Secondary Metabolites from Penicillium pinophilum SD-272, a Marine Sediment-Derived Fungus
Abstract
:1. Introduction
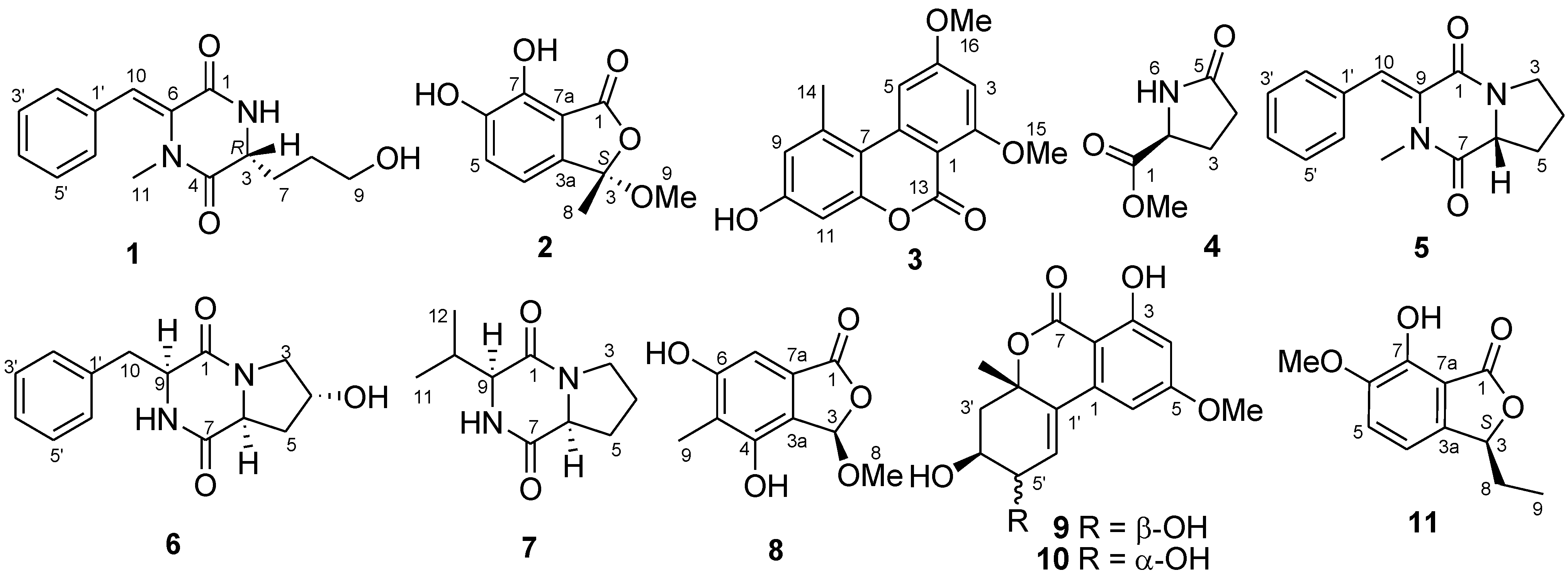
2. Results and Discussion
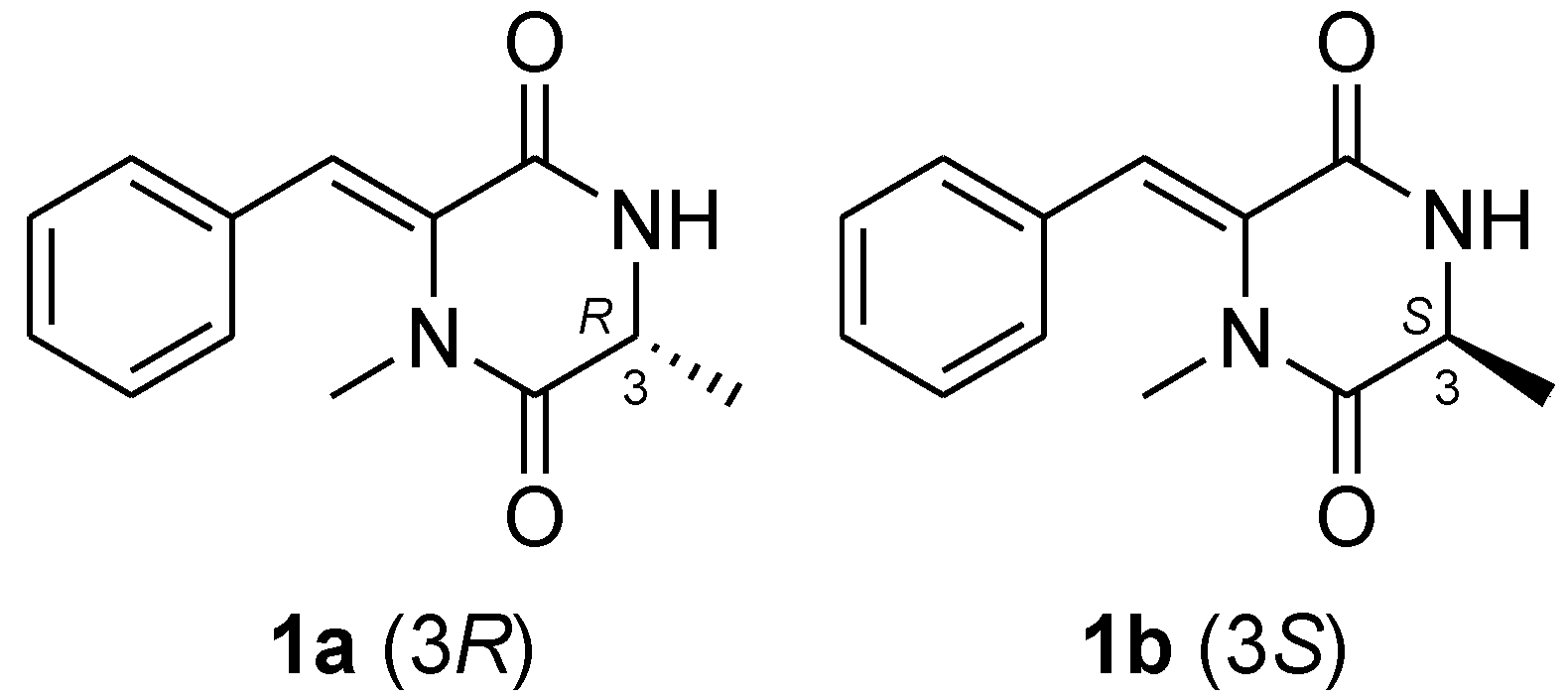
2.1. Structure Elucidation of the New Compounds

| No. | δC | δH ( J in Hz) | No. | δC | δH ( J in Hz) |
|---|---|---|---|---|---|
| 1 | 162.5, C | 9 | 60.1, CH2 | 3.40, m | |
| 2-NH | 8.62, d (2.8) | 10 | 118.1, CH | 6.97, s | |
| 3 | 55.0, CH | 3.94, m | 11 | 34.5, CH3 | 2.73, s |
| 4 | 167.9, C | 1′ | 133.8, C | ||
| 6 | 132.4, C | 2′/6′ | 129.2, CH | 7.42, d (7.5) | |
| 7 | 30.6, CH2 | a 1.79, m | 3′/5′ | 128.3, CH | 7.30, t (7.5) |
| b 1.71, m | 4′ | 128.1, CH | 7.34, t (7.5) | ||
| 8 | 28.0, CH2 | 1.51, m | OH | 4.48, br s |

| No. | δC | δH ( J in Hz) | No. | δC | δH ( J in Hz) |
|---|---|---|---|---|---|
| 1 | 167.6, C | 6 | 146.6, C | ||
| 3 | 108.6, C | 7 | 151.1, C | ||
| 3a | 132.9, C | 7a | 114.6, C | ||
| 4 | 121.2, CH | 6.84, d (8.5) | 8 | 25.6, CH3 | 1.74, s |
| 5 | 125.7, CH | 7.01, d (8.5) | 9 | 52.4, CH3 | 2.94, s |
2.2. Biological Activities of the Isolated Compounds
3. Experimental Section
3.1. General
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
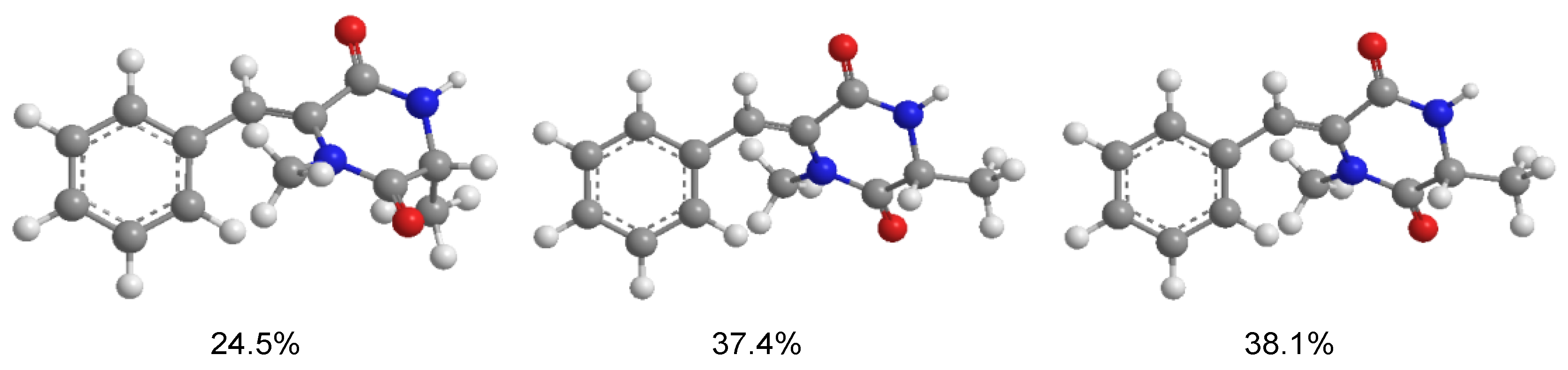
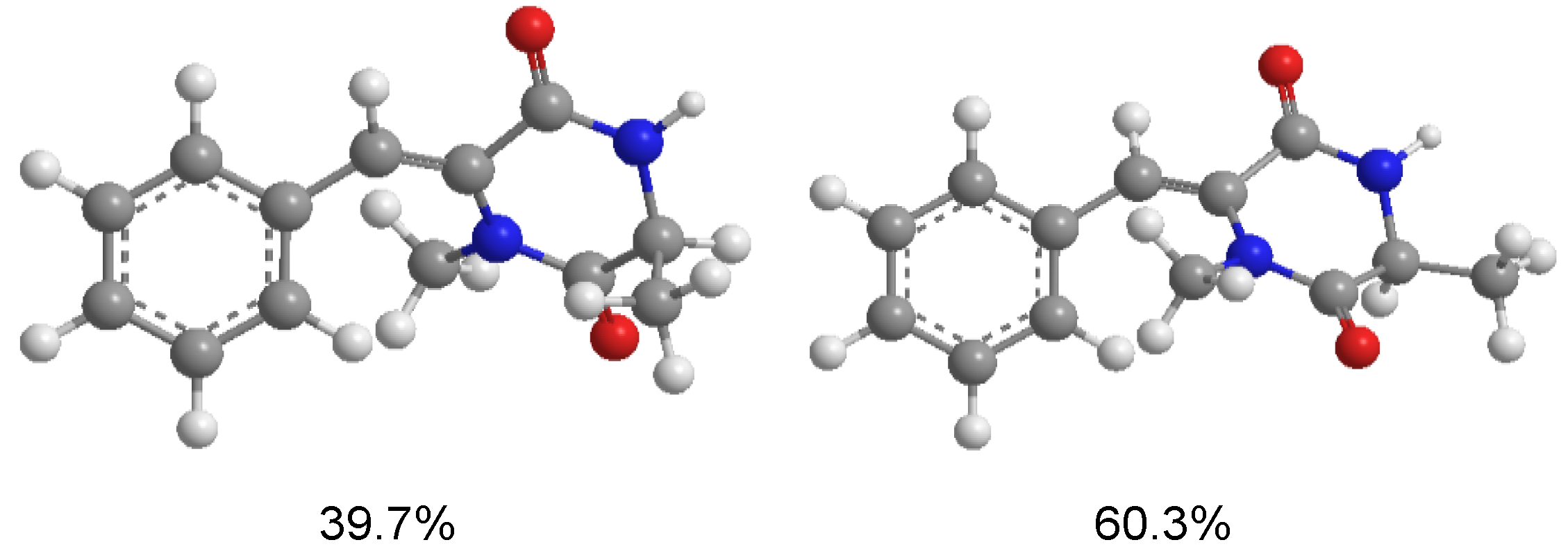
3.5. Computational Details
3.6. Antibacterial Assays
3.7. Brine Shrimp (Artemia salina) Lethality Assay
4. Conclusions
Acknowledgments
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 25, 35–94. [Google Scholar] [CrossRef]
- Wu, Q.X.; Crews, M.S.; Draskovic, M.; Sohn, J.; Johnson, T.A.; Tenney, K.; Valeriote, F.A.; Yao, X.J.; Bjeldanes, L.F.; Crews, P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010, 12, 4458–4461. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Li, C.S.; An, C.Y.; Li, X.M.; Gao, S.S.; Cui, C.M.; Sun, H.F.; Wang, B.G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Du, F.Y.; Li, C.S.; Proksch, P.; Wang, B.G. Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Mar. Drugs 2011, 9, 59–70. [Google Scholar]
- Zhao, Y.; Chen, H.; Shang, Z.; Jiao, B.H.; Yuan, B.; Sun, W.Z.; Wang, B.G.; Miao, M.Y.; Huang, C.G. SD118-Xanthocillin X (1), a novel marine agent extracted from Penicillium commune, induces autophagy through the inhibition of the MEK/ERK pathway. Mar. Drugs 2012, 10, 1345–1359. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Li, C.S.; Shang, Z.; Wang, B.G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar]
- Sun, H.F.; Li, X.M.; Meng, L.; Cui, C.M.; Gao, S.S.; Li, C.S.; Huang, C.G.; Wang, B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012, 75, 148–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.M.; Shang, Z.; Li, C.S.; Ji, N.Y.; Wang, B.G. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J. Nat. Prod. 2012, 75, 1888–1895. [Google Scholar] [CrossRef]
- Sóti, F.; Incze, M.; Kajtár-Peredy, M.; Baitz-Gács, E.; Imre, L.; Farkas, L. Synthese natürlicher dibenzo-α-pyrone, II. Synthese des alternariols und des alternariol-9-methylethers. Chem. Ber. 1977, 110, 979–984. [Google Scholar] [CrossRef]
- Tan, N.; Tao, Y.W.; Pan, J.H.; Wang, S.Y.; Xu, F.; She, Z.G.; Lin, Y.C.; Jones, E.B.G. Isolation, structure elucidation, and mutagenicity of four alternariol derivatives produced by the mangrove endophytic fungus No. 2240. Chem. Nat. Compd. 2008, 44, 296–300. [Google Scholar] [CrossRef]
- Drauz, K.; Kleemann, A.; Martens, J.; Scherberich, P.; Effenberger, F. Amino acids. 7. A novel synthetic route to l-proline. J. Org. Chem. 1986, 51, 3494–3498. [Google Scholar] [CrossRef]
- Liu, M.T; Lin, S.; Gan, M.L.; Chen, M.H.; Li, L.; Wang, S.J.; Zi, J.C.; Fan, X.N.; Liu, Y.; Si, Y.K.; et al. Yaoshanenolides A and B: New spirolactones from the Bark of Machilus yaoshansis. Org. Lett. 2012, 14, 1004–1007. [Google Scholar] [CrossRef]
- Hou, X.F.; Yao, S.; Mándi, A.; Kurtán, T.; Tang, C.P.; Ke, C.Q.; Li, X.Q.; Ye, Y. Bicunningines A and B, two new dimeric diterpenes from Cunninghamia lanceolata. Org. Lett. 2012, 14, 460–463. [Google Scholar] [CrossRef]
- Chou, T.H.; Chen, I.S.; Hwang, T.L.; Wang, T.C.; Lee, T.H.; Cheng, L.Y.; Chang, Y.C.; Cho, J.Y.; Chen, J.J. Phthalides from Pittosporum illicioides var. illicioides with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2008, 71, 1692–1695. [Google Scholar] [CrossRef]
- Poisel, H.; Schmidt, U. Asymmetrische synthese aromatischer α-aminosäuren und N-methyl-α-aminosäuren.—Synthese von l-Dopa.—Über die katalytische hydrierung ungesättigter cyclodipeptide. Chem. Ber. 1973, 106, 3408–3420. [Google Scholar] [CrossRef]
- Jin, S.; Wessig, P.; Liebscher, J. Unusual C=C bond migration in 3-ylidene-2,5-piperazinediones. Eur. J. Org. Chem. 2000, 2000, 1993–1999. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef]
- Kimura, Y.; Yoshinari, T.; Koshino, H.; Fujioka, S.; Okada, K.; Shimada, A. Rubralactone, rubralides A, B and C, and rubramin produced by Penicillium rubrum. Biosci. Biotechnol. Biochem. 2007, 71, 1896–1901. [Google Scholar] [CrossRef]
- Jiao, P.; Gloer, J.B.; Campbell, J.; Shearer, C.A. Altenuene derivatives from an unidentified freshwater fungus in the family Tubeufiaceae. J. Nat. Prod. 2006, 69, 612–615. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef]
- ChemAxon, Marvin 5.9.2; ChemAxon Ltd.: Budapest, Hungary, 2012.
- Bruhn, T.; Hemberger, Y.; Schaumloffel, A.; Bringmann, G. SpecDis; version 1.51; University of Wuerzburg: Wuerzburg, Germany, 2011. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Al-Burtamani, S.K.S.; Fatope, M.O.; Marwah, R.G.; Onifade, A.K.; Al-Saidi, S.H. Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol. 2005, 96, 107–112. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Proteau, P.J.; Nagle, D.G.; Hamel, E.; Blokhin, A.; Slate, D. Structure of curacin A, a novel antimitotic, antiproliferative, and brine shrimp toxic natural product from the marine Cyanobacterium Lyngbya majuscule. J. Org. Chem. 1994, 59, 1243–1245. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, M.-H.; Li, X.-M.; Li, C.-S.; Ji, N.-Y.; Wang, B.-G. Secondary Metabolites from Penicillium pinophilum SD-272, a Marine Sediment-Derived Fungus. Mar. Drugs 2013, 11, 2230-2238. https://doi.org/10.3390/md11062230
Wang M-H, Li X-M, Li C-S, Ji N-Y, Wang B-G. Secondary Metabolites from Penicillium pinophilum SD-272, a Marine Sediment-Derived Fungus. Marine Drugs. 2013; 11(6):2230-2238. https://doi.org/10.3390/md11062230
Chicago/Turabian StyleWang, Ming-Hui, Xiao-Ming Li, Chun-Shun Li, Nai-Yun Ji, and Bin-Gui Wang. 2013. "Secondary Metabolites from Penicillium pinophilum SD-272, a Marine Sediment-Derived Fungus" Marine Drugs 11, no. 6: 2230-2238. https://doi.org/10.3390/md11062230






