Potential Anti-HPV and Related Cancer Agents from Marine Resources: An Overview
Abstract
:1. Introduction
2. Update on Pathogenesis and Therapy for HPV and its Related Cancer
2.1. The Pathogenesis of HPV and Its Related Cancer
2.2. HPV Vaccines
2.3. Current Anti-HPV and Related Cancer Drugs
3. Potential Anti-HPV and Related Cancer Agents from Marine Resources
3.1. Heparin and Marine Heparinoid Polysaccharides
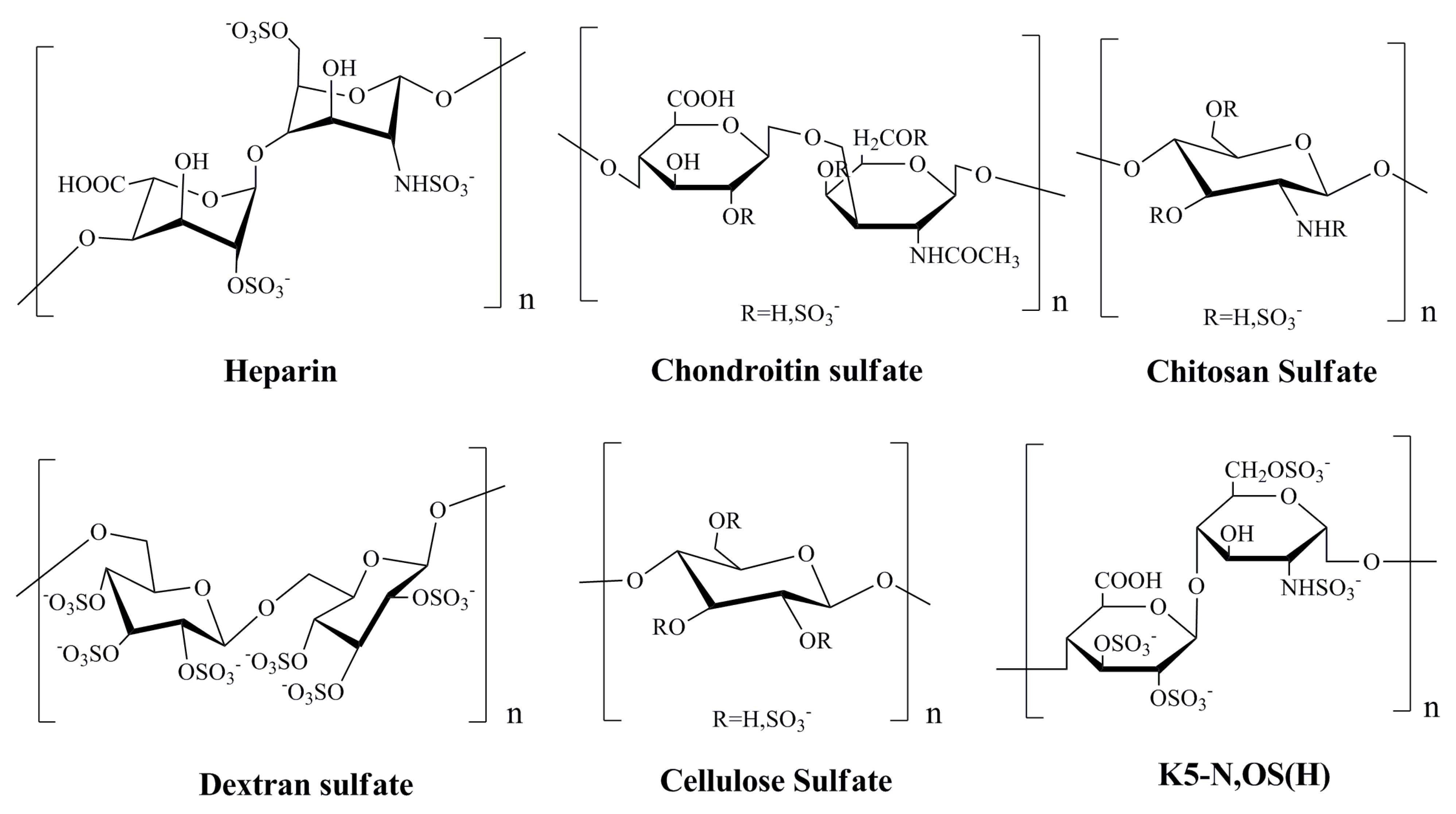
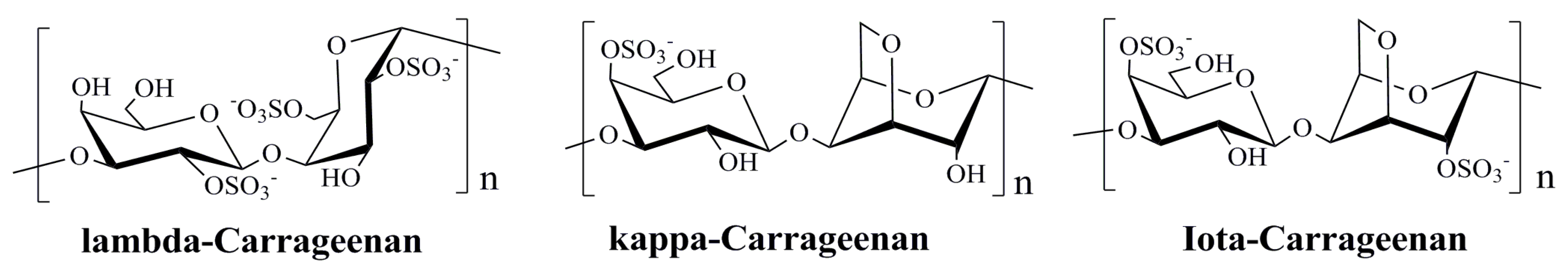
3.2. Polysaccharides Derived from Red Algae
3.3. Sulfated Polysaccharides from Brown Algae
3.4. Agents from Marine Microbes
3.5. Bioactive Compounds from Marine Animals
| Marine Organisms | Specific Compounds | Mechanisms of Action | References |
|---|---|---|---|
| Red Algae | λ-carrageenan | Blocking HPV infection | [7,104] |
| κ-carrageenan | Blocking HPV infection | [7,104] | |
| ι-carrageenan | Blocking HPV infection | [7,74,104] | |
| Agar | Blocking HPV infection | [7] | |
| Brown Algae | Alginic acid | Inhibiting HPV and cancer cell proliferation | [7,87] |
| Fucoidan | Blocking HPV infection | [7] | |
| Marine Fungus | Gliotoxin | Inducing apoptosis in cancer cells | [94] |
| Neoechinulin A | Inducing apoptosis in cancer cells | [95] | |
| Physcion | Inducing apoptosis in cancer cells | [96] | |
| (+)-epoxydon | Inducing apoptosis in cancer cells | [97] | |
| Echinoderm | japonicus polysaccharide | Inducing apoptosis in cancer cells | [98] |
| Shellfish | Clam polypeptide | Inducing apoptosis in cancer cells | [100] |
| Crustacean | Chitosan | Inhibiting HPV and cancer cell proliferation | [101,102,103] |
4. Prospects of Marine Derived Anti-HPV and Related Cancer Agents
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mammas, I.; Sourvinos, G.; Giannoudis, A.; Spandidos, D.A. Human papilloma virus (HPV) and host cellular interactions. Pathol. Oncol. Res. 2008, 14, 345–354. [Google Scholar] [CrossRef]
- Sanclemente, G.; Gill, D.K. Human papillomavirus molecular biology and pathogenesis. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 231–240. [Google Scholar] [CrossRef]
- Zur, H.H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Bosch, F.X.; Munoz, N. The viral etiology of cervical cancer. Virus Res. 2002, 89, 183–190. [Google Scholar] [CrossRef]
- Steben, M.; Duarte, F.E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef]
- Forman, D.; Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet, T.J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Muller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, 69. [Google Scholar] [CrossRef]
- Lowy, D.R.; Schiller, J.T. Reducing HPV-associated cancer globally. Cancer Prev. Res. 2012, 5, 18–23. [Google Scholar] [CrossRef]
- Sidbury, R. What’s new in pediatric dermatology: Update for the pediatrician. Curr. Opin. Pediatr. 2004, 16, 410–414. [Google Scholar] [CrossRef]
- Syrjanen, S.; Puranen, M. Human papillomavirus infections in children: The potential role of maternal transmission. Crit. Rev. Oral. Biol. Med. 2002, 11, 259–274. [Google Scholar] [CrossRef]
- Manhart, L.E.; Koutsky, L.A. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia: A meta-analysis. Sex Transm. Dis. 2002, 29, 725–735. [Google Scholar] [CrossRef]
- Holmes, K.K.; Levine, R.; Weaver, M. Effectiveness of condoms in preventing sexually transmitted infections. Bull. World Health Organ. 2004, 82, 454–461. [Google Scholar]
- Mao, C.; Koutsky, L.A.; Ault, K.A.; Wheeler, C.M.; Brown, D.R.; Wiley, D.J.; Alvarez, F.B.; Bautista, O.M.; Jansen, K.U.; Barr, E. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: A randomized controlled trial. Obstet. Gynecol. 2006, 107, 18–27. [Google Scholar] [CrossRef]
- Bharti, A.C.; Shirish, S.; Sutapa, M.; Suresh, H.; Bhudev, C.D. Anti-human papillomavirus therapeutics: Facts & future. Indian. J. Med. Res. 2009, 130, 296–310. [Google Scholar]
- Lauri, E.M.; Eileen, F.D.; Mona, S.; Herschel, W.L.; Harrell, C.; Elizabeth, R.U. Quadrivalent human Papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007, 56, 1–24. [Google Scholar]
- Wang, Q.; Griffin, N.; Southern, S.; Jackson, D.; Martin, A. Functional analysis of the human Papillomavirus type 16 E1–E4 protein provides a mechanism for in vivo and in vitro keratin filament reorganization. J. Virol. 2004, 78, 821–833. [Google Scholar] [CrossRef]
- Munõz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef]
- Patterson, N.A.; Smith, J.L.; Ozbun, M.A. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. J. Virol. 2005, 79, 6838–6847. [Google Scholar] [CrossRef]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541. [Google Scholar] [CrossRef]
- Day, P.M.; Lowy, D.R.; Schiller, J.T. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 2003, 307, 1–11. [Google Scholar] [CrossRef]
- Bousarghin, L.; Touzé, A.; Sizaret, P.-Y.; Coursaget, P. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J. Virol. 2003, 77, 3846–3850. [Google Scholar] [CrossRef]
- Thierry, F. Transcriptional regulation of the papillomavirus oncogenes by cellular and viral transcription factors in cervical carcinoma. Virology 2009, 384, 375–379. [Google Scholar] [CrossRef]
- Faridi, R.; Zahra, A.; Khan, K.; Idrees, M. Oncogenic potential of Human Papillomavirus (HPV) and its relation with cervical cancer. Virol. J. 2011, 8, 269–277. [Google Scholar] [CrossRef]
- Disbrow, G.L.; Hanover, J.A.; Schlegel, R. Endoplasmic reticulum-localized human Papillomavirus type 16 E5 protein alters endosomal pH but not trans-Golgi pH. J. Virol. 2005, 79, 5839–5846. [Google Scholar] [CrossRef]
- Shai, A.; Brake, T.; Somoza, C.; Lambert, P.F. The human Papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007, 67, 1626–1635. [Google Scholar] [CrossRef]
- Snijders, P.J.; van Duin, M.; Walboomers, J.M.; Steenbergen, R.D.; Risse, E.K.; Helmerhorst, T.J.; Verheijen, R.H.; Meijer, C.J. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: Strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human Papillomavirus DNA. Cancer Res. 1998, 58, 3812–3818. [Google Scholar]
- Horikawa, I.; Barrett, J.C. Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms. Carcinogenesis 2003, 24, 1167–1178. [Google Scholar] [CrossRef]
- Fausch, S.C.; Da, S.D.M.; Kast, W.M. Heterologous papillomavirus virus-like particles and human papillomavirus virus-like particle immune complexes activate human Langerhans cells. Vaccine 2005, 23, 1720–1729. [Google Scholar] [CrossRef]
- Pagliusi, S.R.; Teresa, A.M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004, 23, 569–578. [Google Scholar] [CrossRef]
- Rose, R.C.; White, W.I.; Li, M.; Suzich, J.A.; Lane, C.; Garcea, R.L. Human papillomavirus type 11 recombinant capsomeres induce virus-neutralizing antibodies. J. Virol. 1998, 72, 6151–6154. [Google Scholar]
- White, W.I.; Wilson, S.D.; Frances, J.P.H.; Robert, M.W.; Ghim, S.J.; Lisa, A.H.; Daniel, M.G.; Steven, J.B.; A. Bennett, J.; Scott, K.; et al. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 1999, 73, 4882–4889. [Google Scholar]
- Huh, W.K.; Roden, R.B.S. The future of vaccines for cervical cancer. Gynecol. Oncol. 2008, 109, S48–S56. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.K.H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Munõz, N.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez, A.; MWheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-assocated genital diseases in young women. J. Natl. Cancer Inst. 2010, 102, 325–339. [Google Scholar] [CrossRef]
- Steenbergen, R.D.; De, W.J.; Wilting, S.M.; Brink, A.A.; Snijders, P.J.; Meijer, C.J. HPV-mediated transformation of the anogenital tract. J. Clin. Virol. 2005, 32, 25–33. [Google Scholar] [CrossRef]
- Borysiewicz, L.K.; Fiander, A.; Nimako, M.; Man, S.; Wilkinson, G.W.; Westmoreland, D. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 1996, 347, 1523–1527. [Google Scholar] [CrossRef]
- Greenstone, H.L.; Nieland, J.D.; Visser, K.E.; Bruijn, M.L.; Kirnbauer, R.; Roden, R.B. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA 1998, 95, 1800–1805. [Google Scholar]
- Muderspach, L.; Wilczynski, S.; Roman, L.; Bade, L.; Felix, J.; Small, L.A. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin. Cancer Res. 2000, 6, 3406–3416. [Google Scholar]
- De Jong, A.; O’Neill, T.; Khan, A.Y.; Kwappenberg, K.M.C.; Chisholm, S.E.; Whittle, N.R.; Dobson, J.A.; Jack, L.C.; Roberts, J.A.S.C.; Offringa, R.; et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 2002, 20, 3456–3464. [Google Scholar] [CrossRef]
- Frazer, I.H.; Quinn, M.; Nicklin, J.L.; Tan, J.; Perrin, L.C.; Ng, P. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine 2004, 23, 172–181. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Nieland, J.D.; Jochmus, I.; Baur, S.; Friese, K.; Gabelsberger, J. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). Int. J. Cancer 2007, 121, 2794–2800. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Palmieri, M.; Zanolini, A.; Ravaggi, A.; Siegel, E.R. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: A phase I escalating-dose trial. J. Virol. 2008, 8, 1968–1979. [Google Scholar]
- Welters, M.J.; Kenter, G.G.; Piersma, S.J.; Vloon, A.P.; Lowik, M.J.; Berends-van der, M.D.M.; Drijfhout, J.W.; Valentijn, A.R.; Wafelman, A.R.; Oostendorp, J. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 2008, 14, 178–187. [Google Scholar] [CrossRef]
- Klencke, B.; Matijevic, M.; Urban, R.G.; Lathey, J.L.; Hedley, M.L.; Berry, M. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: A Phase I study of ZYC101. Clin. Cancer Res. 2002, 8, 1028–1037. [Google Scholar]
- Sheets, E.E.; Urban, R.G.; Crum, C.P.; Hedley, M.L.; Politch, J.A.; Gold, M.A. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am. J. Obstet. Gynecol. 2003, 188, 916–926. [Google Scholar] [CrossRef]
- Peng, S.; Trimble, C.; Alvarez, R.D.; Huh, W.K.; Lin, Z.; Monie, A. Cluster intradermal DNA vaccination rapidly induces E7-specific CD8+ T-cell immune responses leading to therapeutic antitumor effects. Gene Ther. 2008, 15, 1156–1166. [Google Scholar] [CrossRef]
- Stern, P.L. Immune control of human papillomavirus (HPV) associated anogenital disease and potential for vaccination. J. Clin. Virol. 2005, 32, 72–81. [Google Scholar] [CrossRef]
- Adams, M.; Navabi, H.; Jasani, B.; Man, S.; Fiander, A.; Evans, A.S. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly[C(12)U] (Ampligen R). Vaccine 2003, 21, 787–790. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Hung, C.F.; Wu, T.C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 2010, 28, 5212–5219. [Google Scholar] [CrossRef]
- Greenfield, I.; Cuthill, S. Antivirals. Clinical and Scientific Advances. In Human Papillomaviruses; Sterling, J.C., Tyring, S.K., Eds.; Arnorld: London, UK, 2001; pp. 120–130. [Google Scholar]
- Cirelli, R.; Tyring, S.K. Interferons in human papillomavirus infections. Antivir. Res. 1994, 24, 191–204. [Google Scholar] [CrossRef]
- Li, J.; Luo, K.Z.; Lin, Y.; Chen, W.N.; Lai, S.P. Laboratory study on anti-human papillomavirus activity of Bupleurum chinese. Chin. J. Dermato. Venerol. Integ. Trad. W. Med. 2005, 4, 171–173. (In Chinese) [Google Scholar]
- Xiao, J.; Wu, J.; Yu, B. Therapeutic efficacy of Youdujing preparation in treating cervical high-risk papilloma virus infection patients. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012, 32, 1212–1215. (In Chinese) [Google Scholar]
- Huang, G.Q. Clinical observation of paiteling on treatment of cervicitis combined with high-risk HPV infection. Chin. J. Woman Child Health Res. 2012, 23, 675–677. (In Chinese) [Google Scholar]
- Song, X.X.; Liu, Y.L.; Hao, Q.Y. Xinfuning combined with Baofukang suppository for 53 cases with cervical high risk human papillomavirus infection. J. Oncol. 2011, 17, 825–827. [Google Scholar]
- Johnson, K.M.; Kines, R.C.; Roberts, J.N.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J. Virol. 2009, 83, 2067–2074. [Google Scholar] [CrossRef]
- Joyce, J.G.; Tung, J.S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef]
- Giroglou, T.; Florin, L.; Schafer, F.; Streeck, R.E.; Sapp, M. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 2001, 75, 1565–1570. [Google Scholar] [CrossRef]
- Drobni, P.; Mistry, N.N.; Millan, M. Carboxy-fluorescein diacetate, succinimidyl ester labeled papillomavirus virus like particles fluoresce after internalization and interact with heparin sulfate for binding and entry. Virology 2003, 310, 163–172. [Google Scholar] [CrossRef]
- Hans, C.S.; Luise, F.; Hetal, D. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J. Virol. 2007, 81, 10970–10980. [Google Scholar] [CrossRef]
- Rommel, O.; Dillner, J.; Fligge, C. Heparan sulfate proteoglycans interact exclusively with conformationally intact HPV L1 assemblies: Basis for a virus-like particle ELISA. J. Med. Virol. 2005, 75, 114–121. [Google Scholar] [CrossRef]
- Saeed, S.K.; Alessandra, H.; Ernst, K.; Guerrino, M.; Katharina, S.; Reinhard, K. Different heparin sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J. Virol. 2003, 77, 13125–13135. [Google Scholar] [CrossRef]
- Malgorzata, B.H.; Patel, H.D.; Sapp, M. Target cell cyclophilins facilitate human papilloma-virus type 16 infection. PLoS Pathog. 2009, 5, e1000524. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Christensen, N.D.; Reed, C.A.; Culp, T.D.; Hermonat, P.L.; Howett, M.K. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 2001, 45, 3427–3432. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Rusnati, M.; Bugatti, A.; Cornaglia, M.; Cappello, P.; Giovarelli, M.; Oreste, P.; Landolfo, S. Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus. Antimicrob. Agents Chemother. 2008, 52, 1374–1381. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Noseda, M.D.; Cerezo, A.S.; Damonte, E.B. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Andreas, L.; Christiane, M.; Marielle, K.S.; Regina, W.; Donata, K.; Bettina, P.; Philipp, G.; Britta, F.G.; Martin, B.; Tamas, F.; et al. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virology 2008, 5, 107. [Google Scholar] [CrossRef]
- Pujol, C.A.; Scolaro, L.A.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiviral activity of a carrageenan from Gigartinatina skottsbergii against intraperitoneal murine Herpes Simplex virus infection. Planta Med. 2006, 72, 121–125. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Hao, C.; Zhang, X.E.; Cui, Z.Q.; Guan, H.S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef]
- Schiller, T.S.; Davies, P. Delivering on the promise: HPV vaccines and cervical cancer. Nat. Rev. Microbiol. 2004, 2, 343–347. [Google Scholar] [CrossRef]
- Van de Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1H and 13C high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- Xin, X.L.; Geng, M.Y.; Guan, H.S.; Li, Z.L. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitro. Chin. J. Mar. Drugs 2000, 19, 15–18. (In Chinese) [Google Scholar]
- Xin, X.L.; Ding, H.; Geng, M.Y.; Liang, P.F.; Li, Y.X.; Guan, H.S. Studies of the anti-AIDS effects of marine polysaccharide drug 911 and its related mechanisms of action. Chin. J. Mar. Drugs 2000, 6, 4–8. (In Chinese) [Google Scholar]
- Geng, M.Y.; Li, F.C.; Xin, X.L.; Li, J.; Yan, Z.W.; Guan, H.S. The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry Interaction between SPMG and HIV-1 gp120 and CD4 molecule. Antivir. Res. 2003, 59, 127–135. [Google Scholar] [CrossRef]
- Miao, B.C.; Geng, M.Y.; Li, J.; Li, F.; Chen, H.; Guan, H.S.; Ding, J. Sulfated polymannuroguluronate, a novel anti-acquired immune deficiency syndrome (AIDS) drug candidate, targeting CD4 in lymphocytes. Biochem. Pharmacol. 2004, 68, 641–649. [Google Scholar] [CrossRef]
- Liu, H.Y.; Geng, M.Y.; Xin, X.L.; Li, F.C.; Chen, H.X.; Guan, H.S.; Ding, J. Multiple and multivalent interactions of novel anti-AIDS drug candidates, sulfated polymannuronate (SPMG)-derived oligosaccharides, with gp120 and their anti-HIV activities. Glycobiology 2005, 15, 501–510. [Google Scholar]
- Dietrich, C.P.; Farias, G.G.M.; Abreu, L.R.; Leite, E.L.; Silva, L.F.; Nader, H.B. A new approach for the characterization of polysaccharides from algae: Presence of four main acidic polysaccharides in three species of the class Phaeophycea. Plant Sci. 1995, 108, 143–153. [Google Scholar] [CrossRef]
- Albuquerque, I.R.L.; Queiroz, K.C.S.; Alves, L.G.; Santos, E.A.; Leite, E.L.; Rocha, H.A.O. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004, 37, 167–171. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.L. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Jailma, A.L.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Stevan, F.R.; Oliveira, M.B.; Bucchi, D.F.; Noseda; Iacomini, M.; Duarte, M.E. Cytotoxic effects against HeLa cells of polysaccharides from seaweeds. J. Submicrosc. Cytol. Pathol. 2001, 33, 477–484. [Google Scholar]
- Shao, C.L.; Wang, C.Y.; Wei, M.Y.; Gu, Y.C.; She, Z.G.; Qian, P.Y.; Lin, Y.C. Aspergilones A and B, two benzylazaphilones with an unprecedented carbon skeleton from the gorgonian-derived fungus Aspergillus sp. Bioorg. Med. Chem. Lett. 2011, 21, 690–693. [Google Scholar] [CrossRef]
- Wei, M.Y.; Wang, C.Y.; Liu, Q.A.; Shao, C.L.; She, Z.G.; Lin, Y.C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a Gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef]
- Lee, D.S.; Jeong, G.S.; Li, B.; Lee, S.U.; Oh, H.C.; Kim, Y.C. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts antiinflammatory effects through heme oxygenase-1 expression in murine macrophages. J. Pharmacol. Sci. 2011, 116, 283–295. [Google Scholar] [CrossRef]
- Axelsson, V.; Holback, S.; Sjogren, M.; Gustafsson, H.; Forsby, A. Gliotoxin induces caspase-dependent neurite degeneration and calpain-mediated general cytotoxicity in differentiated human neuroblastoma SH-SY5Y cells. Biochem. Biophys. Res. Commun. 2006, 345, 1068–1074. [Google Scholar] [CrossRef]
- Comera, C.; Andre, K.; Laffitte, J.; Collet, X.; Galtier, P.; Isabelle, M.P. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes. Infect. 2007, 94, 47–54. [Google Scholar]
- Nieminen, S.M.; Jorm, M.P.; Hirvonen, M.R.; Roponen, M.; Wright, A.V. Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat. Res. 2002, 520, 161–170. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.S.; Qian, Z.J.; Li, Y.X.; Kim, K.N.; Heo, S.J.; Jeon, Y.J.; Park, W.S.; Choi, I.W.; Je, J.Y.; et al. Gliotoxin isolated from marine fungus Aspergillus sp. induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar. Drugs 2014, 12, 69–87. [Google Scholar]
- Isuru, W.; Li, Y.X.; Vo, T.S.; Quang, V.T.; Ngo, D.H.; Kim, S.K. Induction of apoptosis in human cervical carcinoma HeLa cells by neoechinulin A from marine-derived fungus Microsporum sp. Process Biochem. 2013, 48, 68–72. [Google Scholar] [CrossRef]
- Isuru, W.; Zhang, C.; Van Ta, Q.; Vo, T.S.; Li, Y.X.; Kim, S.K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol. Res. 2014, 169, 255–261. [Google Scholar] [CrossRef]
- Jo, M.J.; Bae, S.J.; Son, B.W.; Kim, C.Y.; Kim, G.D. 3,4-dihydroxyphenyl acetic acid and (+) epoxydon isolated from marine algae-derived microorganisms induce down regulation of epidermal growth factor activated mitogenic signaling cascade in Hela cells. Cancer Cell Int. 2013, 13, 49. [Google Scholar] [CrossRef]
- Niu, J.J.; Song, Y. Effect of stichopus Japonicus acidic mucopolysaccharide on cell cycle of Hela cells and its mechanism. Med. J. Qilu 2010, 25, 386–388. (In Chinese) [Google Scholar]
- Wei, N.; Lin, X.K.; Niu, R.L.; Li, H.Y. Overview on anticancer agents from Meretrix meretrix. Food Drug 2007, 9, 63–65. (In Chinese) [Google Scholar]
- Zhang, J.; Kang, J.H.; Liu, F.J.; Fan, C.C.; Li, H.L.; Chen, Q.X. Effect of the polypeptides from Meretrix meretrix Linnaeus on proliferation of cervical cancer Hela cells. J. Xiamen Univ. 2009, 48, 729–732. (In Chinese) [Google Scholar]
- Huang, X.Y.; He, W.M.; Liao, Q.X.; Ruan, G.Y. Clinical nursing observation of the treatment of chronic cervicitis combined HPV infection using chitosan antimicrobial film. China Pract. Med. 2013, 8, 196–198. (In Chinese) [Google Scholar]
- Tong, Y.P.; Shen, S.H.; Zheng, W. Clinical application of chitosan nano-iodine for treatment of cervical CIN and HPV infection. J. Chin. Phys. 2013, 15, 1411–1413. (In Chinese) [Google Scholar]
- Lee, S.H.; Ryu, B.; Je, J.-Y.; Kim, S.K. Diethylaminoethyl chitosan induces apoptosis in HeLa cells via activation of caspase-3 and p53 expression. Carbohydr. Polym. 2011, 84, 571–578. [Google Scholar] [CrossRef]
- Roberts, J.N.; Kines, R.C.; Katki, H.A.; Lowy, D.R.; Schiller, J.T. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J. Natl. Cancer Inst. 2011, 103, 737–743. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, S.H. Chemical structures and bioactivities of sulphated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An Overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.-X.; Zhang, X.-S.; Guan, H.-S.; Wang, W. Potential Anti-HPV and Related Cancer Agents from Marine Resources: An Overview. Mar. Drugs 2014, 12, 2019-2035. https://doi.org/10.3390/md12042019
Wang S-X, Zhang X-S, Guan H-S, Wang W. Potential Anti-HPV and Related Cancer Agents from Marine Resources: An Overview. Marine Drugs. 2014; 12(4):2019-2035. https://doi.org/10.3390/md12042019
Chicago/Turabian StyleWang, Shi-Xin, Xiao-Shuang Zhang, Hua-Shi Guan, and Wei Wang. 2014. "Potential Anti-HPV and Related Cancer Agents from Marine Resources: An Overview" Marine Drugs 12, no. 4: 2019-2035. https://doi.org/10.3390/md12042019





