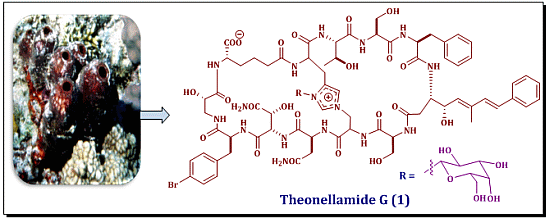
Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of Compound 1
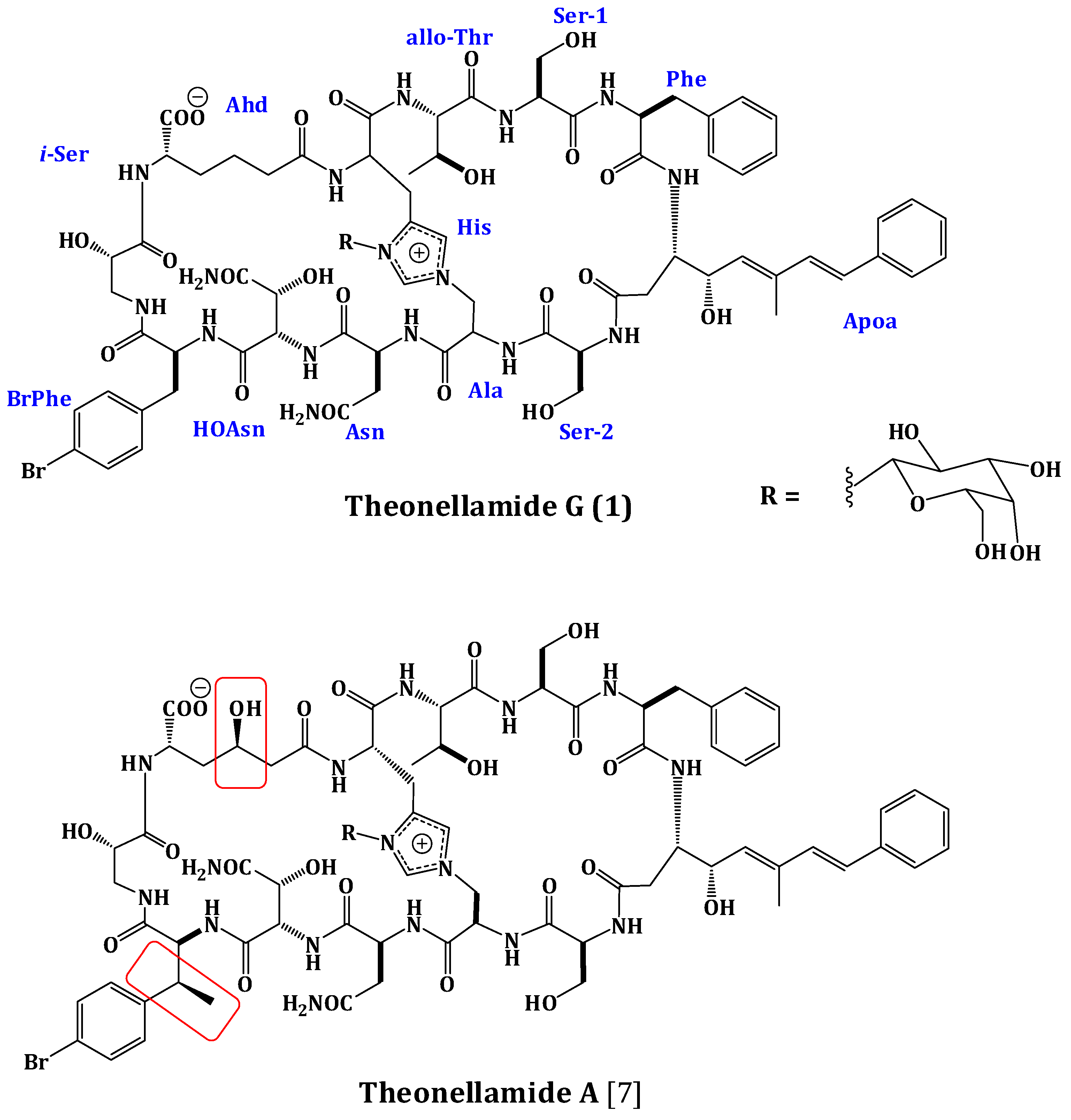
2.2. Structure Elucidation of Compound 1
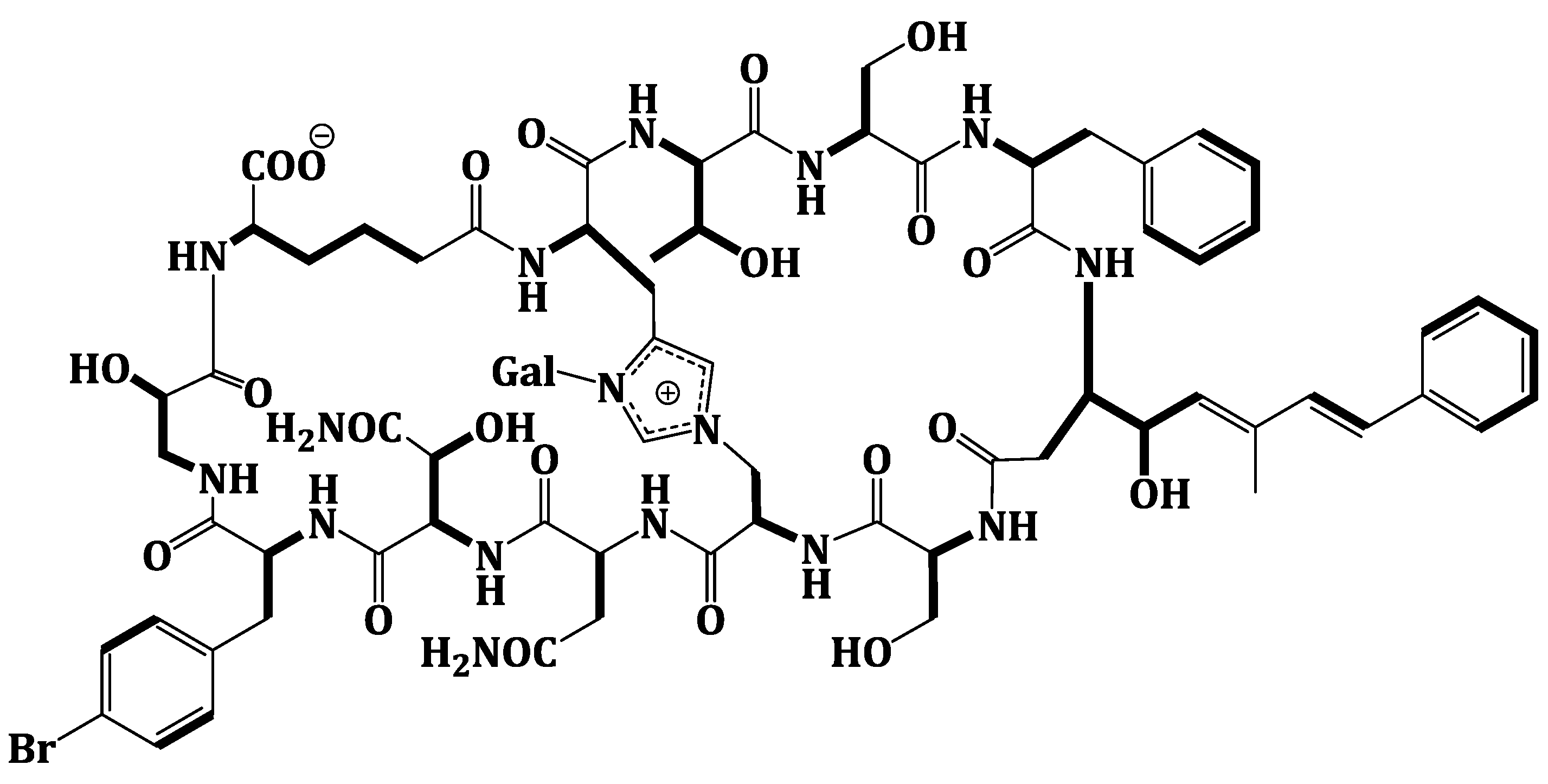

| Amino acid | C | δH m (J in Hz) | δC m | HMBC | NOESY |
|---|---|---|---|---|---|
| allo-Thr1 | CO | − | 173.1 C | − | − |
| α | 4.18 d (9.8) | 58.9 CH | 1 β, 12CO | 1γ, 2α, 12NH | |
| β | 3.55 m | 69.0 CH | 1CO | 12α, 2NH | |
| γ | 0.84 brs | 21.4 CH3 | 1α, 1β | 1α, 2NH, 12α, 12β | |
| NH | 7.65 d (7.8) | − | 12CO | 2α, 2NH, 12α, 5″″ | |
| OH | 5.12 m | − | 1β | − | |
| Ser-12 | CO | − | 170.0 C | − | − |
| α | 4.45 m | 56.6 CH | − | 3α, 3NH | |
| β | 3.64 m | 61.1 CH2 | 2α, 2CO | 2α, 3NH | |
| NH | 7.73 d (3.6) | − | 1CO | 1NH, 3α, 3NH | |
| Phe3 | CO | − | 171.6 C | − | − |
| α | 4.55 t (8.3) | 54.9 CH | 3CO, 1′ | 2α, 2NH | |
| β | 2.81 dd (13.3, 6.8) 2.67 m | 39.3 CH2 | 3α, 3CO, 1′, 2′, 3′ | 2NH | |
| 1′ | − | 137.2 C | − | ||
| 2′, 6′ | 7.12 d (6.6) | 129.9 CH | 1′, 3′, 5′ | ||
| 3′, 5′ | 7.01 t (6.6) | 131.8 CH | 2′, 6′ | ||
| 4″ | 7.28 d (6.6) | 129.7 CH | 2′, 6′ | ||
| NH | 7.93 d (7.8) | − | 2CO | 2α, 2NH, 4NH | |
| Apoa4 | CO | − | 172.6 C | − | |
| α | 2.55 q (10.3) 2.30 brd (13.8) | 36.9 CH2 | 4γ, 4CO | 5NH | |
| β | 4.46 m | 52.2 CH | 3CO, 4CO | 4α, 4γ, 5NH | |
| γ | 4.42 t (8.4) | 68.8 CH | 5NH, 4β, ε-CH3 | ||
| δ | 5.12 m | 132.4 CH | 4ζ, 4ε | 4β, 4ζ, 4γ | |
| ε | − | 137.9 C | − | ||
| ζ | 6.47 d (16.2) | 133.9 CH | 4δ, 4ε, 1″ | 4δ | |
| η | 6.56 d (16.2) | 128.7 CH | 4δ, 4ε, 3ζ, 4ε-CH3, 1″ | ε-CH3 | |
| 1″ | − | 137.2 C | − | − | |
| 2″, 6″ | 7.12 d (6.6) | 129.9 CH | 1″, 3″, 5″ | ||
| 3″, 5″ | 7.01 t (6.6) | 131.8 CH | 2″, 6″ | ||
| 4″ | 7.28 d (6.6) | 129.7 CH | 2″, 6″ | ||
| ε-CH3 | 1.63 s | 13.4 CH3 | 4δ, 4ε, 4ζ | 4η, 4γ | |
| NH | 8.45 brs | − | 4CO | 3NH, 3α, 5NH | |
| Ser-25 | CO | − | 172.8 C | − | − |
| α | 3.74 m | 56.8 CH | 4α, 4β, 4NH, 6α, 6NH | ||
| β | 3.76 m 3.63 m | 62.0 CH2 | 5CO | 4α, 4β, 4NH, 6α, 6NH | |
| NH | 7.78 brs | − | 4CO | 4α, 4NH, 6NH | |
| Ala6 | CO | − | 170.0 C | − | − |
| α | 5.08 m | 51.4 CH | 6CO | 7α, 7NH, 2″″ | |
| β | 4.90 brd (12.6) | 50.6 CH2 | 2″″ | 7α, 7NH, 2″″, 5″″ | |
| NH | 8.27 d (9.6) | − | 5CO | 5α, 7NH, 5″″ | |
| Asn7 | CO | − | 171.4 C | − | − |
| α | 4.11 t (7.2) | 52.9 CH | 6CO, 7-CONH2 | 6α, 8α, 6NH | |
| β | 2.36 dt (13.3, 7.2) 2.12 brd (13.3) | 37.3 CH2 | 7α, 7-CONH2 | 6NH | |
| CONH2 | − | 172.7 C | − | − | |
| NH | 7.67 d (11.2) | − | 6CO | 6α, 6NH | |
| NH2 | 7.69 brs | − | − | 7α, 7β | |
| HOAsn8 | CO | − | 170.9 C | − | − |
| α | 5.34 t (8.4) | 54.9 CH | 8CO, 8-CONH2 | 7α, 9α, 9β, 9NH | |
| β | 4.22 d (11.7) | 72.9 CH | − | − | |
| CONH2 | − | 174.8 C | − | − | |
| NH | 8.32 brs | − | 7CO | 7NH | |
| NH2 | 7.78 s | − | − | 8α, 8NH | |
| OH | 6.78 brs | − | − | − | |
| BrPhe9 | CO | − | 172.6 C | − | − |
| α | 4.34 m | 55.8 CH | 9CO | 10NH | |
| β | 3.01 brd (14.4) 2.65 m | 37.2 CH2 | 9CO, 9α, 1‴, 2‴ | 10NH | |
| 1‴ | − | 137.7 C | − | − | |
| 2‴, 6‴ | 7.21 d (6.6) | 129.2 CH | 1‴, 4‴ | − | |
| 3‴, 5‴ | 7.28 d (6. 6) | 131.8 CH | 1‴, 2‴, 6‴ | − | |
| 4‴ | − | 120.6 C | − | − | |
| NH | 8.71 brs | − | 8CO | 8α, 10NH | |
| i-Ser10 | CO | − | 171.9 C | − | − |
| α | 4.17 d (11.2) | 70.2 CH | 10CO | 11NH | |
| β | 3.95 m 2.96 brd (7.2) | 43.8 CH2 | 10CO | 11δ, 11NH | |
| NH | 7.47 d (7.2) | − | 9CO | 9α, 9β, 9NH, 11γ, 11NH | |
| Ahd−11 | CO | − | 173.1 C | − | − |
| α | 2.22 m 2.01 m | 35.9 CH2 | 11CO, 11β | 12α, 12NH | |
| β | 1.37 m 1.02 m | 22.7 CH2 | 11γ | 12NH | |
| γ | 1.78 m 1.53 m | 32.5 CH2 | 11α | 12NH | |
| δ | 4.57 t (7.3) | 54.9 CH | 11β, 11γ, 10CO, 11-COO− | 10NH, 12NH | |
| COO− | − | 175.0 C * | − | ||
| NH | 7.63 d (6.6) | − | 10CO | 10NH, 12NH | |
| His12 | CO | − | 171.1 C | − | − |
| α | 4.82 m | 54.5 CH | 12CO, 4″″ | 1γ, 1NH, 11α | |
| β | 3.24 t (13.5) 3.01 brd (14.4) | 26.3 CH2 | 12α, 4″″ | 1NH, 1CO, 11α, 2″″, 5″″ | |
| 2″″ | 8.84 s | 137.4 CH | 4″″, 5″″ | 1-Gal, 6β, 6NH, 11β | |
| 4″″ | − | 131.8 C | − | − | |
| 5″″ | 7.26 brs | 124.4 CH | 4″″ | 6α, 6β, 11β, 12β | |
| NH | 8.40 brs | − | 11CO | 1NH, 11α, 11β, 5″″ | |
| Gal13 | 1 | 5.03 d (9.0) | 89.0 CH | 2, 3, 2″″, 4″″ | 12NH, 12β, 2″″ |
| 2 | 3.83 m | 69.5 CH | 4, 3 | ||
| 3 | 3.45 m | 73.7 CH | 4, 5 | ||
| 4 | 3.66 m | 69.8 CH | 3, 6 | ||
| 5 | 3.67 m | 79.0 CH | 3, 4 | ||
| 6 | 3.76 m 3.63 m | 62.0 CH2 | 4, 5 |
| Compound | C. albicans (W.T.) b | C. albicans (AmBR) c | HCT-116 |
|---|---|---|---|
| MIC (μM) | MIC (μM) | IC50 (μM) | |
| Theonellamide G (1) | 4.49 | 2.0 | 6.0 |
| Amphotericin B d | 1.48 | − | |
| Etoposide e | − | − | 2.0 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Materials
3.3. Extraction and Purifications of Compound 1
 +15.85° (C 0.42, MeOH:H2O, 4:1); UV (λmax, MeOH) (log ε) 289 (4.82), 306 (3.57) nm; IR νmax (KBr) 3324, 2965, 1655, 1062 cm−1; NMR data, see Table 1; HRFABMS m/z 1733.5983 (calcd for C75H9879BrN16O27, 1733.5970, [M + H]+).
+15.85° (C 0.42, MeOH:H2O, 4:1); UV (λmax, MeOH) (log ε) 289 (4.82), 306 (3.57) nm; IR νmax (KBr) 3324, 2965, 1655, 1062 cm−1; NMR data, see Table 1; HRFABMS m/z 1733.5983 (calcd for C75H9879BrN16O27, 1733.5970, [M + H]+). 3.4. Acid Hydrolysis and Absolute Configuration of Amino Acids Using LC-MS Analysis of the Marfey Derivatives of 1
3.5. Chiral GC-MS Analysis of 1
3.6. Evaluation of Cytotoxic Activity
3.7. Antifungal Assay with C. albicans
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Winder, P.L.; Pomponi, S.A.; Wright, A.E. Natural Products from the Lithistida: A Review of the Literature since 2000. Mar. Drugs 2011, 9, 2643–2682. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.G.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Harper, M.K.; Faulkner, D.J. Mozamides A and B, cyclic peptides from a Theonellid sponge from Mozambique. J. Nat. Prod. 1997, 60, 779–782. [Google Scholar] [CrossRef]
- Hamada, T.; Suyawara, T.; Matsunaga, S.; Fusetani, N. Polytheonamides, unprecedented highly cytotoxic polypeptides from the marine sponge Theonella swinhoei 2. Structure elucidation. Tetrahedron Lett. 1994, 35, 609–612. [Google Scholar] [CrossRef]
- Nakao, Y.; Oku, N.; Matsunaga, S.; Fusetani, N. Cyclotheonamides E2 and E3, new potent serine protease inhibitors from the marine sponge of the genus Theonella. J. Nat. Prod. 1998, 61, 667–670. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kanzaki, K.; Katayama, S.; Ohashi, K.; Okada, H.; Ikegami, S.; Kitagawa, I. Marine natural products. XXXIII. Theonellapeptolide IId, a new tridecapeptide lactone from the Okinawan marine sponge Theonella swinhoei. Chem. Pharm. Bull. 1994, 42, 1410–1415. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N. Theonellamides A–E, cytotoxic bicyclic peptides, from a marine sponge Theonella sp. J. Org. Chem. 1995, 60, 1177–1181. [Google Scholar] [CrossRef]
- Bewley, C.A.; Faulkner, D.J. Theonegramide, an antifungal glycopeptides from the Philippine Lithistid Sponge Theonella swinhoei. J. Org. Chem. 1994, 59, 4849–4852. [Google Scholar] [CrossRef]
- Kobayashi, J.; Itagaki, F.; Shigemori, H.; Takao, T.; Shimonishi, Y. Keramamides E, G, H, and J, new cyclic peptides containing an oxazole or a thiazole ring from a Theonella sponge. Tetrahedron 1995, 51, 2525–2532. [Google Scholar] [CrossRef]
- de Silva, E.D.; Williams, D.E.; Andersen, R.J.; Klix, H.; Holmes, C.F.B.; Allen, T.M. A potent protein phosphatase inhibitor isolated from the Papau New Guinea sponge Theonella swinhoei Gray. Tetrahedron Lett. 1992, 33, 1561–1564. [Google Scholar]
- Schmidt, E.W.; Faulkner, D.J. Microsclerodermins C–E, antifungal cyclic peptides from the lithistid sponges Theonella sp. and Microscleroderma sp. Tetrahedron 1998, 54, 3043–3056. [Google Scholar] [CrossRef]
- Bonnington, L.S.; Tanaka, J.; Higa, T.; Kimura, J.; Yoshimura, Y.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J. Cupolamide A: A cytotoxic cyclic heptapeptide from two samples of the sponge Theonella cupola. J. Org. Chem. 1997, 62, 7765–7767. [Google Scholar] [CrossRef]
- Chill, L.; Kashman, Y.; Schleyer, M. Oriamide, a new cytotoxic cyclic peptide containing a novel amino acid from the marine sponge Theonella sp. Tetrahedron 1997, 53, 16147–16152. [Google Scholar] [CrossRef]
- Clark, D.P.; Carroll, J.; Naylor, S.; Crews, P. An antifungal cyclodepsipeptide, cyclolithistide A from the sponge Theonella swinhoei. J. Org. Chem. 1998, 63, 8757–8764. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Bewley, C.A.; Faulkner, D.J. Theopalauamide, a bicyclic glycopeptide from filamentous bacterial symbionts of the Lithistid sponge Theonella swinhoei from Palau and Mozambique. J. Org. Chem. 1998, 63, 1254–1258. [Google Scholar] [CrossRef]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; de Silva, E.D.; Lassota, P.; Allen, T.M.; et al. Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Mooberry, S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Hashimoto, K.; Wӓlchlit, M. A novel antifungal bicyclic peptide from a marine sponge Theonella sp. J. Am. Chem. Soc. 1989, 111, 2582–2588. [Google Scholar] [CrossRef]
- Nishimura, S.; Arita, Y.; Honda, M.; Iwamoto, K.; Matsuyama, A.; Shirai, A.; Kawasaki, H.; Kakeya, H.; Kobayashi, T.; Matsunaga, S.; et al. Marine antifungal theonellamides target 3β-hydroxysterol to activate Rho1 signaling. Nat. Chem. Biol. 2010, 6, 519–526. [Google Scholar] [CrossRef]
- Espiritu, R.A.; Matsumori, N.; Murata, M.; Nishimura, S.; Kakeya, H.; Matsunaga, S.; Yoshida, M. Interaction between the marine sponge cyclic peptide theonellamide A and sterols in lipid bilayers as viewed by surface plasmon resonance and solid-state (2)H nuclear magnetic resonance. Biochemistry 2013, 52, 2410–2418. [Google Scholar] [CrossRef]
- Nishimura, S.; Ishii, K.; Iwamoto, K.; Arita, Y.; Matsunaga, S.; Ohno-Iwashita, Y.; Sato, S.B.; Kakeya, H.; Kobayashi, T.; Yoshida, M. Visualization of sterol-rich membrane domains with fluorescently-labeled theonellamides. PLoS One 2013, 8, e83716. [Google Scholar]
- Ibrahim, S.R.M.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Edrada-Ebel, R.; Mohamed, G.A.; Youssef, D.T.A.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. ARKIVOC 2008, 2008, 164–171. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W. Antibiotic terpenoid chlorodihydroquinones from a new marine actinomycete. J. Nat. Prod. 2005, 68, 904–910. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; CLSI Document M7-A8; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Youssef, D.T.A.; Shaala, L.A.; Mohamed, G.A.; Badr, J.M.; Bamanie, F.H.; Ibrahim, S.R.M. Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei. Mar. Drugs 2014, 12, 1911-1923. https://doi.org/10.3390/md12041911
Youssef DTA, Shaala LA, Mohamed GA, Badr JM, Bamanie FH, Ibrahim SRM. Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei. Marine Drugs. 2014; 12(4):1911-1923. https://doi.org/10.3390/md12041911
Chicago/Turabian StyleYoussef, Diaa T. A., Lamiaa A. Shaala, Gamal A. Mohamed, Jihan M. Badr, Faida H. Bamanie, and Sabrin R. M. Ibrahim. 2014. "Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei" Marine Drugs 12, no. 4: 1911-1923. https://doi.org/10.3390/md12041911
APA StyleYoussef, D. T. A., Shaala, L. A., Mohamed, G. A., Badr, J. M., Bamanie, F. H., & Ibrahim, S. R. M. (2014). Theonellamide G, a Potent Antifungal and Cytotoxic Bicyclic Glycopeptide from the Red Sea Marine Sponge Theonella swinhoei. Marine Drugs, 12(4), 1911-1923. https://doi.org/10.3390/md12041911