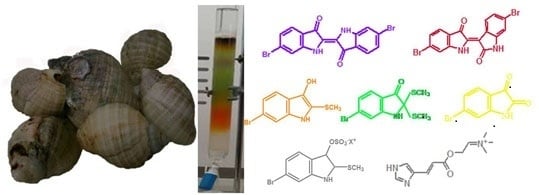
Natural Product Research in the Australian Marine Invertebrate Dicathais orbita
Abstract
:1. Introduction

2. Secondary Metabolites from Dicathais orbita
2.1. Brominated Indole Derivatives
| Compound | MW/Formula | Log pa | Polar surface area/volume | No. non-H atoms | No. H bond acceptors b | No. H bond donors c | Rotatable bonds | No. rule of 5 violations d |
|---|---|---|---|---|---|---|---|---|
Tyrindoxyl sulfate | 337.196 C9H7BrNO4S2− | −0.346 | 82.224/211.287 | 17 | 5 | 1 | 3 | 0 |
Tyrindoxyl | 258.14 C9H8BrNOS | 3.375 | 36.019/173.614 | 13 | 2 | 2 | 1 | 0 |
6 Bromoisatin | 226.029 C8H4BrNOS | 1.615 | 49.933/141.457 | 12 | 3 | 1 | 0 | 0 |
Tyrindoleninone | 256.124 C9H6BrNOS | 2.889 | 29.963/168.021 | 13 | 2 | 0 | 1 | 0 |
Tyrindolinone | 304.234 C10H10BrNOS2 | 2.999 | 29.098/208.356 | 15 | 2 | 1 | 2 | 0 |
Tyriverdin | 514.264 C18H14Br2N2O2S2 | 4.66 | 58.196/334.697 | 26 | 4 | 2 | 3 | 1 |
Tyrian purple 6,6′ dibromoindigo | 420.06 C16H8Br2N2O2 | 4.47 | 65.724/259.728 | 22 | 4 | 2 | 0 | 0 |
6,6′ Dibromoindirubin | 420.06 C16H8Br2N2O2 | 4.47 | 65.724/259.728 | 22 | 4 | 2 | 0 | 0 |
| Compound | MW/Formula | Log pa | Polar surface area/volume | No. non-H atoms | No. H bond acceptors b | No. H bond donors c | Rotatable bonds | No. rule of 5 violations d |
|---|---|---|---|---|---|---|---|---|
Murexine | 224.284 C11H18N3O2+ | −3.373 | 54.988/219.763 | 16 | 5 | 1 | 5 | 0 |
Senecoiycholine | 186.275 C10H20NO2+ | −2.096 | 26.305/200.647 | 13 | 3 | 0 | 5 | 0 |
Tigloylcholine | 186.275 C10H20NO2+ | −2.33 | 26.305/200.647 | 13 | 3 | 0 | 5 | 0 |
Choline | 104.173 C5H14NO+ | −4.236 | 20.228/120.158 | 7 | 2 | 1 | 2 | 0 |
2.2. Choline Esters
2.3. Mycosporine-Like Amino Acids, Fatty Acids and Sterols in the Egg Masses
3. Bioactivity of Dicathais orbita Extracts and Compounds
3.1. Drug-Likeness of D. orbita Secondary Metabolites
| Compound | GPCR ligand | Ion channel modulator | Kinase inhibitor | Nuclear receptor ligand | Protease inhibitor | Enzyme inhibitor | Other known bioactivity |
|---|---|---|---|---|---|---|---|
Tyrindoxyl sulfate | 0.22 * | 0.02 | −0.13 | −0.36 | 0.10 | 0.73 ** | - |
Tyrindoxyl | −0.56 | −0.09 | −0.41 | −0.71 | −1.00 | −0.11 | Unstable in O2 |
6 Bromoisatin | −1.08 | −0.49 | −0.50 | −1.62 | −1.07 | −0.39 | Anticancer, induces apoptosis, anti-bacterial [12,71] |
Tyrindoleninone | −0.93 | −0.39 | −0.69 | −1.16 | −1.15 | −0.43 | Anticancer, induces apoptosis, anti-bacterial [12,71] |
Tyrindolinone | −0.87 | −0.54 | −0.89 | −1.03 | −0.93 | −0.51 | Unstable in O2 |
Tyriverdin | −0.23 | −0.23 | −0.29 | −0.34 | −0.17 | −0.17 | Bacteriostatic, inhibits FDA hydrolysis [12] |
Tyrian purple 6,6′ Dibromoindigo | −0.32 | −0.30 | 0.22 * | −0.05 | −0.36 | −0.01 | Highly insoluble, no apparent antibacterial or anticancer activity [4] |
6,6′ Dibromoindirubin | −0.78 | −0.74 | 0.45 * | −0.28 | −0.61 | 0.01 | GSK-3 inhibitor [72] |
| Compound | GPCR ligand | Ion channel modulator | Kinase inhibitor | Nuclear receptor ligand | Protease inhibitor | Enzyme inhibitor | Other known bioactivity |
|---|---|---|---|---|---|---|---|
Murexine | 0.38 * | 0.50 * | −0.16 | −1.70 | −0.36 | 0.84 ** | Neuromuscular blocking and nicotinic action. No muscarinic effects. Paralysis of the skeletal musculature, toxic to mice at high doses (i.v. LD50 8.5 mg/kg, s.c. LD50 = 50 mg/kg); human clinical dose (EC50 = 1 mg/kg) [63,73] |
Senecoiycholine | −0.39 | 0.33 * | −1.04 | −1.28 | −0.95 | 0.35 * | Neuromuscular blocking and nicotinic action. No muscarinic effects [63] |
Tigloylcholine | −0.45 | 0.32 * | −1.37 | −1.31 | −1.35 | 0.41 * | Toxic to mice (i.v. LD50 = 0.92 mg/kg) [64] |
Choline | −2.64 | −2.21 | −3.84 | −4.93 | −3.94 | −2.18 | Essential nutrient, precursor for the neurotransmitter acetyl choline [74] |
3.2. Bioactivity of D. orbita Brominated Indoles
3.3. Bioactivity of Choline Esters
3.4. Antibacterial Activity and Chemical Ecology of the Egg Masses
3.5. Anti-Cancer Extracts, Toxicity & Nutraceutical Potential
4. A Biological Basis for Future Natural Products Research
4.1. Biosynthesis of D. orbita Brominated Indoles
| Precursor/Substrate | Enzyme | Product |
|---|---|---|
| Tryptophan | Trytophanase | Indole |
| Indole | Dioxygenases | Indoxyl sulfate |
| Indole/Indoxyl sulfate | Bromoperoxidase | 6 Bromoindole/Indoxyl |
| (6 Bromo) Indoxyl sulfate | Sulfur transferase & Sulfur reductase | (6 Bromo) Methylthio indolone/Tyrindoxyl sulfate |
| Tyrindoxyl sulfate | Aryl sulfatase | Tyrindoxyl |
4.2. Biodistribution of the Secondary Metabolites in D. orbita
4.3. Microbial Symbionts
4.4. Sustainable Supply
5. Conclusions
Acknowledgments
References
- Baker, J.T. Tyrian purple: An ancient dye, a modern problem. Endeavour 1974, 33, 11–17. [Google Scholar] [CrossRef]
- Cooksey, C.J. Tyrian purple: 6,6′-Dibromoindigo and related compounds. Molecules 2001, 6, 736–769. [Google Scholar]
- Freidlander, P. Ueber den farbstoff des antiken purpura aus Murex brandaris. Chem. Ber. 1909, 42, 765–770. [Google Scholar] [CrossRef]
- Westley, C.B.; Vine, K.L.; Benkendorff, K. A Proposed Functional Role for Indole Derivatives in Reproduction and Defense of the Muricidae (Neogastropoda: Mollusca). In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, L., Eisenbrand, G., Eds.; Station Biologique de Roscoff: Roscoff, France, 2006; pp. 31–44. [Google Scholar]
- Laffy, P.W.; Benkendorff, K.; Abbott, C.A. Suppressive subtractive hybridization transcriptomics provides a novel insight into the functional role of the hypobranchial gland in a marine mollusc. Comp. Biochem. Physiol. Part D Genomics Proteomics 2013, 8, 111–122. [Google Scholar]
- Baker, J.; Duke, C. Chemistry of the indoleninones. II. Isolation from the hypobranchial glands of marine molluscs of 6-bromo-2,2-dimethylthioindolin-3-one and 6-bromo-2-methylthioindoleninone as alternative precursors to Tyrian purple. Aust. J. Chem. 1973, 26, 2153–2157. [Google Scholar]
- Baker, J.; Duke, C. Isolation of choline and choline ester salts of tyrindoxyl sulphate from the marine molluscs Dicathais orbita and Mancinella keineri. Tetrahedron Lett. 1976, 15, 1233–1234. [Google Scholar] [CrossRef]
- Baker, J.; Sutherland, M. Pigments of marine animals VIII. Precursors of 6,6′-dibromoindigotin (Tyrian purple) from the mollusc Dicathais orbita (Gmelin). Tetrahedron Lett. 1968, 1, 43–46. [Google Scholar] [CrossRef]
- Baker, J.T. Studies on Tyrian Purple and Its Precursors from Australian Molluscs. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 1967. [Google Scholar]
- Baker, J.T.; Duke, C.C. Precursors of Tyrian Purple. In Food-Drugs Sea; Marine Technology Society: Washington, DC, USA, 1974; pp. 345–354. [Google Scholar]
- Baker, J.T.; Duke, C.C. Isolation from the hypobranchial glands of marine molluscs of 6-bromo-2,2-dimethylthioindolin-3-one and 6-bromo-2-methylthioindoleninone as alternative precursors to Tyrian purple. Tetrahedron Lett. 1973, 14, 2481–2482. [Google Scholar] [CrossRef]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Tyrian purple precursors in the egg masses of the Australian muricid, Dicathais orbita: A possible defensive role. J. Chem. Ecol. 2000, 26, 1037–1050. [Google Scholar] [CrossRef]
- Westley, C.; Benkendorff, K. Sex-specific Tyrian purple genesis: Precursor and pigment distribution in the reproductive system of the marine mollusc, Dicathais orbita. J. Chem. Ecol. 2008, 34, 44–56. [Google Scholar] [CrossRef]
- Westley, C.; Benkendorff, K. The distribution of precursors and biosynthetic enzymes required for Tyrian purple genesis in the hypobranchial gland, gonoduct, an egg masses of Dicathais orbita (Gmelin, 1791) (Neogastropoda: Muricidae. Nautilus 2009, 123, 148–153. [Google Scholar]
- Westley, C.B.; Lewis, M.C.; Benkendorff, K. Histomorphology of the hypobranchial gland in Dicathais orbita (Gmelin, 1791) (Neogastropoda: Muricidae). J. Moll. Stud. 2010, 76, 186–195. [Google Scholar] [CrossRef]
- Duke, C.C. A Study of Precursors to Purple Dyes from Australian Gastropod Molluscs and of Analogous Synthetic Compounds. Ph.D. Thesis, James Cook University, Townsville, Qld, Australia, 1973. [Google Scholar]
- Benkendorff, K. Bioactive Molluscan Resources and Their Conservation: Biological and Chemical Studies on the Egg Masses of Marine Molluscs. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 1999. [Google Scholar]
- Westley, C.B. The Distribution, Biosynthetic Origin and Functional Significance of Tyrian Purple Precursors in the Australian Muricid Dicathais orbita (Neogastropoda: Muricidae). Ph.D. Thesis, Flinders University, Adelaide, SA, Australia, 2008. [Google Scholar]
- Edwards, V. The Effects of Bioactive Compounds from the Marine Mollusc Dicathais orbita on Human Reproductive Cells and Human Reproductive Cancer Cells. Ph.D. Thesis, Flinders University, Adelaide, SA, Australia, 2012. [Google Scholar]
- Vine, K.L. An Investigation into the Cytotoxic Properties of Isatin-Derived Compounds: Potential Use in Targeted Cancer Therapy. Ph.D. Thesis, University of Wollongong, Wollongong, NSW, Australia, 2007. [Google Scholar]
- Laffy, P.W. Evolution, Gene Expression and Enzymatic Production of Tyrian Purple: A Molecular Study of the Australian Muricid Dicathais orbita (Neogastropoda: Muricidae). Ph.D. Thesis, Flinders University, Adelaide, SA, Australia, 2012. [Google Scholar]
- Wang, R. Effects of Marine Mollusc Extracts on Human Immune Function. Master’s Thesis, Flinders University, Adelaide, SA, Australia, 2009. [Google Scholar]
- Vine, K.L. Cytotoxicity of Molluscan Extracts and Natural Products. Biotechnology Honours Thesis, University of Wollongong, Wollongong, NSW, Australia, 2002. [Google Scholar]
- Noble, W.J. Survey Methodologies and the Distribution and Abundance of Dicathais orbita in South Australia: Population Characteristics and Appropriateness of Techniques. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2004. [Google Scholar]
- Laffy, P.W. Genes Expressed in the Hypobranchial Gland of Dicathais orbita. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2004. [Google Scholar]
- Cocks, R.R. In Vitro Bioactivity of Extracts from the Mucus of Dicathais orbita against the MCF-7 Breast Cancer Cell Line. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2008. [Google Scholar]
- Bogdanovic, A. Isolation of Bioactive Compounds from the Egg Masses of Dicathais orbita. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2007. [Google Scholar]
- Lim, S.H. Microbial Fouling and Antifouling Properties of Molluscan Egg Masses. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2006. [Google Scholar]
- Woodcock, S.H. Dietary Preferences and the Impact of Diet on the Growth and Proximate Composition of the Marine Whelk Dicathais orbita. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2007. [Google Scholar]
- Roberts, B. Bacterial Communities Associated with the Marine Snail Dicathais orbita. Honours Thesis, Flinders University, Adelaide, SA, Australia, 2009. [Google Scholar]
- Phillips, B. The Biology of the Whelk Dicathais in Western Australia. Ph.D. Thesis, University of Western Australia, Perth, Australia, 1968. [Google Scholar]
- Gibson, C.P. The Current Status of Imposex in the Intertidal Gastropod, Thais orbita Gmelin (Muricidae), along the New South Wales Coast, Australia. Ph.D. Thesis, Australian Catholic University, Sydney, Australia, 1999. [Google Scholar]
- Fairweather, P.G. Interactions between Predators and Prey, and the Structure of Rocky Intertidal Communities. Ph.D. Thesis, University of Sydney, Sydney, Australia, 1985. [Google Scholar]
- Przeslawski, R. The Effects of UV Radiation on the Egg Masses of Intertidal Molluscs. Ph.D. Thesis, University of Wollongong, Wollongong, NSW, Australia, 2005. [Google Scholar]
- Brown, E. Effects of Human Access on the Size Distribution and Abundance of Intertidal Molluscs along the Fleurieu Peninsula. Honours Thesis, Flinders University, Adelaide, NSW, Australia, 2009. [Google Scholar]
- Rittschof, D.; McClellan-Green, P. Molluscs as multidisciplinary models in environment toxicology. Mar. Poll. Bull. 2005, 50, 369–373. [Google Scholar] [CrossRef]
- Phillips, B.F.; Campbell, N.A.; Phillips, B. Mortality and longevity in the whelk Dicathais orbita (Gmelin). Mar. Freshw. Res. 1974, 25, 25–33. [Google Scholar] [CrossRef]
- Phillips, B.F.; Campbell, N.A.; Wilson, B.R. A multivariate study of geographic variation in the whelk Dicathais. J. Exp. Mar. Biol. Ecol. 1973, 11, 27–69. [Google Scholar] [CrossRef]
- Fairweather, P. Movements of intertidal whelks (Morula marginalba and Thais orbita) in relation to availability of prey and shelter. Mar. Biol. 1988, 100, 63–68. [Google Scholar]
- Benkendorff, K. Aquaculture and the Production of Pharmaceuticals and Nutraceuticals. In New Technologies in Aquaculture: Improving Production Efficiency, Quality and Environmental Management; Burnell, G., Allen, G., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 866–891. [Google Scholar]
- Phillips, B.F. The population of the whelk Dicathais aegrota in Western Australia. Mar. Freshw. Res. 1969, 20, 225–266. [Google Scholar]
- Westley, C.B.; Lewis, M.C.; Benkendorff, K. Histomorphology of the female pallial gonoduct in Dicathais orbita (Neogastropoda, Muricidae): Sperm passage, fertilization, and sperm storage potential. Invert. Biol. 2010, 129, 138–150. [Google Scholar]
- Westley, C.B.; Benkendorff, K. Histochemical correlations between egg capsule laminae and the female gonoduct reveal the process of capsule formation in the Muricidae (Neogastropoda: Mollusca). Invert. Reprod. Dev. 2008, 52, 81–92. [Google Scholar] [CrossRef]
- Woodcock, S.H.; Benkendorff, K. The impact of diet on the growth and proximate composition of juvenile whelks, Dicathais orbita (Gastropoda: Mollusca). Aquaculture 2008, 276, 162–170. [Google Scholar] [CrossRef]
- Kool, S.P. Phylogenetic analysis of the Rapaninae (Neogastropoda: Muricidae). Malacologia 1993, 35, 155–259. [Google Scholar]
- Barco, A.; Claremont, M.; Reid, D.G.; Houart, R.; Bouchet, P.; Williams, S.T.; Cruaud, C.; Couloux, A.; Oliverio, M. A molecular phylogenetic framework for the Muricidae, a diverse family of carnivorous gastropods. Mol. Phylogenet. Evol. 2010, 56, 1025–1039. [Google Scholar] [CrossRef]
- Colgan, D.; Ponder, W.; Beacham, E.; Macaranas, J. Gastropod phylogeny based on six segments from four genes representing coding or non-coding and mitochondrial or nuclear DNA. Moll. Res. 2003, 23, 123–148. [Google Scholar]
- Colgan, D.J.; Ponder, W.F.; Beacham, E.; Macaranas, J. Molecular phylogenetics of Caenogastropoda (Gastropoda: Mollusca). Mol. Phylogenet. Evol. 2007, 42, 717–737. [Google Scholar] [CrossRef]
- Colgan, D.J.; Ponder, W.F.; Eggler, P.E. Gastropod evolutionary rates and phylogenetic relationships assessed using partial 28S rDNA and histone H3 sequences. Zool. Scripta 2000, 29, 29–63. [Google Scholar] [CrossRef]
- Laffy, P.W.; Benkendorff, K.; Abbott, C.A. Trends in molluscan gene sequence similiarity: An observation from genes expressed within the hypobranchial gland of Dicathais orbita (Gmelin, 1791) (Neogastropoda: Muricidae). Nautilus 2009, 123, 154–158. [Google Scholar]
- Cardenas, L.; Sanchez, R.; Gomez, D.; Fuenzalida, G.; Gallardo-Escarate, C.; Tanguy, A. Transcriptome analysis in Concholepas concholepas (Gastropoda, Muricidae): Mining and characterization of new genomic and molecular markers. Mar. Genomics 2011, 4, 197–205. [Google Scholar] [CrossRef]
- Ronci, M.; Rudd, D.; Guinan, T.; Benkendorff, K.; Voelcker, N.H. Mass spectrometry imaging on porous silicon: Investigating the distribution of bioactives in marine mollusc tissues. Anal. Chem. 2012, 84, 8996–9001. [Google Scholar]
- Cole, W. Letter to the Philosophical Society of Oxford containing observations on the purple fish. Phil. Trans. R. Soc. Lond. 1685, 15, 1278–1286. [Google Scholar] [CrossRef]
- Christophersen, C.; Watjen, F.; Buchardt, O.; Anthoni, U. A revised structure of tyriverdin: The precursor to Tyrian purple. Tetrahedron Lett. 1978, 34, 2779–2781. [Google Scholar] [CrossRef]
- Cooksey, C.J. Marine Indirubins. In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, L., Eisenbrand, G., Eds.; Progress in Life Series: Roscoff, France, 2006; pp. 23–30. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv.Drug Del. Rev. 1997, 23, 4–25. [Google Scholar]
- Baker, J.T. Some metabolites from Australian marine organisms. Pure Appl. Chem. 1976, 48, 35–44. [Google Scholar] [CrossRef]
- Fouquet, H.; Bielig, H.J. Biological precursors and genesis of Tyrian Purple. Angew. Chem. Int. Ed. 1971, 10, 816–817. [Google Scholar] [CrossRef]
- Benkendorff, K.; Westley, C.B.; Gallardo, C.S. Observations on the production of purple pigments in the egg capsules, hypobranchial and reproductive glands from seven species of Muricidae (Gastropoda: Mollusca). Invert. Reprod. Dev. 2004, 46, 93–102. [Google Scholar] [CrossRef]
- Benkendorff, K.; Bremner, J.; Davis, A. Indole derivatives from the egg masses of muricid molluscs. Molecules 2001, 6, 70–78. [Google Scholar] [CrossRef]
- Benkendorff, K.; Pillai, R.; Bremner, J.B. 2,4,5-Tribromo-1H-Imidazole in the egg masses of three muricid molluscs. Nat. Prod. Res. 2004, 18, 427–431. [Google Scholar] [CrossRef]
- Duke, C.C.; Eichholzer, J.V.; Macleod, J.K. The synthesis of the isomeric N-methyl derivatives of murexine. Aust. J. Chem. 1981, 34, 1739–1744. [Google Scholar] [CrossRef]
- Duke, C.C.; Eichholzer, J.V.; Macleod, J.K. N-Methylmurexine-naturally occuring marine compound. Tetrahedron Lett. 1978, 50, 5047–5048. [Google Scholar]
- Roseghini, M.; Severini, C.; Erspamer, G.F.; Erspamer, V. Choline esters and biogenic amines in the hypobranchial gland of 55 molluscan species of the neogastropod Muricoidea superfamily. Toxicon 1996, 34, 33–55. [Google Scholar] [CrossRef]
- Shiomi, K.; Ishii, M.; Shimakura, K.; Nagashima, Y.; Chino, M. Tigloycholine: A new choline ester toxin from the hypobranchial gland of two species of muricid gastropods (Thais clavigera and Thais bronni). Toxicon 1998, 36, 795–798. [Google Scholar]
- Benkendorff, K.; Davis, A.; Bremner, J. Rapid screening for antimicrobial agents in the egg masses of marine muricid molluscs. J. Med. Appl. Malac. 2000, 10, 211–223. [Google Scholar]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–171. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Przeslawski, R.; Benkendorff, K.; Davis, A. A quantitative survey of mycosporine-like amino acids (MAAS) in intertidal egg masses from temperate rocky shores. J. Chem. Ecol. 2005, 31, 2417–2438. [Google Scholar] [CrossRef]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Edwards, V.; Benkendorff, K.; Young, F. Marine compounds selectively induce apoptosis in female reproductive cancer cells but not in primary-derived human reproductive granulosa cells. Mar. Drugs 2012, 10, 64–83. [Google Scholar] [CrossRef]
- Meijer, L.; Skaltsounis, A.L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.Z.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R.; et al. GSK-3-Selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef]
- Erspamer, V.; Glasser, A. The pharmacological actions of murexine (urocanylcholine). Br. J. Pharmcol. Chemother. 1957, 12, 176–184. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Cooksey, C. The synthesis and properties of 6-bromoindigo: Indigo blue or Tyrian purple? The effect of physical state on the colours of indigo and bromoindigos. Dyes Hist. Archaeol. 2001, 16–17, 97–104. [Google Scholar]
- Benkendorff, K.; Davis, A.R.; Bremner, J.B. Chemical defense in the egg masses of benthic invertebrates: An assessment of antibacterial activity in 39 mollusks and 4 polychaetes. J. Invert. Path. 2001, 78, 109–118. [Google Scholar] [CrossRef]
- Benkendorff, K.; McIver, C.M.; Abbott, C.A. Bioactivity of the Murex homeopathic remedy and of extracts from an Australian muricid mollusc against human cancer cells. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Westley, C.B.; McIver, C.M.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. Enhanced acute apoptotic response to azoxymethane-induced DNA damage in the rodent colonic epithelium by Tyrian purple precursors A potential colorectal cancer chemopreventative. Cancer Biol. Ther. 2010, 9, 371–379. [Google Scholar]
- Magiatis, P.; Skaltsounis, A.L. From Hexaplex trunculus to New Kinase Inhibitory Indirubins. In In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, A.-L., Eisenbrand, G., Eds.; Life in Progress Editions: Roscoff, France, 2006; pp. 147–156. [Google Scholar]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Benkendorff, K.; Pyne, S.G.; Bremner, J.B. In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 2007, 15, 931–938. [Google Scholar] [CrossRef]
- Esmaeelian, B. In-Vitro and In-Vivo Testing of Purified Muricid Mollusc Extract on Colorectal Cancer. Ph.D. Thesis, Flinders University, Adelaide, SA, Australia, 2013. [Google Scholar]
- Keyl, M.J.; Whittaker, P. Some pharmacological properties of murexine (urocanoylcholine). Br. J. Pharmcol. 1958, 13, 103–106. [Google Scholar]
- Quilliam, J.P. The mechanism of action of murexine on neuromescular transmission in the frog. Br. J. Pharmcol. 1957, 12, 338–392. [Google Scholar]
- Benkendorff, K. Molluscan Resources: The Past Present and Future Value of Molluscs. In The Other 99%. The Conservation and Biodiversity of Invertebrates; Ponder, W., Lunney, D., Eds.; Mosman: Sydney, Australia, 1999; p. 454. [Google Scholar]
- Lim, N.S.H.; Everuss, K.J.; Goodman, A.E.; Benkendorff, K. Comparison of surface microfouling and bacterial attachment on the egg capsules of two molluscan species representing Cephalopoda and Neogastropoda. Aquat. Microb. Ecol. 2007, 47, 275–287. [Google Scholar] [CrossRef]
- Przeslawski, R.; Benkendorff, K. The role of surface fouling in the development of encapsulated gastropod embryos. J. Moll. Stud. 2005, 71, 75–83. [Google Scholar] [CrossRef]
- Westley, C.B.; Benkendorff, K.; McIver, C.; Leu, R.K.; Abbott, C.A. Gastrointestinal and hepatotoxicity assessment of an anticancer extract from muricid molluscs. Evid. Based Complement. Altern. Med. 2013, in press. [Google Scholar]
- Barnes, D.K.A.; Corrie, A.; Whittington, M.; Carvalho, M.A.; Gell, F. Coastal shellfish resource use in the Quirimba Archipelago, Mozambique. J. Shellfish Res. 1998, 17, 51–58. [Google Scholar]
- Leiva, G.E.; Castilla, J.C. A review of the world marine gastropod fishery: Evolution of catches, management and the Chilean experience. Rev. Fish Biol. Fish. 2001, 11, 283–300. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Carvalho, S.; Castro, M.; Gaspar, M. The artisanal fishery for muricid gastropods (banded murex and purple dye murex) in the Ria Formosa lagoon (Algrave coast, Southern Portugal). Sci. Mar. 2008, 72, 287–298. [Google Scholar]
- Jennings, S.; Kaiser, M.; Reynolds, J. Marine Fisheries Ecology; Blackwell Science: Oxford, UK, 2001; p. 11. [Google Scholar]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar]
- Debat, J. Promotion of Analgesic and Sedative Action with 5-Bromoistain. U.S. Patent 3,659,011, 1972. [Google Scholar]
- Ryan, W.L. Immunization of animals using choline esters as an immunological adjuvant. U.S. Patent 4,171,353, 1979. [Google Scholar]
- O’Connor, K.; Hartmans, E.; Hartmans, S. Indigo formation by aromatic hydrocarbon-degrading bacteria. Biotechnol. Lett. 1998, 20, 219–223. [Google Scholar]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leas with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef]
- Wagner, C.; El Omari, M.; Koenig, G.M. Biohalogenation: Nature’s way to synthesize halogenated metabolites. J. Nat. Prod. 2009, 72, 540–553. [Google Scholar]
- Neumann, C.S.; Fujimori, D.G.; Walsh, C.T. Halogenation strategies in natural product biosynthesis. Chem. Biol. 2008, 15, 99–109. [Google Scholar] [CrossRef]
- Jannun, R.; Coe, E.L. Bromoperoxidase from the marine snail, Murex trunculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1987, 88, 917–922. [Google Scholar]
- Jannun, R.; Nuwayhid, N.; Coe, E. Biological bromination-bromoperoxidase activity in the murex sea-snail. Fed. Proc. 1981, 40, 1774. [Google Scholar]
- Winter, J.M.; Moore, B.S. Exploring the chemistry and biology of vanadium-dependent haloperoxidases. J. Biol. Chem. 2009, 284, 18577–18581. [Google Scholar] [CrossRef]
- Butler, A.; Carter-Franklin, J.N. The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat. Prod. Rep. 2004, 21, 180–188. [Google Scholar] [CrossRef]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000–2008. Anticancer Agents Med. Chem. 2009, 9, 397–414. [Google Scholar]
- Rudd, D. Marine Natural Products of Dicathais orbita: Developing a Potential Nutraceutical and Understanding the Ecological Significance of Active Compounds within This Species. Flinders University: Adealide, SA, Australia, Unpublished work. 2013. [Google Scholar]
- Hsieh, Y.; Li, F.; Korfmacher, W. Mapping pharmaceuticals in rat brain sections using MALDI imaging mass spectrometry. Methods Mol. Biol. 2010, 656, 147–158. [Google Scholar] [CrossRef]
- Balfour-Paul, J. Indigo; Fitzroy Dearborn: Chicargo, IL, USA, 2000. [Google Scholar]
- Lim, H.K.; Chung, E.J.; Kim, J.C.; Choi, G.J.; Jang, K.S.; Chung, Y.R.; Cho, K.Y.; Lee, S.W. Characterization of a forest soil metagenome clone that confers indirubin and indigo production on Escherichia coli. Appl. Environ. Microb. 2005, 71, 7768–7777. [Google Scholar] [CrossRef]
- Lane, A.L.; Moore, B.S. A sea of biosynthesis: Marine natural products meet the molecular age. Nat. Prod. Rep. 2011, 28, 411–428. [Google Scholar] [CrossRef]
- Andrews, E.B. The fine structure and function of the anal gland of the muricid, Nucella lapillus (Neogastropoda) and a comparison with that of the trochid Gibbula cineraria. J. Moll. Stud. 1992, 58, 297–313. [Google Scholar] [CrossRef]
- Rappe, M.S.; Giovannoni, S.J. The uncultured microbial majority. Ann. Rev. Microb. 2003, 57, 369–394. [Google Scholar] [CrossRef]
- Kremer-Pigmente. Tyrian Purple. Available online: http://www.kremer-pigmente.com/en/pigments/tyrian-purple-36010.html (accessed on 24 January 2012).
- Noble, W.J.; Cocks, R.R.; Harris, J.O.; Benkendorff, K. Application of anaesthetics for sex identification and bioactive compound recovery from wild Dicathais orbita. J. Exp. Mar. Biol. Ecol. 2009, 380, 53–60. [Google Scholar] [CrossRef]
- Phillips, B.F.; Campbell, N.A. A new method of fitting the von Bertalanffy growth curve using data on the whelk Dicathais. Growth 1968, 32, 317–329. [Google Scholar]
- Phillips, B.F.; Campbell, N.A. Comparison of methods of estimating population size using data on the whelk Dicathais aegrota (Reeve). J. Anim. Ecol. 1970, 39, 753–759. [Google Scholar] [CrossRef]
- Gibson, C.P.; Wilson, S.P. Imposex still evident in eastern Australia 10 years after tributyltin restrictions. Mar. Environ. Res. 2003, 55, 101–112. [Google Scholar] [CrossRef]
- Rees, C.M.; Brady, B.A.; Fabris, G.J. Incidence of imposex in Thais orbita from Port Phillip Bay (Victoria, Australia), following 10 years of regulation on use of TBT. Mar. Poll. Bull. 2001, 42, 873–878. [Google Scholar] [CrossRef]
- Noble, W.J. Life History Assessment and Larval Culture of Dicathais orbita. Flinders University: Adelaide, SA, Australia, Unpublished work. 2013. [Google Scholar]
- Morton, B. Competitive grazers and the predatory whelk (Gastropoda: Muricidae) structure a mussel bed on a southwest Australian shore. J. Moll. Stud. 1999, 65, 435–452. [Google Scholar] [CrossRef]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis. Biotechnol. Bioeng. 2005, 90, 201–222. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Benkendorff, K. Natural Product Research in the Australian Marine Invertebrate Dicathais orbita. Mar. Drugs 2013, 11, 1370-1398. https://doi.org/10.3390/md11041370
Benkendorff K. Natural Product Research in the Australian Marine Invertebrate Dicathais orbita. Marine Drugs. 2013; 11(4):1370-1398. https://doi.org/10.3390/md11041370
Chicago/Turabian StyleBenkendorff, Kirsten. 2013. "Natural Product Research in the Australian Marine Invertebrate Dicathais orbita" Marine Drugs 11, no. 4: 1370-1398. https://doi.org/10.3390/md11041370
APA StyleBenkendorff, K. (2013). Natural Product Research in the Australian Marine Invertebrate Dicathais orbita. Marine Drugs, 11(4), 1370-1398. https://doi.org/10.3390/md11041370