Gliotoxin Isolated from Marine Fungus Aspergillus sp. Induces Apoptosis of Human Cervical Cancer and Chondrosarcoma Cells
Abstract
:1. Introduction
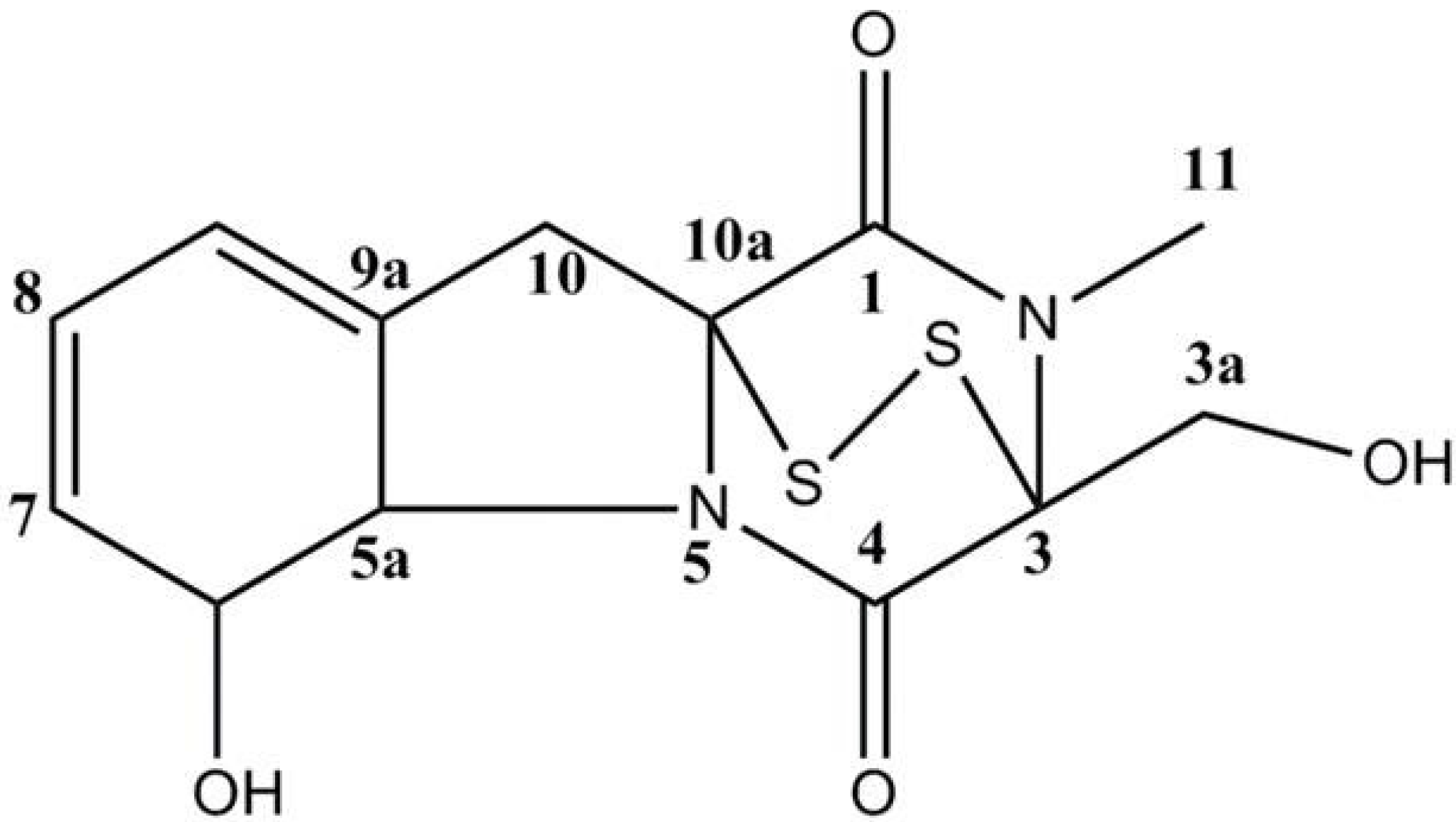
2. Results and Discussion
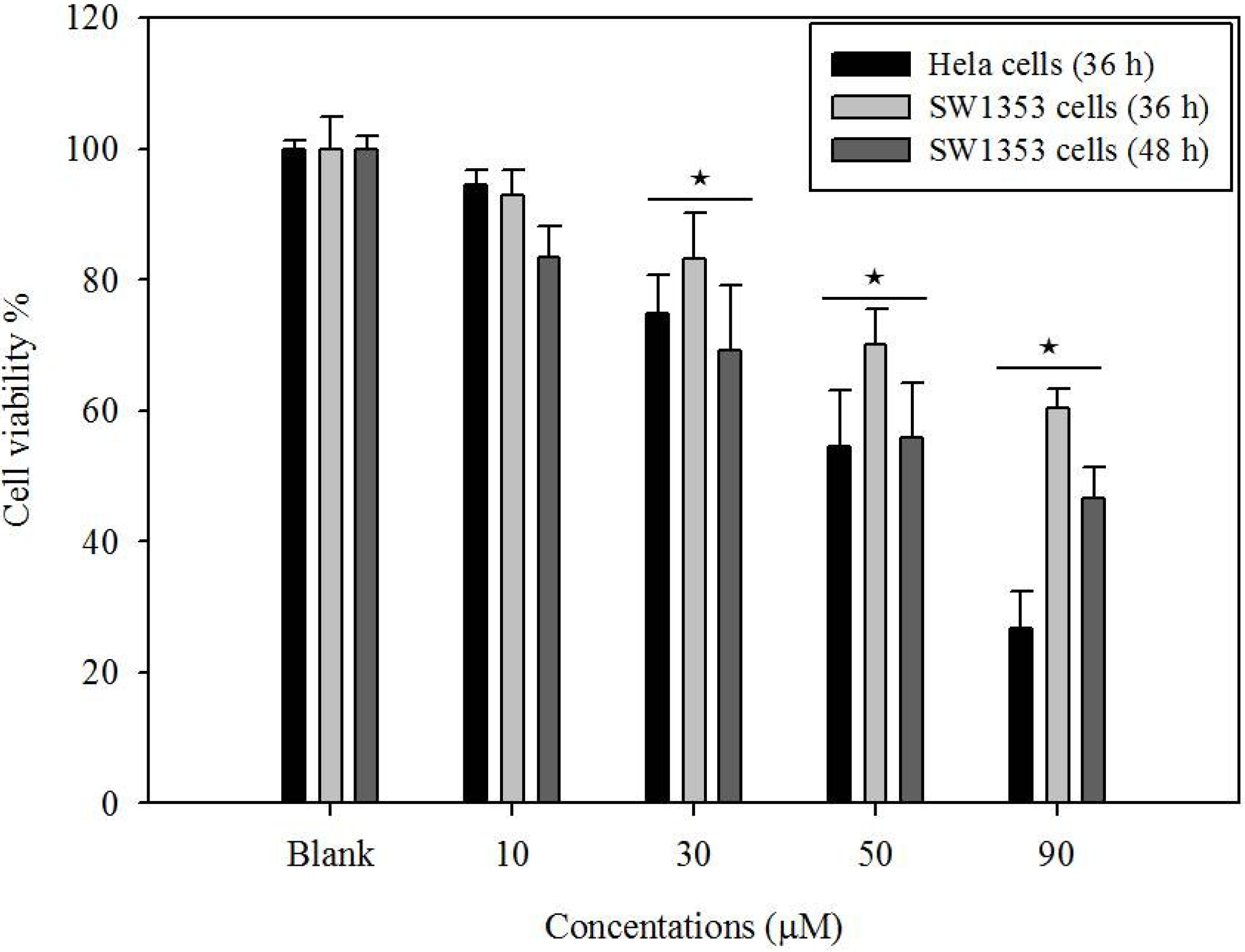
2.1. Cytotoxicity of Gliotoxin
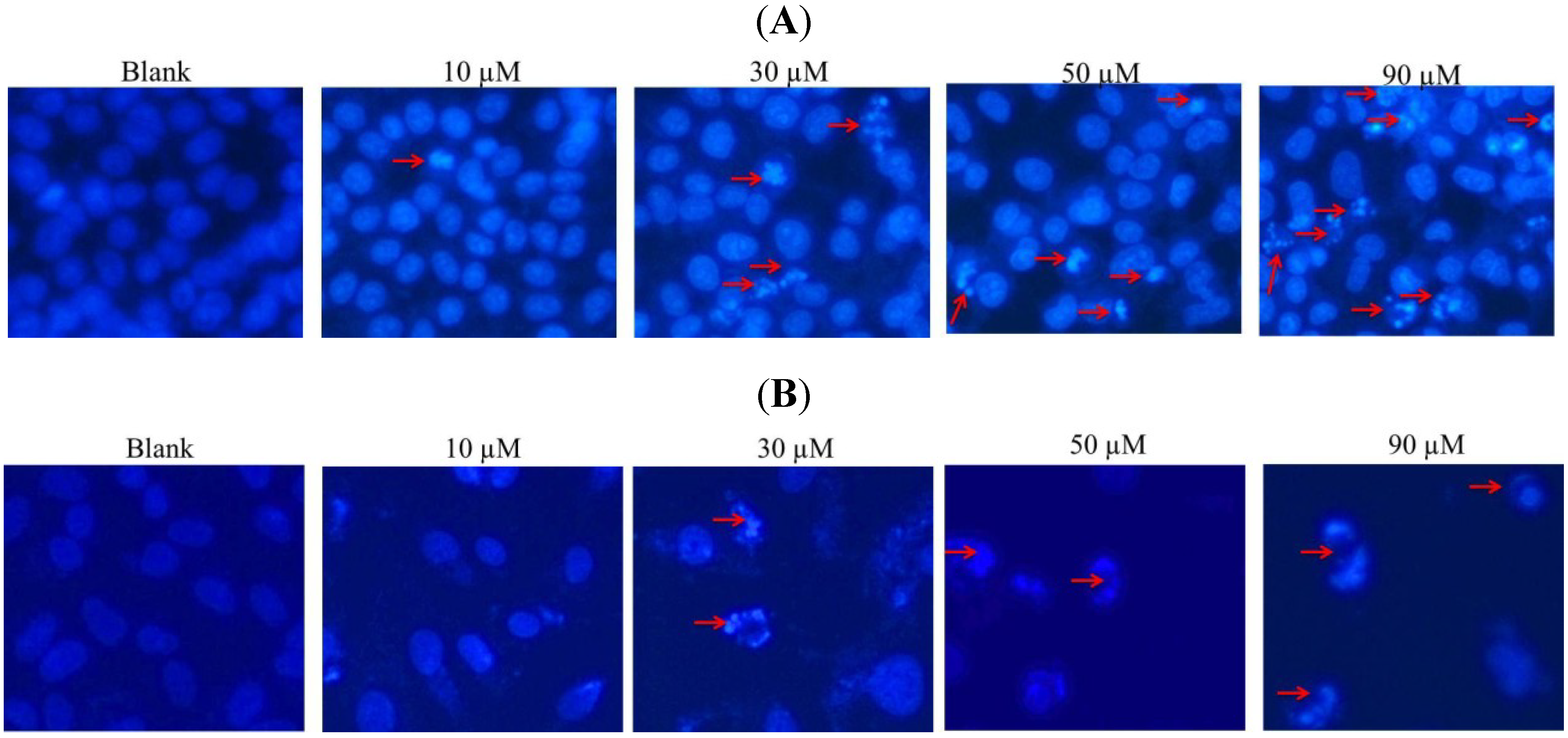
2.2. DAPI Staining of Hela and SW1353 Cells with Gliotoxin
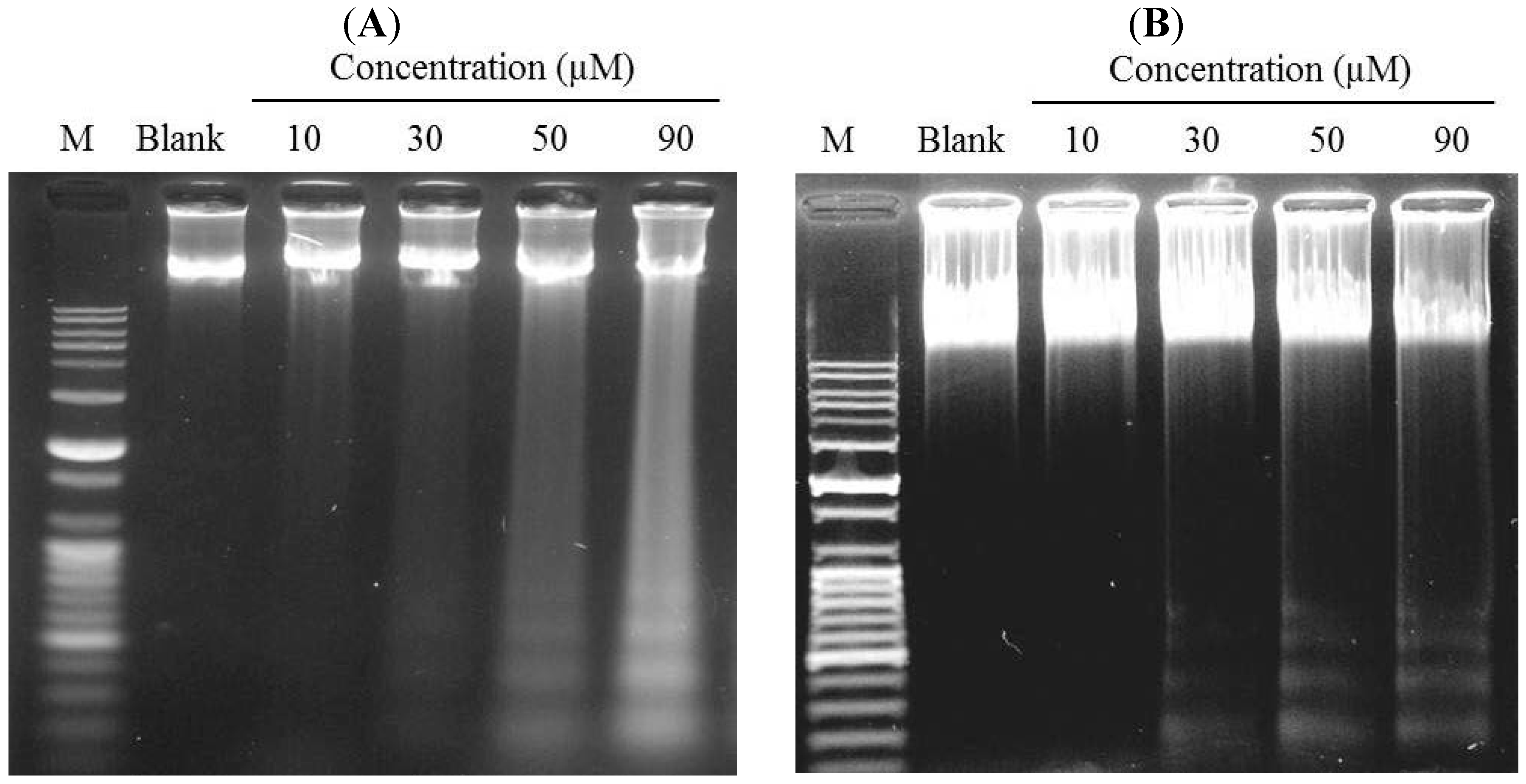
2.3. Induction of DNA Fragmentation
2.4. GuL Effects on Hela and SW1353 Cells Membrane Phosphatidylserine

2.5. Gliotoxin Disrupted ΔΨm in Hela and SW1353 Cells
2.6. Effects of Gliotoxin on Protein and Gene Expression Levels in HeLa and SW1353 Cells
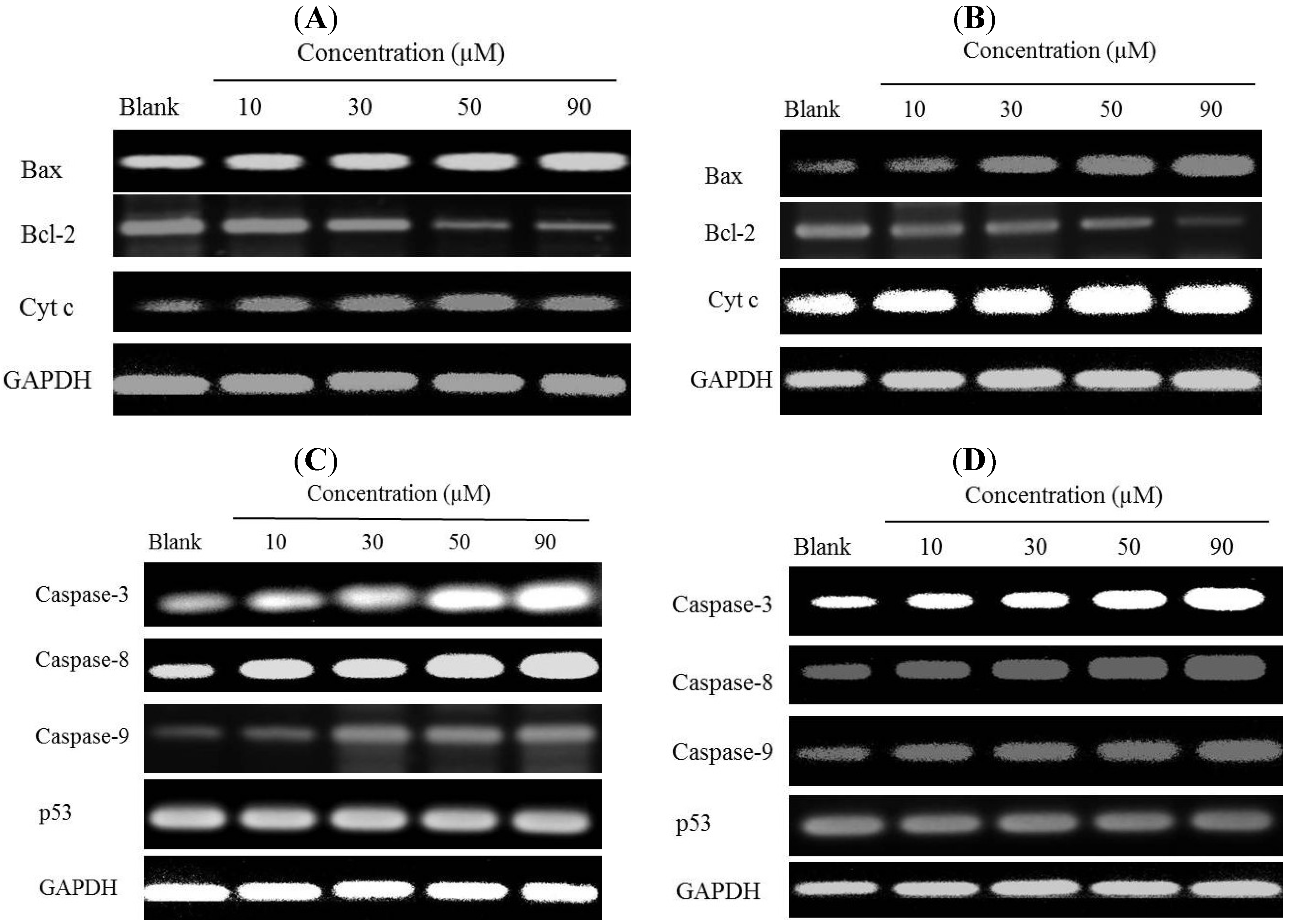

2.7. Discussion
3. Experimental Section
3.1. Materials
3.2. Extraction and Isolation of Gliotoxin
| Position | δH | δC |
|---|---|---|
| 1 | 166.0 (s) | |
| 3 | 77.2 (s) | |
| 3a | 3.44 (1H, dd, J = 4.8), 4.28 (1H, dd, J = 9.9) | 60.5 (t) |
| 4 | 165.2 (s) | |
| 5a | 4.39 (1H, dd, J = 6.8) | 69.8 (d) |
| 6 | 4.84 (1H, m ) | 75.6 (d) |
| 7 | 5.78(1H, d, J = 9.9) | 129.9 (d) |
| 8 | 5.95 (1H, m) | 123.4 (d) |
| 9 | 6.00 (1H, m) | 120.2 (d) |
| 9a | 130.7 (s) | |
| 10 | 2.96 (1H, d, J = 18.1), 3.73(1H, d, J = 18.1) | 36.6 (t) |
| 10a | 73.1 (s) | |
| 11 | 3.20 (3H, s) | 27.5 (q) |
3.3. Cell Culture and Treatment
3.4. Cell Viability Assay
3.5. Nuclear Staining with DAPI
3.6. Annexin V-FITC Labeling
3.7. DNA Fragmentation Assay
3.8. Measurement of Mitochondrial Membrane Potential (ΔΨm)
3.9. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.10. Protein Extraction and Western Blotting Assay
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Choi, Y.W.; Park, C.; Jin, C.Y.; Lee, Y.J.; Park, D.J.; Kim, S.G.; Kim, G.Y.; Choi, I.W.; Hwang, W.D.; et al. Apoptosis induction of human leukemia U937 cells by gomisin N, a dibenzocyclooctadiene lignan, isolated from Schizandra chinensis Baill. Food Chem. Toxicol. 2010, 48, 807–813. [Google Scholar] [CrossRef]
- Susan, E. Apoptosis, a review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Oikawa, Y.; Matsuda, E.; Nishii, T.; Ishida, Y.; Kawaichi, M. Down-regulation of CIBZ, a novel substrate of caspase-3, induces apoptosis. J. Biol. Chem. 2008, 283, 14242–14247. [Google Scholar] [CrossRef]
- Park, C.; Shin, H.J.; Kim, G.Y.; Kwon, T.K.; Nam, T.J.; Kim, S.K.; Cheong, J.; Choi, I.W.; Choi, Y.H. Induction of apoptosis by streptochlorin isolated from Streptomyces sp. in human leukemic U937 cells. Toxicol. In Vitro 2008, 22, 1573–1581. [Google Scholar] [CrossRef]
- Li, H.N.; Nie, F.F.; Liu, W.; Dai, Q.S.; Lu, N.; Qi, Q.; Li, Z.Y.; You, Q.D.; Guo, Q.L. Apoptosis induction of oroxylin A in human cervical cancer HeLa cell line in vitro and in vivo. Toxicology 2009, 257, 80–85. [Google Scholar] [CrossRef]
- Barisić, K.; Petrik, J.; Rumora, L. Biochemistry of apoptotic cell death. Acta Pharm. 2003, 53, 151–164. [Google Scholar]
- Srivastava, R. Apoptosis, Cell Signaling, and Human Diseases Molecular Mechanisms, 2nd ed.; Humana Press: New York, NY, USA, 2007; p. 384. [Google Scholar]
- Yang, W.; Gong, X.; Zhao, X.; An, W.; Wang, X.; Wang, M. Capsaicin induces apoptosis in HeLa cells via Bax/Bcl-2 and caspase-3 pathways. Asian Pac. J. Trop. Med. 2006, 1, 3–4. [Google Scholar]
- Karagozlua, M.Z.; Kim, J.A.; Karadeniz, F.; Kong, C.S.; Kim, S.K. Antiproliferative effect of aminoderivatized chitooligosaccharides on AGS human gastric cancer cells. Process Biochem. 2010, 45, 1523–1528. [Google Scholar] [CrossRef]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis the p53 network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Dai, X. Apoptosis of Hela cell induced by celastrus orbiculatus thunb extract and primary mechanisms. Chin.-Ger. J. Clin. Oncol. 2011, 10, 666–668. [Google Scholar] [CrossRef]
- Li, Z.B.; Wang, J.Y.; Jiang, B.; Zhang, X.L.; An, L.J.; Bao, Y.M. Benzobijuglone, a novel cytotoxic compound from Juglans mandshurica, induced apoptosis in HeLa cervical cancer cells. Phytomed 2007, 14, 846–852. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, C.L.; Liu, J.F.; Fong, Y.C.; Hsu, S.F.; Li, T.M.; Su, Y.C.; Liu, S.H.; Tang, C.H. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Lett. 2010, 291, 20–30. [Google Scholar] [CrossRef]
- Krajarng, A.; Nakamura, Y.; Suksamrarn, S.; Watanapokasin, R. α-Mangostin Induces Apoptosis in Human Chondrosarcoma Cells through Down-regulation of ERK/JNK and Akt Signaling Pathway. J. Agric. Food Chem. 2011, 59, 5746–5754. [Google Scholar] [CrossRef]
- Shao, C.L.; Wang, C.Y.; Wei, M.Y.; Gu, Y.C.; She, Z.G.; Qian, P.Y.; Lin, Y.C. Aspergilones A and B, two benzylazaphilones with an unprecedented carbon skeleton from the gorgonian-derived fungus Aspergillus sp. Bioorg. Med. Chem. Lett. 2011, 21, 690–693. [Google Scholar]
- Wei, M.Y.; Wang, C.Y.; Liu, Q.A.; Shao, C.L.; She, Z.G.; Lin, Y.C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a Gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef]
- Lee, D.S.; Jeong, G.S.; Li, B.; Lee, S.U.; Oh, H.C.; Kim, Y.C. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts antiinflammatory effects through heme oxygenase-1 expression in murine macrophages. J. Pharmacol. Sci. 2011, 116, 283–295. [Google Scholar] [CrossRef]
- Axelsson, V.; Holback, S.; Sjogren, M.; Gustafsson, H.; Forsby, A. Gliotoxin induces caspase-dependent neurite degeneration and calpain-mediated general cytotoxicity in differentiated human neuroblastoma SH-SY5Y cells. Biochem. Biophys. Res. Commun. 2006, 345, 1068–1074. [Google Scholar] [CrossRef]
- Comera, C.; Andre, K.; Laffitte, J.; Collet, X.; Galtier, P.; Isabelle, M.P. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes Infect. 2007, 94, 47–54. [Google Scholar]
- Nieminen, S.M.; Jorm, M.P.; Hirvonen, M.R.; Roponen, M.; Wright, A.V. Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat. Res. 2002, 520, 161–170. [Google Scholar] [CrossRef]
- Anselmi, K.; Stolz, D.B.; Nalesnik, M.; Watkins, S.C.; Kamath, R.; Gandhi, C.R. Gliotoxin causes apoptosis and necrosis of rat Kupffer cells in vitro and in vivo in the absence of oxidative stress, Exacerbation by caspase and serine protease inhibition. J. Hepatol. 2007, 47, 103–113. [Google Scholar] [CrossRef]
- Kweon, Y.O.; Paik, Y.H.; Schnabl, B.; Qian, T.; Lemasters, J.J.; Brenner, D.A. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J. Hepatol. 2003, 39, 38–46. [Google Scholar] [CrossRef]
- Xiaoming, Z.; Zhao, A.; Goping, G.; Hirszel, P. Gliotoxin-induced cytotoxicity proceeds via apoptosis and is mediated by caspases and reactive oxygen species in LLC-PK1 cells. Toxicol. Sci. 2000, 54, 194–202. [Google Scholar] [CrossRef]
- Qu, X.; Qing, L. Abrin Induces HeLa cell apoptosis by cytochrome c release and caspase activation. J. Biochem. Mol. Biol. 2004, 37, 445–453. [Google Scholar] [CrossRef]
- Li, X.; Ye, H.; Cai, L.; Yu, F.; Chen, W.; Lin, R.; Zheng, C.; Xu, H.; Ye, J.; Wu, G.; Liu, X. Millimeter wave radiation induces apoptosis via affecting the ratio of Bax/Bcl-2 in SW1353 human chondrosarcoma cells. Oncol. Rep. 2012, 27, 664–672. [Google Scholar]
- Ju, H.K.; Lee, H.W.; Chung, K.S.; Choi, J.H.; Cho, J.G.; Baek, N.I.; Chung, H.G.; Lee, K.T. Standardized flavonoid-rich fraction of Artemisia princeps Pampanini cv. Sajabal induces apoptosis via mitochondrial pathway in human cervical cancer HeLa cells. J. Ethnopharmacol. 2012, 141, 460–468. [Google Scholar] [CrossRef]
- Pardo, J.; Urban, C.; Galvez, E.M.; Ekert, P.G.; Müller, U.; Kwon-Chung, J.; Lobigs, M.; Müllbacher, A.; Wallich, R.; Borner, C.; et al. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J. Cell Biol. 2006, 174, 509–519. [Google Scholar] [CrossRef]
- Geissler, A.; Haun, F.; Frank, D.O.; Wieland, K.; Simon, M.M.; Idzko, M.; Davis, R.J.; Maurer, U.; Borner, C. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 2013, 20, 1317–1329. [Google Scholar] [CrossRef]
- Wright, M.C.; Issa, R.; Smart, D.E.; Trim, N.; Murray, G.I.; Primrose, J.N.; Arthur, M.J.; Iredale, J.P.; Mann, D.A. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 2001, 121, 685–698. [Google Scholar] [CrossRef]
- Upperman, J.S.; Potoka, D.A.; Zhang, X.R.; Wong, K.; Zamora, R.; Ford, H.R. Mechanism of intestinal-derived fungal sepsis by gliotoxin, a fungal metabolite. J. Pediatr. Surg. 2003, 38, 966–970. [Google Scholar] [CrossRef]
- Qian, Z.J.; Zhang, C.; Li, Y.X.; Je, J.Y.; Kim, S.K.; Jung, W.K. Protective effects of emodin and chrysophanol isolated from marine fungus Aspergillus sp. on ethanol-induced toxicity in HepG2/CYP2E1 cells. Evid. Based Complement Alternat. Med. 2011, 2011, 1–7. [Google Scholar]
- Mou, H.; Zheng, Y.; Zhao, P.; Bao, H.; Fang, W.; Xu, N. Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol. In Vitro 2011, 25, 1027–1032. [Google Scholar] [CrossRef]
- Gupta, S.; Radha, V.; Furukawa, Y.; Swarup, G. Direct transcriptional activation of human caspase-1 by tumor suppressor p53. J. Biol. Chem. 2001, 276, 10585–10588. [Google Scholar] [CrossRef]
- Lee, S.H.; Ryu, B.M.; Je, J.Y.; Kim, S.K. Diethylaminoethyl chitosan induces apoptosis in HeLa cells via activation of caspase-3 and p53 expression. Carbohydr. Polym. 2011, 84, 571–578. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nguyen, V.-T.; Lee, J.S.; Qian, Z.-J.; Li, Y.-X.; Kim, K.-N.; Heo, S.-J.; Jeon, Y.-J.; Park, W.S.; Choi, I.-W.; Je, J.-Y.; et al. Gliotoxin Isolated from Marine Fungus Aspergillus sp. Induces Apoptosis of Human Cervical Cancer and Chondrosarcoma Cells. Mar. Drugs 2014, 12, 69-87. https://doi.org/10.3390/md12010069
Nguyen V-T, Lee JS, Qian Z-J, Li Y-X, Kim K-N, Heo S-J, Jeon Y-J, Park WS, Choi I-W, Je J-Y, et al. Gliotoxin Isolated from Marine Fungus Aspergillus sp. Induces Apoptosis of Human Cervical Cancer and Chondrosarcoma Cells. Marine Drugs. 2014; 12(1):69-87. https://doi.org/10.3390/md12010069
Chicago/Turabian StyleNguyen, Van-Tinh, Jung Suck Lee, Zhong-Ji Qian, Yong-Xin Li, Kil-Nam Kim, Soo-Jin Heo, You-Jin Jeon, Won Sun Park, Il-Whan Choi, Jae-Young Je, and et al. 2014. "Gliotoxin Isolated from Marine Fungus Aspergillus sp. Induces Apoptosis of Human Cervical Cancer and Chondrosarcoma Cells" Marine Drugs 12, no. 1: 69-87. https://doi.org/10.3390/md12010069







