Apo-9′-Fucoxanthinone, Isolated from Sargassum muticum, Inhibits CpG-Induced Inflammatory Response by Attenuating the Mitogen-Activated Protein Kinase Pathway
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effects of SME on Pro-Inflammatory Cytokine Production in CpG DNA-Stimulated BMDMs and BMDCs
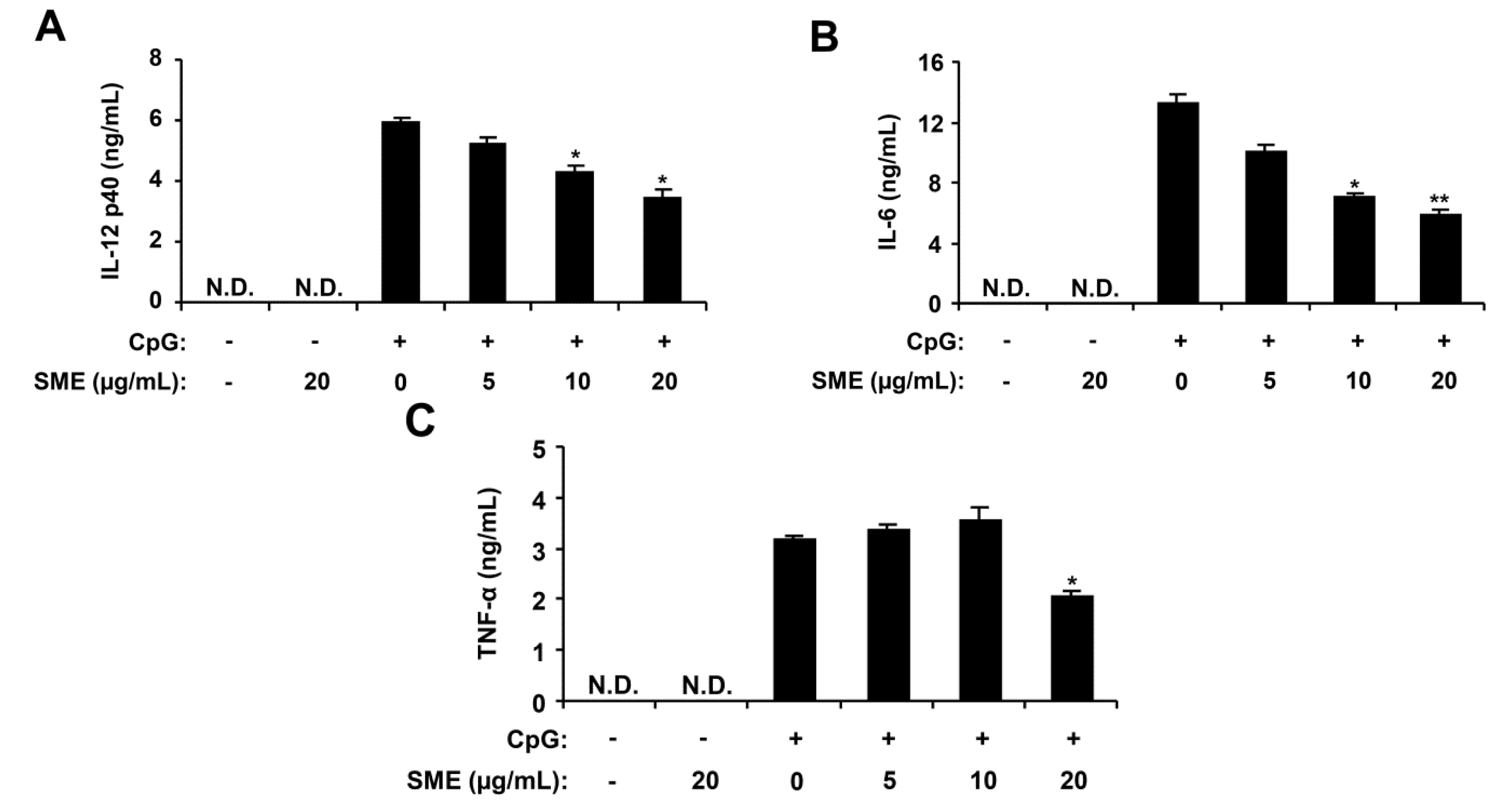
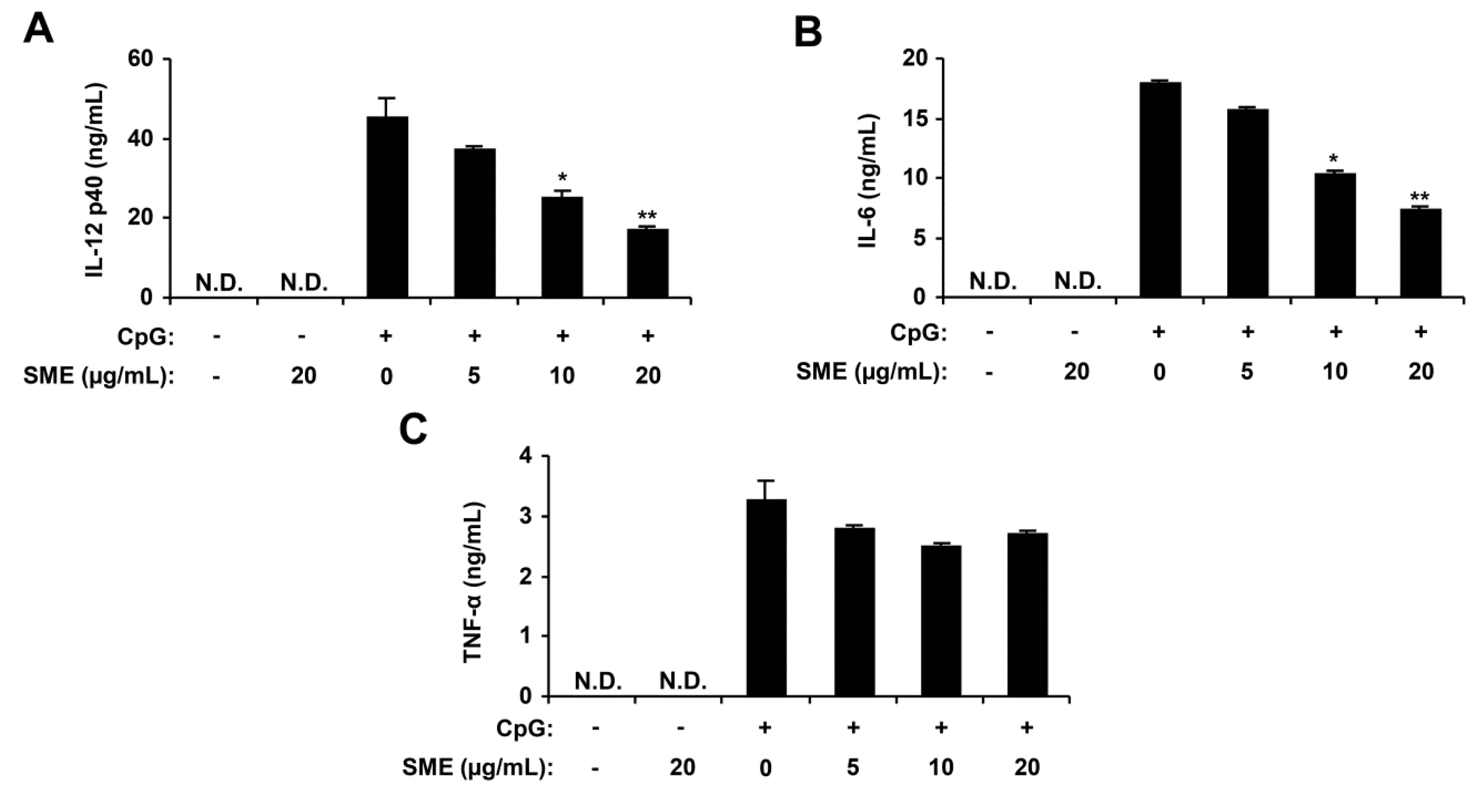
2.2. Inhibitory Effects of APO-9′ on Pro-Inflammatory Cytokine Production in CpG DNA-Stimulated BMDMs and BMDCs
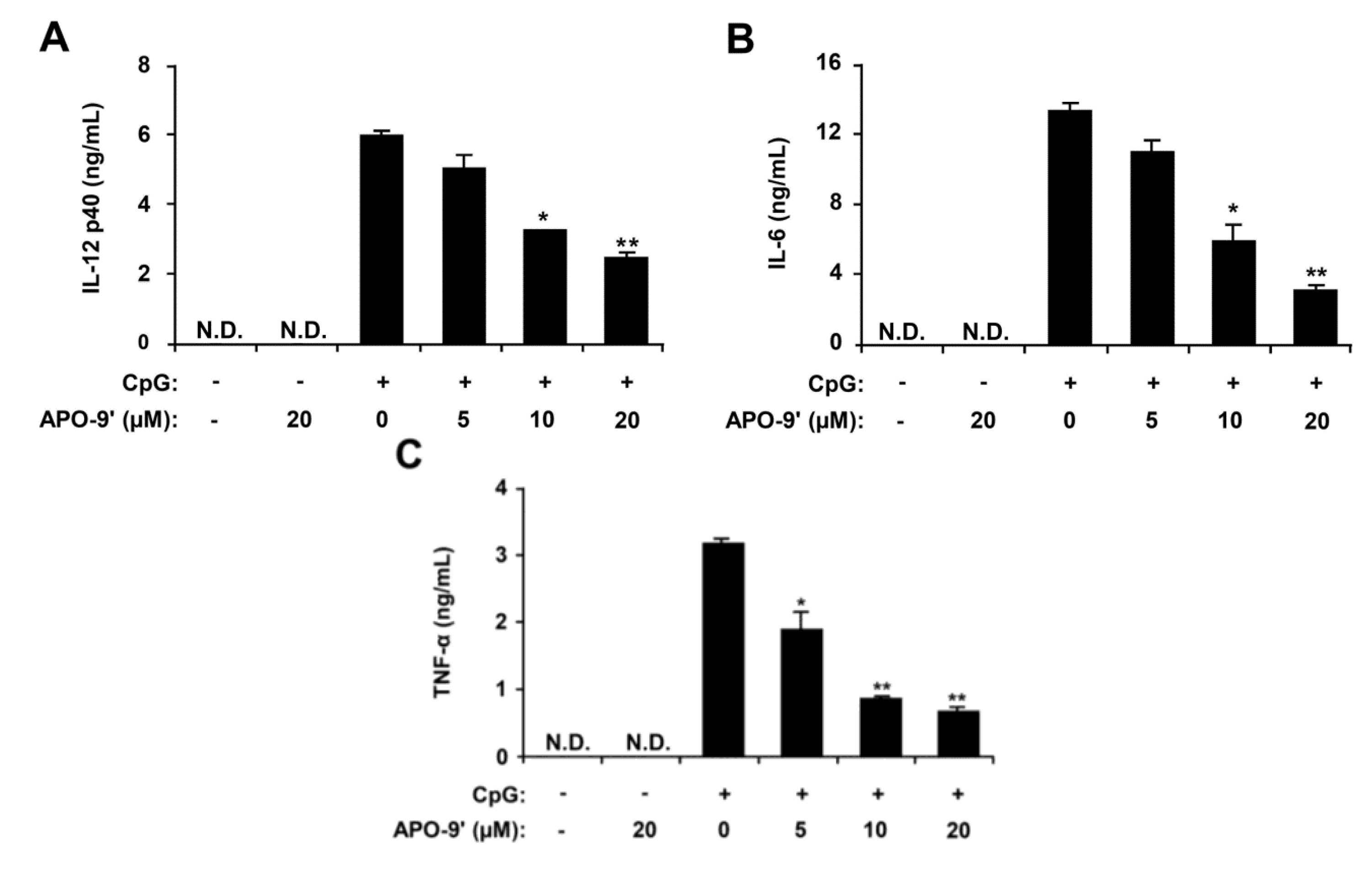

2.3.Effects of APO-9′ on the Phosphorylation of MAPK and the Degradation of IκBα by CpG-Stimulated BMDMs
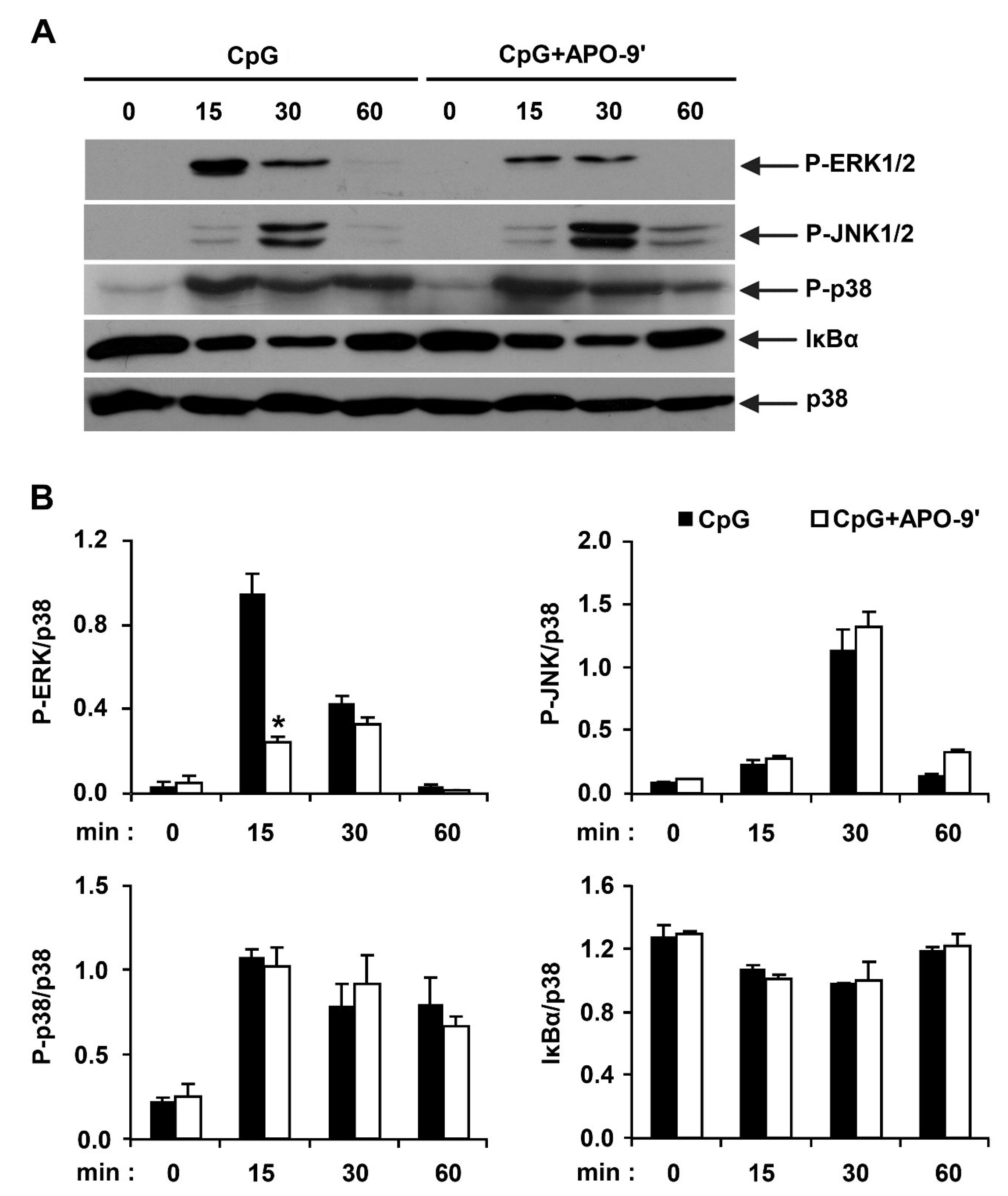
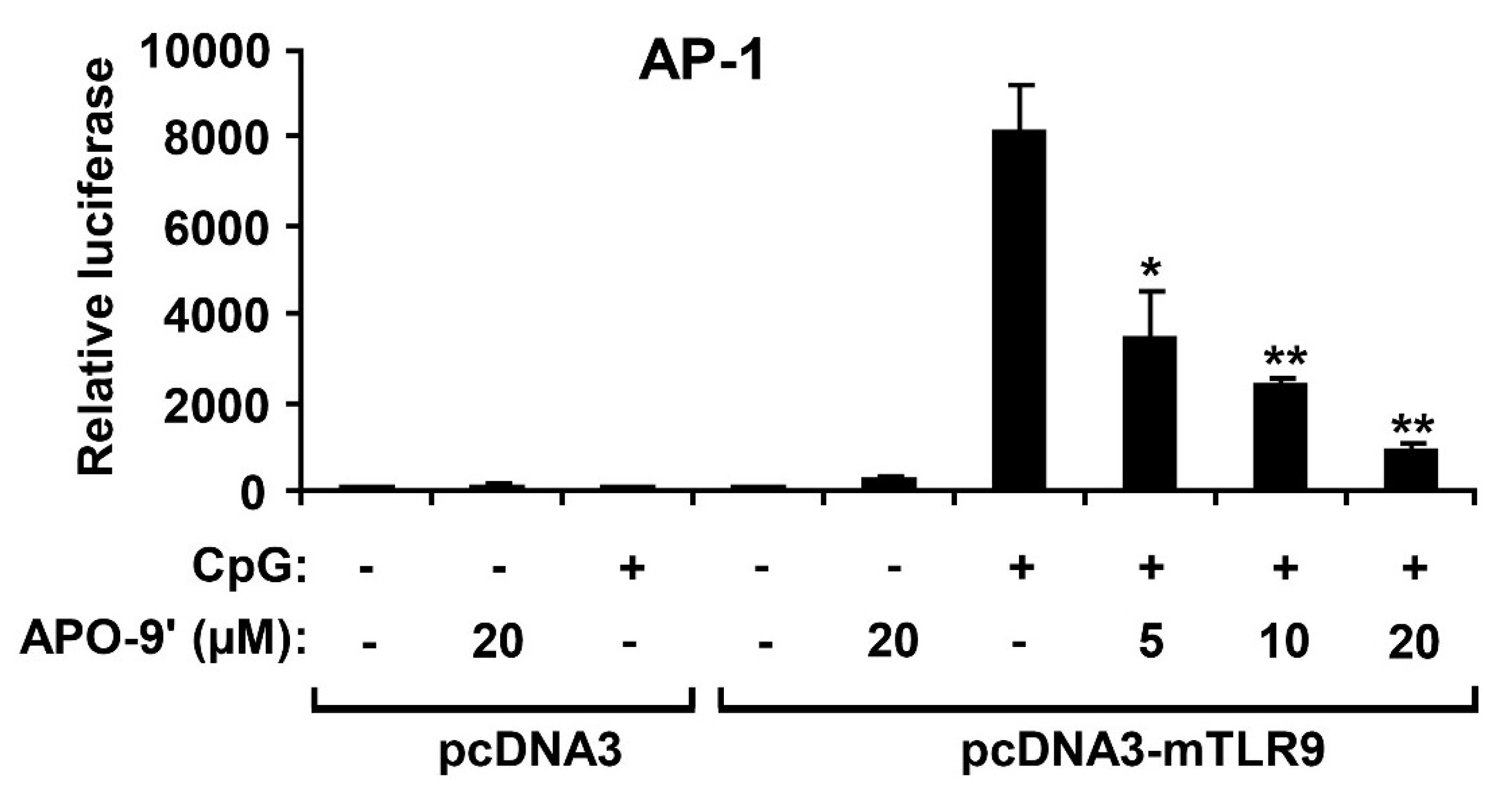
2.4. APO-9′ Treatment Inhibited AP-1 Reporter Activity in HEK293T Cells
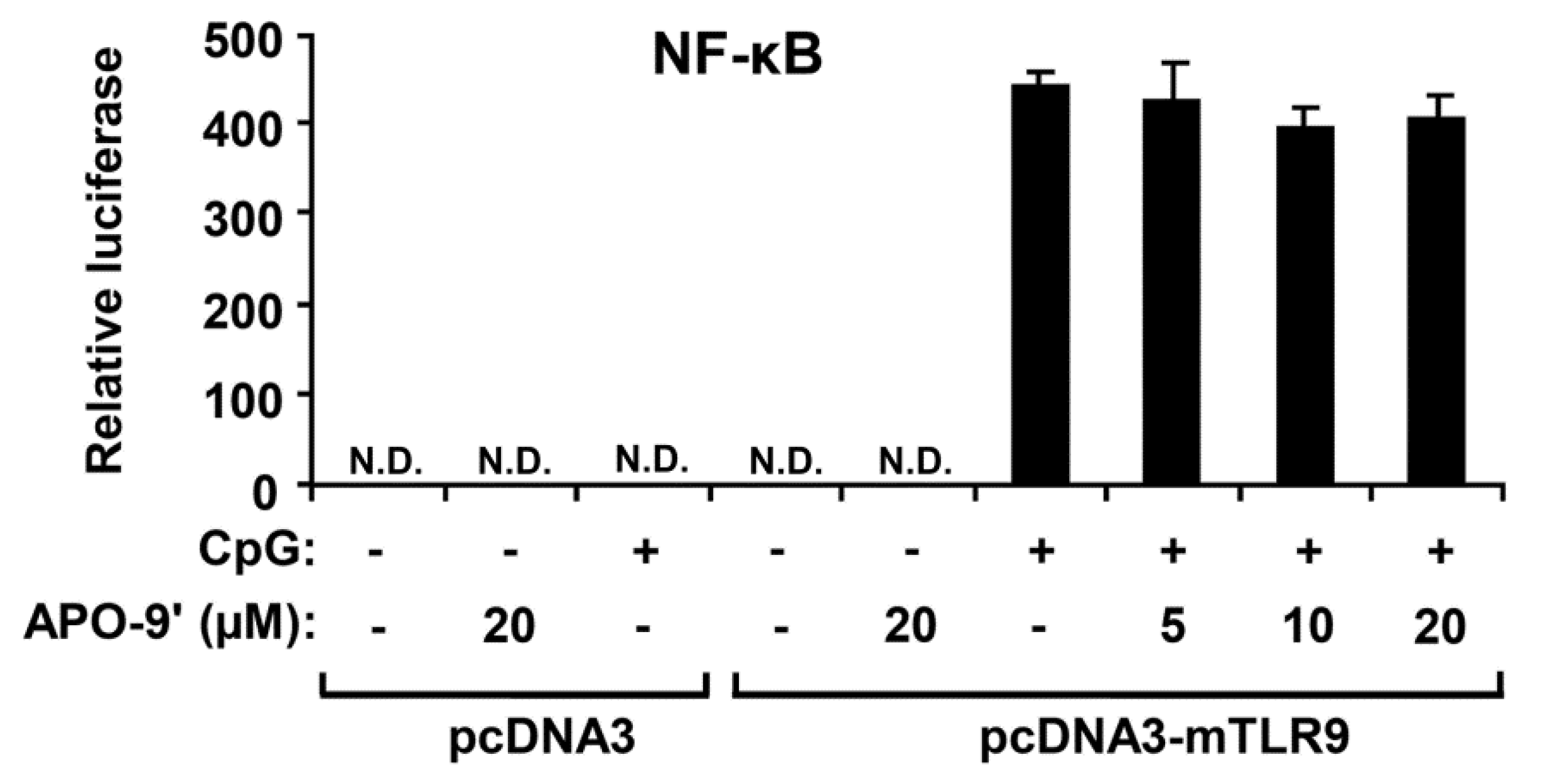
2.5.APO-9′ Treatment Did Not Inhibit NF-κB Reporter Activity in HEK293T Cells
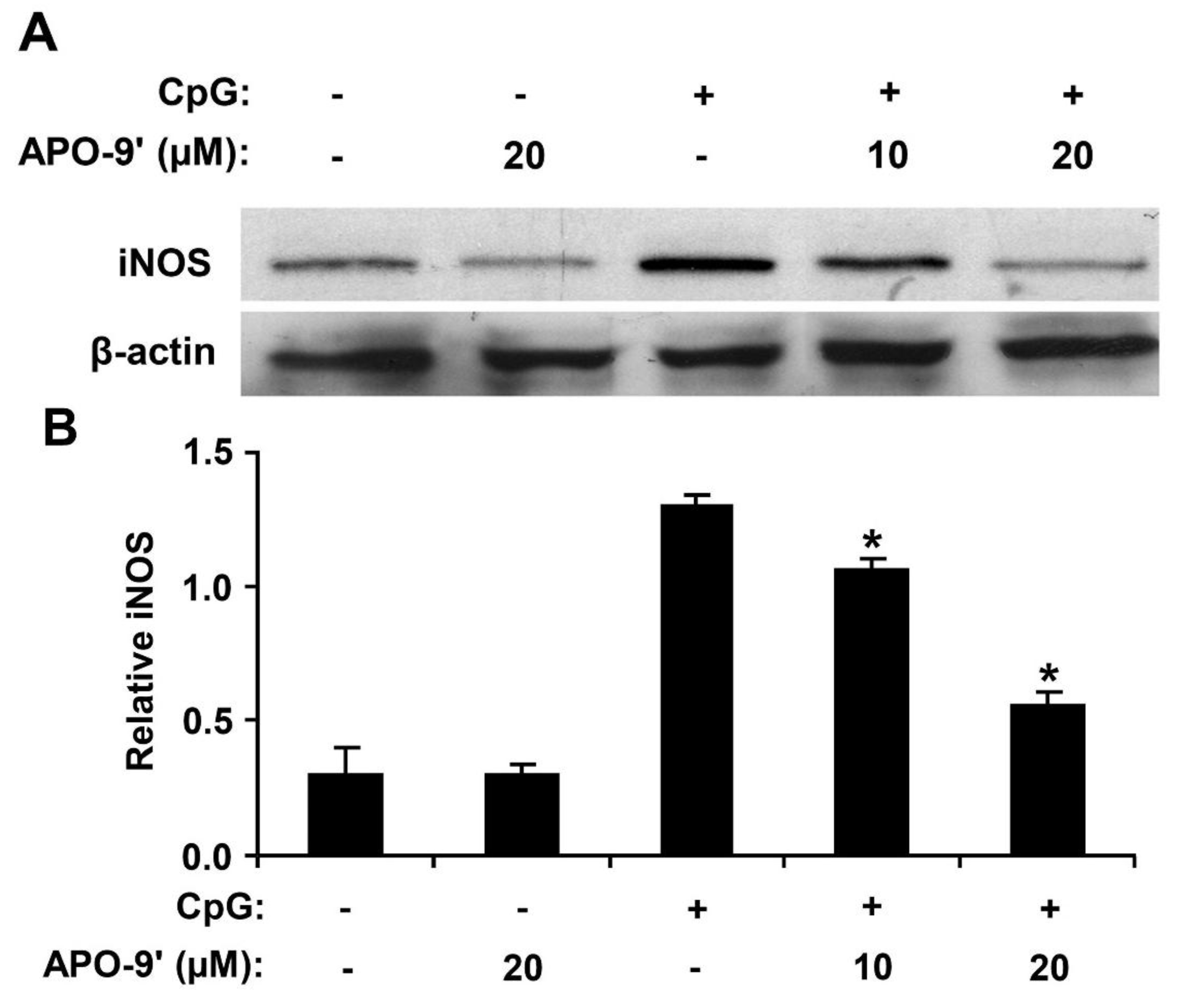
2.6. Effect of APO-9′ on the Production of iNOS in CpG DNA-Stimulated RAW264.7 Cells
3. Discussion
4. Experimental Section
4.1. Preparation of S. muticum Extract (SME)
4.2. Isolation of Apo-9′-fucoxanthinone from SME
4.3. Mice
4.4. Cell Cultures and Measurement of Cytokine Production
4.5. Cell Viability Assay
4.6. Western Blot Analysis
4.7. Luciferase Assay
4.8. Data Analysis
5. Conclusions
Acknowledgment
Abbreviations
| IC50 | half maximal inhibitory concentration |
| N.D. | not detectable |
| NMR | nuclear magnetic resonance |
| AP-1 | activator protein-1 |
| DMSO | dimethyl sulfoxide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| RPMI | Roswell park memorial institute medium |
| FBS | fetal bovine serum |
| SDS | sodium dodecyl sulfate |
Conflicts of Interest
References
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Roach, J.C.; Glusman, G.; Rowen, L.; Kaur, A.; Purcell, M.K.; Smith, K.D.; Hood, L.E.; Aderem, A. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 9577–9582. [Google Scholar]
- Efron, P.A.; Tsujimoto, H.; Bahjat, F.R.; Ungaro, R.; Debernardis, J.; Tannahill, C.; Baker, H.V.; Edwards, C.K.; Moldawer, L.L. Differential maturation of murine bone-marrow derived dendritic cells with lipopolysaccharide and tumor necrosis factor-α. J. Endotoxin Res. 2005, 11, 145–160. [Google Scholar]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptorssignalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J.H. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2002, 52, 144–151. [Google Scholar]
- Manzoor, Z.; Koh, Y.S. Mitogen-activated protein kinases in inflammation. J. Bacteriol. Virol. 2012, 42, 189–195. [Google Scholar] [CrossRef]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Yasuda, K.; Richez, C.; Uccellini, M.B.; Richards, R.J.; Bonegio, R.G.; Akira, S.; Monestier, M.; Corley, R.B.; Viglianti, G.A.; Marshak-Rothstein, A.; et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J. Immunol. 2009, 183, 3109–3117. [Google Scholar] [CrossRef]
- Koh, Y.S. Nucleic acid recognition and signaling by Toll-like receptor 9: Compartment-dependent regulation. J. Bacteriol. Virol. 2011, 41, 131–132. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 2008, 10 (Suppl. 2), S2. [Google Scholar] [CrossRef]
- Hesslinger, C.; Strub, A.; Boer, R.; Ulrich, W.R.; Lehner, M.D.; Braun, C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem. Soc. Trans. 2009, 37, 886–891. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar]
- Lee, H.J.; Hyun, E.A.; Yoon, W.J.; Kim, B.H.; Rhee, M.H.; Kang, H.K.; Cho, J.Y.; Yoo, E.S. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J. Ethnopharmacol. 2006, 103, 208–216. [Google Scholar]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.A.; Kim, K.N.; Yoon, W.J.; Lee, W.J.; Park, S.Y. Antioxidative and antimicrobial activities of Sargassum muticum extracts. J. Korean Soc. Food Sci. Nutr. 2007, 36, 663–669. [Google Scholar] [CrossRef]
- Yoon, W.J.; Ham, Y.M.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Brown alga Sargassum muticum inhibits proinflammatory cytokines, iNOS, and COX-2 expression in macrophage RAW 264.7 cells. Turk. J. Biol. 2010, 34, 25–34. [Google Scholar]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. In Vitro 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 371–392. [Google Scholar]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Doi, Y.; Ishibashi, M.; Yamaguchi, N.; Kobayashi, J. Isolation of Apo-9′-fucoxanthinone from the cultured marine Dinoflagellate amphidinium sp. J. Nat. Prod. 1995, 58, 1097–1099. [Google Scholar]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and Its Metabolites in Edible Brown Algae Cultivated in Deep Seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar]
- Bromberg, J.; Wang, T.C. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009, 15, 79–80. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Bao, L.; Lindgren, J.U.; van der Meide, P.; Zhu, S.; Ljunggren, H.G.; Zhu, J. The critical role of IL-12 p40 in initiating, enhancing, and perpetuating pathogenic events in murine experimental autoimmune neuritis. Brain Pathol. 2002, 12, 420–429. [Google Scholar]
- Ishii, K.J.; Koyama, S.; Nakagawa, A.; Coban, C.; Akira, S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 2008, 3, 352–363. [Google Scholar]
- Heinrich, P.C.; Behrmann, I.; Muller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar]
- Trikha, M.; Corringham, R.; Klein, B.; Rossi, J.F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin. Cancer Res. 2003, 9, 4653–4665. [Google Scholar]
- Gadó, K.; Domján, G.; Hegyesi, H.; Falus, A. Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell Biol. Int. 2000, 24, 195–209. [Google Scholar] [CrossRef]
- McInnes, I.B.; Leung, B.P.; Sturrock, R.D.; Field, M.; Liew, F.Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat. Med. 1997, 3, 189–195. [Google Scholar] [CrossRef]
- González, S.; Rodrigo, L.; Martínez-Borra, J.; López-Vázquez, A.; Fuentes, D.; Niño, P.; Cadahía, V.; Saro, C.; Dieguez, M.A.; López-Larrea, C. TNF-alpha -308A promoter polymorphism is associated with enhanced TNF-alpha production and inflammatory activity in Crohn's patients with fistulizing disease. Am. J. Gastroenterol. 2003, 98, 1101–1106. [Google Scholar]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar]
- Kroncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Clancy, R.M.; Aminm, A.R.; Abramsonm, S.B. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998, 41, 1141–1151. [Google Scholar]
- Park, K.E.; Kim, Y.A.; Jung, H.A.; Lee, H.J.; Ahn, J.W.; Lee, B.J.; Seo, Y. Three Norisoprenoids from the brown alga Sargassum thunbergii. J. Korean Chem. Soc. 2004, 48, 394–398. [Google Scholar] [CrossRef]
- Koo, J.E.; Hong, H.J.; Dearth, A.; Kobayashi, K.S.; Koh, Y.S. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS ONE 2012, 7, e39042. [Google Scholar]
- Koo, J.E.; Hong, H.J.; Mathema, V.B.; Kang, H.K.; Hyun, J.W.; Kim, G.Y.; Kim, Y.R.; Maeng, Y.H.; Hyun, C.L.; Chang, W.Y.; et al. Inhibitory effects of Carpinus tschonoskii leaves extract on CpG-stimulated pro-inflammatory cytokine production in murine bone marrow-derived macrophages and dendritic cells. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 197–202. [Google Scholar] [CrossRef]
- Manzoor, Z.; Kim, S.; Chae, D.; Yoo, E.S.; Kang, H.K.; Hyun, J.W.; Lee, N.H.; Suh, I.S.; Koh, Y.S. Sea lettuce (Ulva fasciata) extract has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived macrophages and dendritic cells. Food Sci. Biotechnol. 2013, 22, 781–786. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chae, D.; Manzoor, Z.; Kim, S.C.; Kim, S.; Oh, T.-H.; Yoo, E.-S.; Kang, H.-K.; Hyun, J.-W.; Lee, N.H.; Ko, M.-H.; et al. Apo-9′-Fucoxanthinone, Isolated from Sargassum muticum, Inhibits CpG-Induced Inflammatory Response by Attenuating the Mitogen-Activated Protein Kinase Pathway. Mar. Drugs 2013, 11, 3272-3287. https://doi.org/10.3390/md11093272
Chae D, Manzoor Z, Kim SC, Kim S, Oh T-H, Yoo E-S, Kang H-K, Hyun J-W, Lee NH, Ko M-H, et al. Apo-9′-Fucoxanthinone, Isolated from Sargassum muticum, Inhibits CpG-Induced Inflammatory Response by Attenuating the Mitogen-Activated Protein Kinase Pathway. Marine Drugs. 2013; 11(9):3272-3287. https://doi.org/10.3390/md11093272
Chicago/Turabian StyleChae, Doobyeong, Zahid Manzoor, Sung Chun Kim, Sohyun Kim, Tae-Heon Oh, Eun-Sook Yoo, Hee-Kyoung Kang, Jin-Won Hyun, Nam Ho Lee, Mi-Hee Ko, and et al. 2013. "Apo-9′-Fucoxanthinone, Isolated from Sargassum muticum, Inhibits CpG-Induced Inflammatory Response by Attenuating the Mitogen-Activated Protein Kinase Pathway" Marine Drugs 11, no. 9: 3272-3287. https://doi.org/10.3390/md11093272



