The Lipid A from the Haloalkaliphilic Bacterium Salinivibrio sharmensis Strain BAGT
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of Lipid A and Its Compositional Analysis
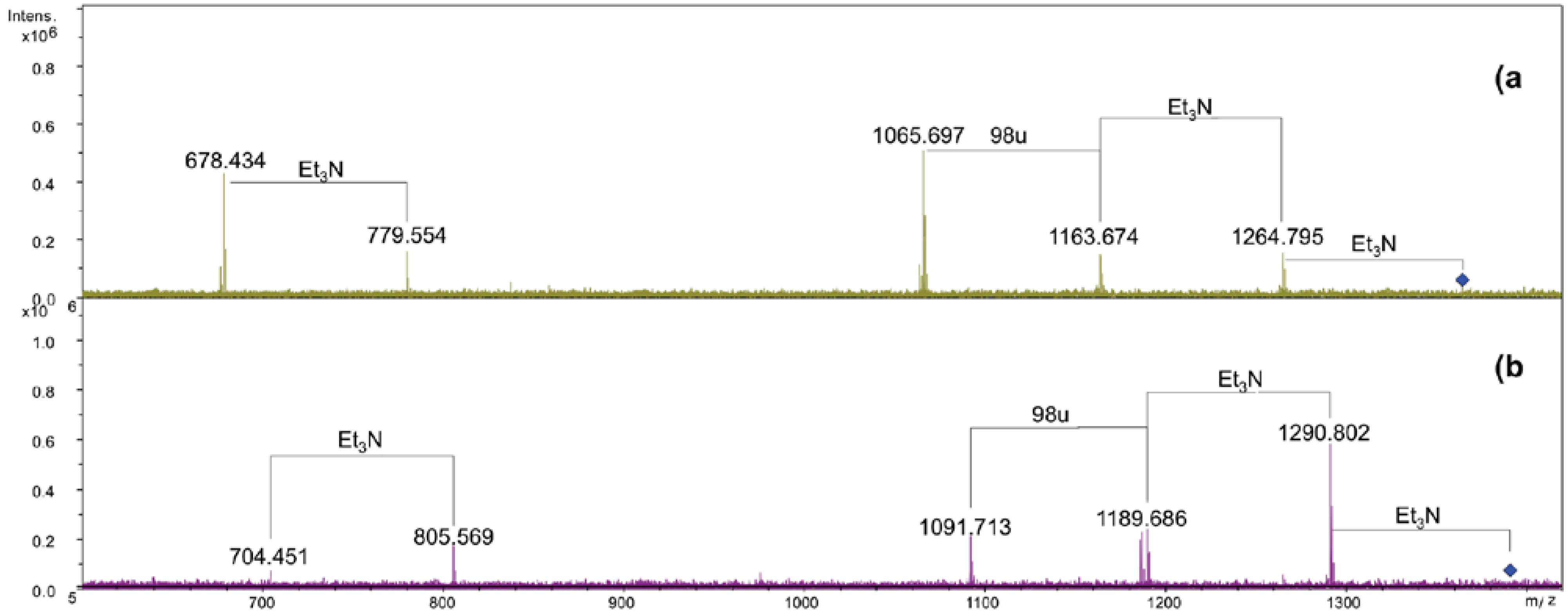
2.2. ESI FT-ICR Mass Spectrometric Analysis of Lipid A

| Species | Mobs | Macc | Composition |
|---|---|---|---|
| M1 | 1741.14 | 1741.17 | GlcN2P2[C12:0(3-OH)]2[C14:0(3-OH)]2(C12:0)(C14:0) |
| M2 | 1661.18 | 1661.21 | GlcN2P[C12:0(3-OH)]2[C14:0(3-OH)]2(C12:0)(C14:0) |
| M3 | 1558.99 | 1559.00 | GlcN2P2[C12:0(3-OH)]2[C14:0(3-OH)]2(C14:0) |
| M4 | 1479.01 | 1479.04 | GlcN2P[C12:0(3-OH)]2[C14:0(3-OH)]2(C14:0) |
| M5 | 1360.82 | 1360.84 | GlcN2P2[C12:0(3-OH)][C14:0(3-OH)]2(C14:0) |
| M6 | 1280.86 | 1280.87 | GlcN2P[C12:0(3-OH)][C14:0(3-OH)]2(C14:0) |
| M7 | 1150.62 | 1150.64 | GlcN2P2[C12:0(3-OH)][C14:0(3-OH)]2 |
| M8 | 1070.66 | 1070.67 | GlcN2P[C12:0(3-OH)][C14:0(3-OH)]2 |
| M9 | 872.50 | 872.51 | GlcN2P[C14:0(3-OH)]2 |
2.3. Analysis of NH4OH Product
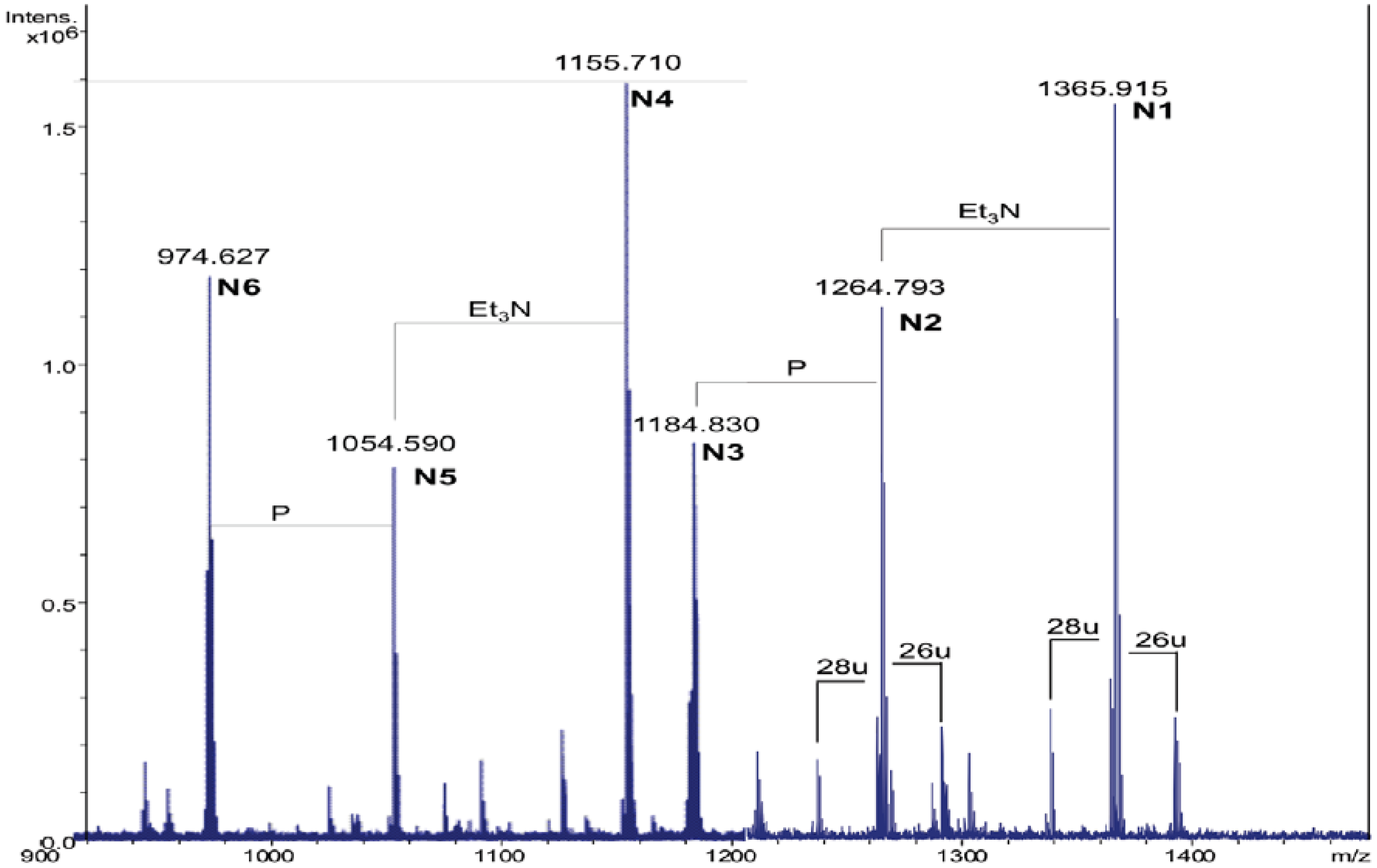
| Species | Observed m/z | Calculated m/z | Composition |
|---|---|---|---|
| N1 | 1365.91 a | 1365.92 | GlcN2P2[C14:0(3-OH)]2(C14:0) |
| N2 | 1264.79 b | 1264.80 | GlcN2P2[C14:0(3-OH)]2(C14:0) |
| N3 | 1184.83 b | 1184.84 | GlcN2P[C14:0(3-OH)]2(C14:0) |
| N4 | 1155.71 a | 1155.72 | GlcN2P2[C14:0(3-OH)]2 |
| N5 | 1054.59 b | 1054.60 | GlcN2P2[C14:0(3-OH)]2 |
| N6 | 974.63 b | 974.64 | GlcN2P[C14:0(3-OH)]2 |

3. Experimental Section
3.1. Isolation of the LPS
3.2. Mild Acid Hydrolysis of the LPS
3.3. Chemical Analysis
3.4. NH4OH Hydrolysis of Lipid A
3.5. ESI FT-ICR Mass Spectrometry
4. Conclusions
References
- Galinsky, E.A. Compatible solutes of halophilic eubacteria: Molecular principles, water-solute interaction, stress protection. Experientia 1993, 49, 487–496. [Google Scholar] [CrossRef]
- Galinsky, E.A.; Trüper, H.G. Microbial behavior in salt-stressed ecosystems. FEMS Microbiol. Rev. 1994, 15, 95–108. [Google Scholar] [CrossRef]
- Van de Burg, B. Extremophiles as a source for novel enzymes. Curr. Opin. Microbiol. 2003, 6, 213–218. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Comp. Rev. Food Sci. Food Safety 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Russell, N.J.; Evans, R.I.; ter Steeg, P.F.; Hellemons, J.; Verheul, A.; Abee, T. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 1995, 28, 255–261. [Google Scholar] [CrossRef]
- Alexander, C.; Zähringer, U. Chemical Structure of Lipid A. The Primary Immunomodulatory Center of Bacterial Lipopolysaccharides. Trends Glycosci. Glycotechnol. 2002, 14, 69–86. [Google Scholar] [CrossRef]
- Endotoxin in Health and Disease; Brade, H.; Opal, S.M.; Vogel, S.N.; Morrison, D.C. (Eds.) Marcel Dekker: New York, NY, USA, 1999; pp. 93–114.
- Holst, O. The structures of core regions from enterobacterial lipopolysaccharides—an update. FEMS Microbiol. Lett. 2007, 271, 3–11. [Google Scholar] [CrossRef]
- Izquierdo, L.; Coderch, N.; Piqué, N.; Bedini, E.; Corsaro, M.M.; Merino, S.; Fresno, S.; Tomás, J.M.; Regué, M. The Klebsiella pneumonia wabG gene: Its role in the biosynthesis of the core lipopolysaccharide and virulence. J. Bacteriol. 2003, 185, 7213–7221. [Google Scholar] [CrossRef]
- Fresno, S.; Jiménez, N.; Izquierdo, L.; Merino, S.; Corsaro, M.M.; de Castro, C.; Parrilli, M.; Naldi, T.; Regué, M.; Tomás, J.M. The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide. Microbiology 2006, 152, 1807–1818. [Google Scholar] [CrossRef]
- Pieretti, G.; Carillo, S.; Lindner, B.; Kim, K.K.; Lee, K.C.; Lee, J.S.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Characterization of the core oligosaccharide and the O-antigen biological repeating unit from Halomonas stevensii LPS: The first case of O-antigen linked to the inner core. Chem. Eur. J. 2012, 18, 3729–3735. [Google Scholar]
- Carillo, S.; Pieretti, G.; Parrilli, E.; Tutino, M.L.; Gemma, S.; Molteni, M.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Structural Investigation and Biological Activity of the Lipooligosaccharide from the Psychrophilic Bacterium Pseudoalteromonas haloplanktis TAB 23. Chem. Eur. J. 2011, 17, 7053–7060. [Google Scholar]
- Romano, I.; Orlando, P.; Gambacorta, A.; Nicolaus, B.; Dipasquale, L.; Pascual, J.; Giordano, A.; Lama, L. Salinivibrio sharmensis sp. nov., a novel haloalkaliphilic bacterium from a saline lake in Ras Mohammed Park (Egypt). Extremophiles 2011, 15, 213–220. [Google Scholar] [CrossRef]
- Galanos, C.; Lüderitz, O.; Westphal, O. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 1969, 9, 245–249. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Kondakova, A.; Lindner, B. Structural characterization of complex bacterial glycolipids by Fourier transform mass spectrometry. Eur. J. Mass Spectrom. 2005, 11, 535–546. [Google Scholar] [CrossRef]
- Silipo, A.; Lanzetta, R.; Amoresano, A.; Parrilli, M.; Molinaro, A. Ammonium hydroxide hydrolysis: A valuable support in the MALDI-TOF mass spectrometry analysis of Lipid A fatty acid distribution. J. Lipid Res. 2002, 43, 2188–2195. [Google Scholar] [CrossRef]
- Carillo, S.; Pieretti, G.; Lindner, B.; Romano, I.; Nicolaus, B.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Structural characterization of the core oligosaccharide isolated from the lipopolysaccharide of the haloalkaliphilic bacterium Salinivibrio sharmensis strain BAGT. Carbohydr. Res. 2012. [Google Scholar] [CrossRef]
- Holst, O.; Müller-Loennies, S.; Lindner, B.; Brade, H. Chemical structure of the lipid A of Escherichia coli J-5. Eur. J. Biochem. 1993, 214, 695–701. [Google Scholar] [CrossRef]
- Kulshin, V.A.; Zaehringer, U.; Lindner, B.; Frasch, C.E.; Tsai, C.M.; Dmitriev, B.A.; Rietschel, E.T. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J. Bacteriol. 1992, 174, 1793–1800. [Google Scholar]
- Zughaier, S.; Steeghs, L.; van der Ley, P.; Stephens, D.S. TLR4-dependent adjuvant activity of Neisseria meningitidis lipid A. Vaccine 2007, 25, 4401–4409. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carillo, S.; Pieretti, G.; Lindner, B.; Romano, I.; Nicolaus, B.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. The Lipid A from the Haloalkaliphilic Bacterium Salinivibrio sharmensis Strain BAGT. Mar. Drugs 2013, 11, 184-193. https://doi.org/10.3390/md11010184
Carillo S, Pieretti G, Lindner B, Romano I, Nicolaus B, Lanzetta R, Parrilli M, Corsaro MM. The Lipid A from the Haloalkaliphilic Bacterium Salinivibrio sharmensis Strain BAGT. Marine Drugs. 2013; 11(1):184-193. https://doi.org/10.3390/md11010184
Chicago/Turabian StyleCarillo, Sara, Giuseppina Pieretti, Buko Lindner, Ida Romano, Barbara Nicolaus, Rosa Lanzetta, Michelangelo Parrilli, and Maria Michela Corsaro. 2013. "The Lipid A from the Haloalkaliphilic Bacterium Salinivibrio sharmensis Strain BAGT" Marine Drugs 11, no. 1: 184-193. https://doi.org/10.3390/md11010184






