7, CH3 | 18.3, CH3 | 18.4, CH3 |
| 17 | 113.2, CH2 | 116.7, CH2 | 117.8, CH2 | 110.7, CH2 |
| 18 | 28.6, CH | 28.5, CH | 28.6, CH | 29.0, CH |
| 19 | 21.8, CH3 | 21.7, CH3 | 21.7, CH3 | 21.9, CH3 |
| 20 | 15.6, CH3 | 15.3, CH3 | 15.4, CH3 | 15.6, CH3 |
| 3-n-butyrate | 172.3, C | |||
| 37.2, CH2 | ||||
| 18.3, CH2 | ||||
| 13.6, CH3 | ||||
| 3-OAc | 169.5, C | 169.4, C | 169.6, C | |
| 22.4, CH3 | 22.4, CH3 | 22.4, CH3 | ||
| 6-OAc | 171.8, C | 171.8, C | 171.8, C | 171.8, C |
| 21.4, CH3 | 21.4, CH3 | 21.4, CH3 | 21.4, CH3 | |
| 12-OAc | 170.4, C | 170.8, C | ||
| 21.6, CH3 | 21.4, CH3 |
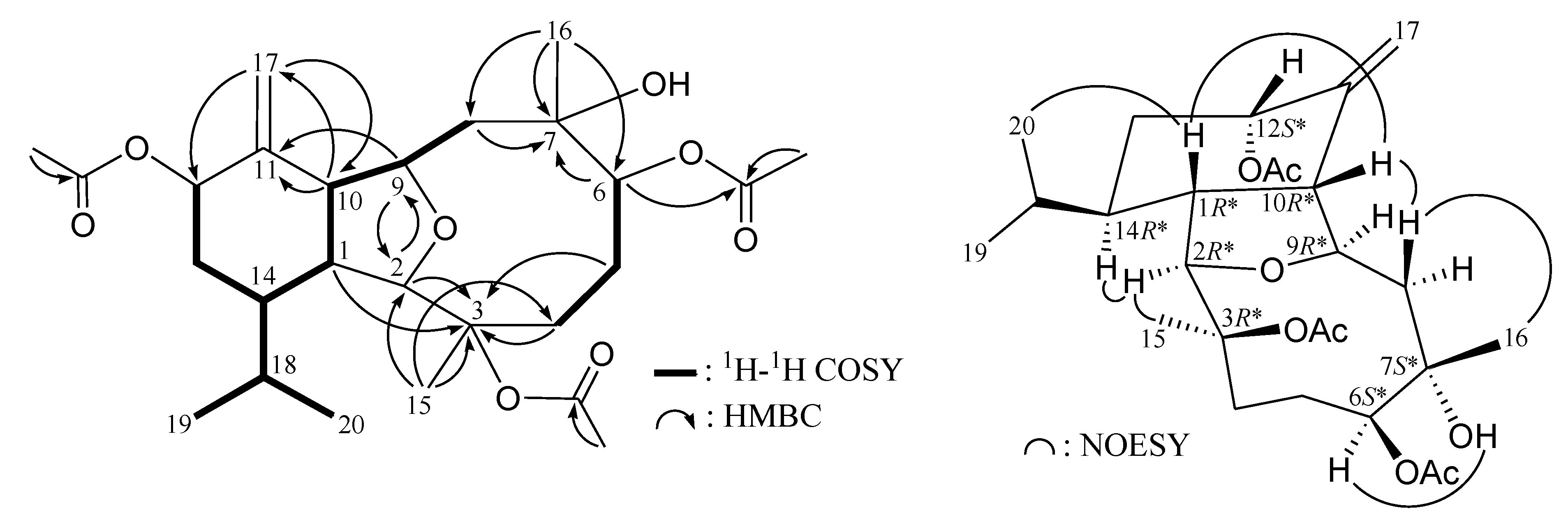
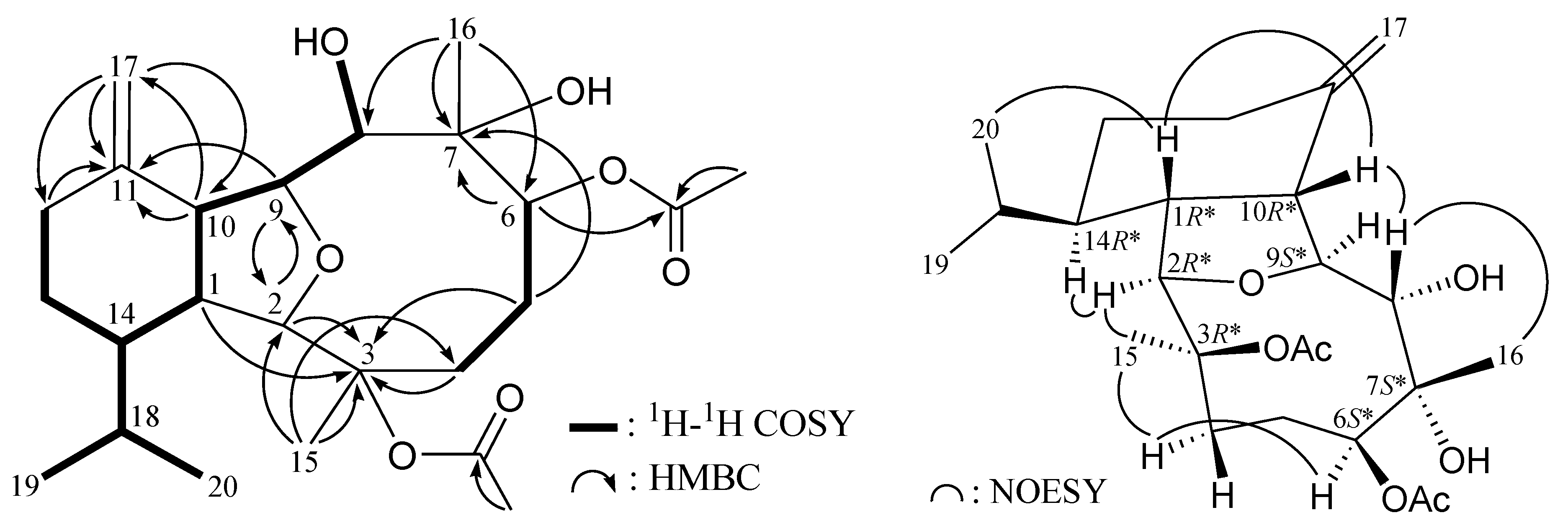
| Cell Lines IC50 (μM) | ||
|---|---|---|
| Compounds | Molt 4 | HL 60 |
| 1 | 16.43 | >20 |
| 2 | >20 | >20 |
| 3 | 14.17 | >20 |
| 4 | >20 | >20 |
| 5 | 15.55 | >20 |
| 8 a | 14.42 | >20 |
| Doxorubicin b | 0.02 | 0.02 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
 −10 (c 0.1, CHCl3); IR (neat) υmax 3462, 1734 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 547 [M + Na]+; HRESIMS: m/z 547.28760 (calcd for C28H44O9Na, 547.28775).
−10 (c 0.1, CHCl3); IR (neat) υmax 3462, 1734 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1; ESIMS: m/z 547 [M + Na]+; HRESIMS: m/z 547.28760 (calcd for C28H44O9Na, 547.28775). +31 (c 0.8, CHCl3); IR (neat) υmax 3437, 1729 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 461 [M + Na]+; HRESIMS: m/z 461.25067 (calcd for C24H38O7Na, 461.25097).
+31 (c 0.8, CHCl3); IR (neat) υmax 3437, 1729 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 461 [M + Na]+; HRESIMS: m/z 461.25067 (calcd for C24H38O7Na, 461.25097). +14 (c 0.4, CHCl3); IR (neat) υmax 3478, 1729 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 503 [M + Na]+; HRESIMS: m/z 503.26152 (calcd for C26H40O8Na, 503.26154).
+14 (c 0.4, CHCl3); IR (neat) υmax 3478, 1729 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 503 [M + Na]+; HRESIMS: m/z 503.26152 (calcd for C26H40O8Na, 503.26154). −7 (c 3.0, CHCl3); IR (neat) υmax 3448, 1733 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 547 [M + Na]+; HRESIMS: m/z 547.28755 (calcd for C28H44O9Na, 547.28775).
−7 (c 3.0, CHCl3); IR (neat) υmax 3448, 1733 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 547 [M + Na]+; HRESIMS: m/z 547.28755 (calcd for C28H44O9Na, 547.28775). +24 (c 0.6, CHCl3); IR (neat) υmax 3462, 1732 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 461 [M + Na]+; HRESIMS: m/z 461.25110 (calcd for C24H38O7Na, 461.25097).
+24 (c 0.6, CHCl3); IR (neat) υmax 3462, 1732 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 2 and ; ESIMS: m/z 461 [M + Na]+; HRESIMS: m/z 461.25110 (calcd for C24H38O7Na, 461.25097).3.4. MTT Antiproliferative Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, T.-H.; Lu, M.-C.; Chang, Y.-C.; Su, Y.-D.; Chen, Y.-H.; Lin, N.-C.; Fang, L.-S.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellin-based diterpenoids from a Formosan soft coral Cladiella sp. Mar. Drugs 2013, 11, 4585–4593. [Google Scholar] [CrossRef]
- Shih, F.-Y.; Chen, T.-H.; Lu, M.-C.; Chen, W.-F.; Wen, Z.-H.; Kuo, Y.-H.; Sung, P.-J. Cladieunicellins K and L, new eunicellin-based diterpenoids from an octocoral Cladiella sp. Int. J. Mol. Sci. 2013, 14, 21781–21789. [Google Scholar]
- Chen, T.-H.; Cheng, C.-H.; Chen, Y.-H.; Lu, M.-C.; Fang, L.-S.; Chen, W.-F.; Wen, Z.-H.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-J. Cladieunicellin J, a new hydroperoxyeunicellin from Cladiella sp. Nat. Prod. Commun. 2014, 9. in press. [Google Scholar]
- Radhika, P. Chemical constituents and biological activities of the soft corals of genus Cladiella: A review. Biochem. Syst. Ecol. 2006, 34, 781–789. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2011, 9, 2036–2045. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Tai, C.-J.; Hwang, T.-L.; Sheu, J.-H. Krempfielins J–M, new eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 2741–2750. [Google Scholar] [CrossRef]
- Ochi, M.; Yamada, K.; Kataoka, K.; Kotsuki, H.; Shibata, K. Litophynins I and J, two new biologically active diterpenoids from the soft coral Litophyton sp. Chem. Lett. 1992, 1992, 155–158. [Google Scholar]
- Friedrich, D.; Paquette, L.A. Structural and stereochemical reassessment of sclerophytin-type diterpenes. J. Nat. Prod. 2002, 65, 126–130. [Google Scholar] [CrossRef]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed.; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 84–85. [Google Scholar]
- Su, J.-H.; Chen, Y.-C.; El-Shazly, M.; Du, Y.-C.; Su, C.-W.; Tsao, C.-W.; Liu, L.-L.; Chou, Y.; Chang, W.-B.; Su, Y.-D.; et al. Towards the small and the beautiful: A small dibromotyrosine derivative from Pseudoceratina sp. sponge exhibits potent apoptotic effect through targeting IKK/NFκB signaling pathway. Mar. Drugs 2013, 11, 3168–3185. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, T.-H.; Chen, W.-F.; Wen, Z.-H.; Lu, M.-C.; Wang, W.-H.; Li, J.-J.; Wu, Y.-C.; Sung, P.-J. Cladieunicellins M–Q, New Eunicellins from Cladiella sp. Mar. Drugs 2014, 12, 2144-2155. https://doi.org/10.3390/md12042144
Chen T-H, Chen W-F, Wen Z-H, Lu M-C, Wang W-H, Li J-J, Wu Y-C, Sung P-J. Cladieunicellins M–Q, New Eunicellins from Cladiella sp. Marine Drugs. 2014; 12(4):2144-2155. https://doi.org/10.3390/md12042144
Chicago/Turabian StyleChen, Tsung-Hung, Wu-Fu Chen, Zhi-Hong Wen, Mei-Chin Lu, Wei-Hsien Wang, Jan-Jung Li, Yang-Chang Wu, and Ping-Jyun Sung. 2014. "Cladieunicellins M–Q, New Eunicellins from Cladiella sp." Marine Drugs 12, no. 4: 2144-2155. https://doi.org/10.3390/md12042144





