Briarenolides F and G, New Briarane Diterpenoids from a Briareum sp. Octocoral
Abstract
:1. Introduction
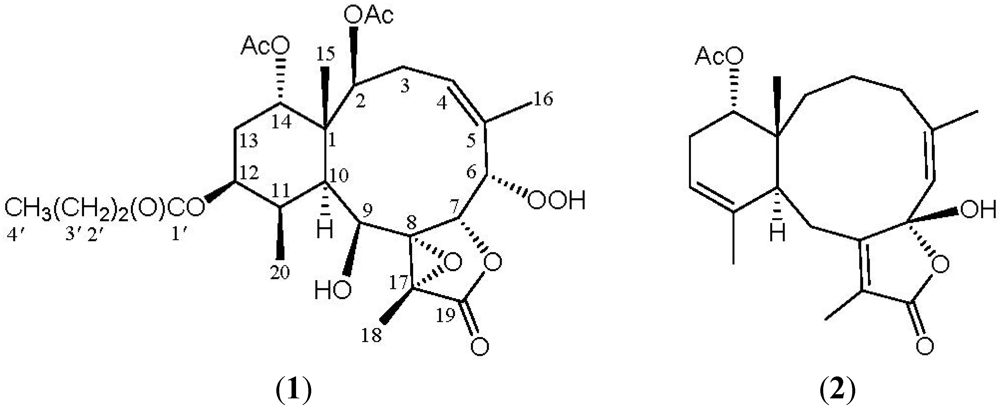
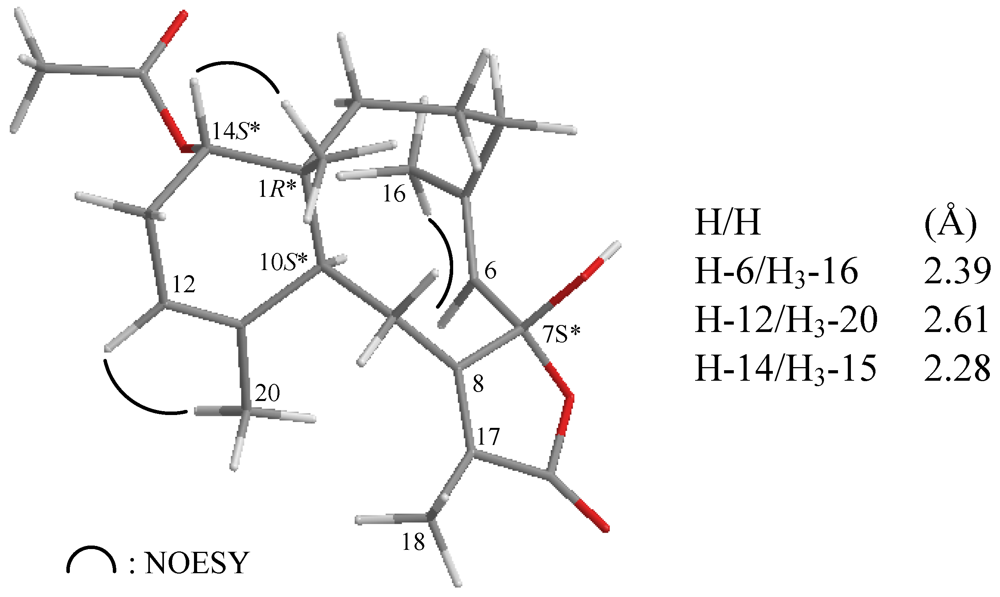
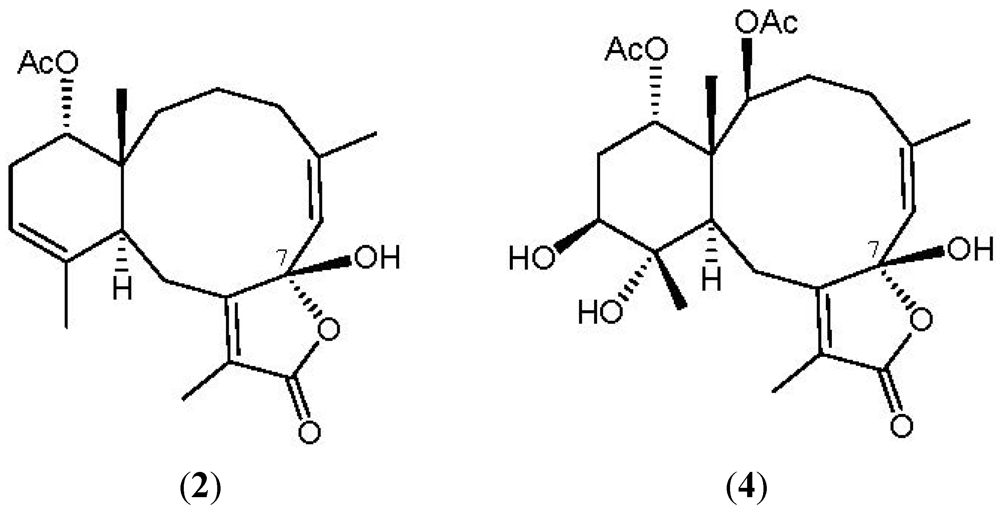
2. Results and Discussion
| C/H | δ (J in Hz) | δC, Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 44.9, qC | |||
| 2 | 5.22 d (8.0) | 75.4, CH | H2-3 | C-1, -4, -10, -15, acetate carbonyl |
| 3α | 1.89 m | 33.3, CH2 | H-2, H-3β, H-4 | N.O. |
| β | 3.81 dd (17.2, 13.6) | H-2, H-3α, H-4 | C-4, -5 | |
| 4 | 5.65 br d (13.6) | 130.3, CH | H2-3, H3-16 | N.O. |
| 5 | 128.8, qC | |||
| 6 | 4.65 d (2.4) | 84.9, CH | H-7 | C-4, -5, -7, -8, -16 |
| 7 | 5.33 d (2.4) | 77.2, CH | H-6 | C-5, -6 |
| 8 | 68.8, qC | |||
| 9 | 4.59 d (4.8) | 75.1, CH | OH-9 | C-1, -8, -10, -11, -17 |
| 10 | 2.06 d (4.0) | 39.4, CH | H-11 | C-1, -8, -9, -15, -20 |
| 11 | 2.28 m | 40.2, CH | H-10, H-12, H3-20 | C-1, -10, -12 |
| 12 | 4.98 ddd (12.4, 5.2, 5.2) | 70.7, CH | H-11, H2-13 | C-20, n-butyrate carbonyl |
| 13 | 1.87–1.97 m | 26.5, CH2 | H-12, H-14 | C-12 |
| 14 | 4.88 dd (3.2, 2.4) | 74.9, CH | H2-13 | N.O. |
| 15 | 1.37 s | 15.9, CH3 | C-1, -2, -10, -14 | |
| 16 | 1.77 br s | 25.4, CH3 | H-4 | C-4, -5, -6 |
| 17 | 58.4, qC | |||
| 18 | 1.49 s | 8.8, CH3 | C-8, -17, -19 | |
| 19 | 171.0, qC | |||
| 20 | 1.22 d (7.2) | 10.6, CH3 | H-11 | C-10, -11, -12 |
| 2-OAc | 1.99 s | 170.6, qC21.3, CH3 | Acetate carbonyl | |
| 6-OOH | 8.71 br s | N.O. | ||
| 9-OH | 2.95 d (4.8) | H-9 | N.O. | |
| 12-OC(O)Pr | 173.1, qC | |||
| 2.27 t (7.2) | 36.3, CH2 | H2-3 ' | C-1 ' , -3 ' , -4 ' | |
| 1.63 sext (7.2) | 18.4, CH2 | H2-2 ' , H3-4 ' | C-1 ' , -2 ' , -4 ' | |
| 0.94 t (7.2) | 13.7, CH3 | H2-3 ' | C-2 ' , -3 ' | |
| 14-OAc | 170.6, qC | |||
| 2.01 s | 21.3, CH3 | Acetate carbonyl |


| C/H | δΗ (J in Hz) | δC, Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 39.1, qC | |||
| 2α | 1.69 m | 35.4, CH2 | H-2β, H2-3 | C-3, -14 |
| β | 1.29 m | H-2α, H2-3 | C-1, -3, -10, -14 | |
| 3 | 1.72 m | 23.7, CH2 | H2-2, H2-4 | C-2 |
| 4α | 1.90 m | 29.6, CH2 | H2-3, H-4β | N.O. |
| β | 3.72 m | H2-3, H-4α | C-3, -16 | |
| 5 | 144.4, qC | |||
| 6 | 5.29 br s | 124.6, CH | H3-16 | C-4, -7, -16 |
| 7 | 106.7, qC | |||
| 8 | 160.8, qC | |||
| 9α | 2.54 br d (15.2) | 25.3, CH2 | H-9β, H-10 | C-8, -10, -11, -17 |
| β | 2.37 dd (15.2, 10.8) | H-9α, H-10 | C-7, -8, -10, -11, -17 | |
| 10 | 3.76 d (10.8) | 35.9, CH | H2-9 | N.O. |
| 11 | 136.3, qC | |||
| 12 | 5.12 m | 117.8, CH | H2-13, H3-20 | N.O. |
| 13α | 2.06 m | 29.3, CH2 | H-12, H-13β | C-11, -12, -14 |
| β | 2.33 m | H-12, H-13α | C-11 | |
| 14 | 4.78 br s | 77.2, CH | H2-13 | C-12 |
| 15 | 0.77 s | 21.9, CH3 | C-1, -2, -10, -14 | |
| 16 | 1.78 br s | 23.9, CH3 | H-6 | C-4, -5, -6 |
| 17 | 125.1, qC | |||
| 18 | 1.87 d (1.6) | 9.1, CH3 | C-8, -17, -19 | |
| 19 | 171.7, qC | |||
| 20 | 1.63 d (0.8) | 21.9, CH3 | H-12 | C-10, -11, -12 |
| 7-OH | 3.35 s | C-6, -7, -8 | ||
| 14-OAc | 170.9, qC | |||
| 2.03 s | 21.7, CH3 | Acetate carbonyl |
| Superoxide Anion | Elastase Release | |||
|---|---|---|---|---|
| Compounds | IC50 (µg/mL) | Inh% a | IC50 (µg/mL) | Inh% a |
| 1 | 3.82 ± 0.45 | 76.65 ± 4.21 | >10.0 | 27.48 ± 6.60 |
| 2 | >10.0 | 22.04 ± 3.43 | >10.0 | 12.98 ± 4.68 |
| DPI b | 0.82 ± 0.31 | |||
| Elastatinal b | 31.82 ± 5.92 | |||
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Molecular Mechanics Calculations
3.5. Superoxide Anion Generation and Elastase Release by Human Neutrophils
4. Conclusions
Acknowledgments
- Samples Availability: Not available.
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2009, 26, 1156–1171. [Google Scholar] [CrossRef]
- Sung, P.-J.; Sheu, J.-H.; Xu, J.-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chang, P.-C.; Fang, L.-S.; Sheu, J.-H.; Chen, W.-C.; Chen, Y.-P.; Lin, M.-R. Survey of briarane-related diterpenoids—Part II. Heterocycles 2005, 65, 195–204. [Google Scholar] [CrossRef]
- Sung, P.-J.; Sheu, J.-H.; Wang, W.-H.; Fang, L.-S.; Chung, H.-M.; Pai, C.-H.; Su, Y.-D.; Tsai, W.-T.; Chen, B.-Y.; Lin, M.-R.; et al. Survey of briarane-type diterpenoids—Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Wang, W.-H.; Sheu, J.-H.; Fang, L.-S.; Wu, Y.-C.; Chen, Y.-H.; Chung, H.-M.; Su, Y.-D.; Chang, Y.-C. Survey of briarane-type diterpenoids—Part IV. Heterocycles 2011, 83, 1241–1258. [Google Scholar]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New cytotoxic briaran diterpenes from the Formosan gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar]
- Sheu, J.-H.; Sung, P.-J.; Cheng, M.-C.; Liu, H.-Y.; Fang, L.-S.; Duh, C.-Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A-E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Wang, G.-H.; Lin, S.-F.; Duh, C.-Y.; Sheu, J.-H. Excavatolides F-M, new briarane diterpenes from the gorgonian Briareum excavatum. J. Nat. Prod. 1999, 62, 457–463. [Google Scholar]
- Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Wang, G.-H.; Duh, C.-Y.; Shen, Y.-C.; Chiang, M.Y.; Chen, I.-T. Excavatolides U-Z, new briarane diterpenes from the Gorgonian Briareum excavatum. J. Nat. Prod. 1999, 62, 1415–1420. [Google Scholar]
- Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Liu, H.-Y.; Duh, C.-Y.; Chiang, M.Y. Briaexcavatolides A-J, new diterpenes from the gorgonian Briareum excavatum. Tetrahedron 1999, 55, 14555–14564. [Google Scholar]
- Sung, P.-J.; Su, J.-H.; Duh, C.-Y.; Chiang, M.Y.; Sheu, J.-H. Briaexcavatolides K-N, new briarane diterpenes from the gorgonian Briareum excavatum. J. Nat. Prod. 2001, 64, 318–323. [Google Scholar]
- Wu, S.-L.; Sung, P.-J.; Chiang, M.Y.; Wu, J.-Y.; Sheu, J.-H. New polyoxygenated briarane diterpenoids, briaexcavatolides O-R, from the gorgonian Briareum excavatum. J. Nat. Prod. 2001, 64, 1415–1420. [Google Scholar] [CrossRef]
- Wu, S.-L.; Sung, P.-J.; Su, J.-H.; Sheu, J.-H. Briaexcavatolides S-V, four new briaranes from a Formosan gorgonian Briareum excavatum. J. Nat. Prod. 2003, 66, 1252–1256. [Google Scholar] [CrossRef]
- Wu, S.-L.; Sung, P.-J.; Su, J.-H.; Wang, G.-H.; Sheu, J.-H. Briaexcavatolide W, a new diterpenoid from Briareum excavatum. Heterocycles 2004, 63, 895–898. [Google Scholar] [CrossRef]
- Sung, P.-J.; Hu, W.-P.; Wu, S.-L.; Su, J.-H.; Fang, L.-S.; Wang, J.-J.; Sheu, J.-H. Briaexcavatolides X-Z, three new briarane-related derivatives from the gorgonian coral Briareum excavatum. Tetrahedron 2004, 60, 8975–8979. [Google Scholar]
- Sung, P.-J.; Hu, W.-P.; Fang, L.-S.; Fan, T.-Y.; Wang, J.-J. Briarenol A, a new diterpenoid from a gorgonian Briareum sp. (Briareidae). Nat. Prod. Res. 2005, 19, 689–694. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chao, C.-H.; Chen, Y.-P.; Su, J.-H.; Hu, W.-P.; Sheu, J.-H. Briaexcavatins A and B, novel briaranes from the octocoral Briareum excavatum. Tetrahedron Lett. 2006, 47, 167–170. [Google Scholar]
- Sung, P.-J.; Chen, Y.-P.; Hwang, T.-L.; Hu, W.-P.; Fang, L.-S.; Wu, Y.-C.; Li, J.-J.; Sheu, J.-H. Briaexcavatins C-F, four new briarane-related diterpenoids from the Formosan octocoral Briareum excavatum (Briareidae). Tetrahedron 2006, 62, 5686–5691. [Google Scholar]
- Chen, Y.-P.; Wu, S.-L.; Su, J.-H.; Lin, M.-R.; Hu, W.-P.; Hwang, T.-L.; Sheu, J.-H.; Fan, T.-Y.; Fang, L.-S.; Sung, P.-J. Briaexcavatins G and H, two new briaranes from the octocoral Briareum excavatum. Bull. Chem. Soc. Jpn. 2006, 79, 1900–1905. [Google Scholar] [CrossRef]
- Su, J.-H.; Sung, P.-J.; Kuo, Y.-H.; Hsu, C.-H.; Sheu, J.-H. Briarenolides A-C, briarane diterpenoids from the gorgonian coral Briareum sp. Tetrahedron 2007, 63, 8282–8285. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Su, Y.-D.; Chiang, M.Y.; Hu, W.-P.; Su, J.-H.; Cheng, M.-C.; Hwang, T.-L.; Sheu, J.-H. New briaranes from the octocorals Briareum excavatum (Briareidae) and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 2596–2604. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Hwang, T.-L.; Fan, T.-Y.; Su, W.-C.; Ho, C.-C.; Fang, L.-S.; Wang, W.-H. Briaexcavatins M-P, four new briarane-related diterpenoids from cultured octocoral Briareum excavatum (Briareidae). Chem. Pharm. Bull. 2008, 56, 930–935. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Lin, M.-R.; Tsai, W.-T.; Yeh, H.-C.; Hu, W.-P.; Sheu, J.-H.; Sung, P.-J. New polyoxygenated briaranes from octocorals Briareum excavatum and Ellisella robusta. Bull. Chem. Soc. Jpn. 2008, 81, 1638–1646. [Google Scholar] [CrossRef]
- Sung, P.-J.; Lin, M.-R.; Chiang, M.Y. The structure and absolute stereochemistry of briaexcavatin U, a new chlorinated briarane from a cultured octocoral Briareum excavatum. Chem. Lett. 2009, 38, 154–155. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Chiang, M.Y.; Hwang, T.-L. Briaexcavatins V-Z, discovery of new briaranes from a cultured octocoral Briareum excavatum. Bull. Chem. Soc. Jpn. 2009, 82, 987–996. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, Y.-D.; Li, G.-Y.; Chiang, M.Y.; Lin, M.-R. Excavatoids A-D, new polyoxygenated briaranes from the octocoral Briareum excavatum. Tetrahedron 2009, 65, 6918–6924. [Google Scholar]
- Sung, P.-J.; Chen, B.-Y.; Lin, M.-R.; Hwang, T.-L.; Wang, W.-H.; Sheu, J.-H.; Wu, Y.-C. Excavatoids E and F: Discovery of two new briaranes from the cultured octocoral Briareum excavatum. Mar. Drugs 2009, 7, 472–482. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chen, B.-Y.; Chiang, M.Y.; Hou, C.-H.; Su, Y.-D.; Hwang, T.-L.; Chen, Y.-H.; Chen, J.-J. Excavatoids G-K, new 8,17-epoxybriaranes from the cultured octocoral Briareum excavatum (Briareidae). Bull. Chem. Soc. Jpn. 2010, 83, 539–545. [Google Scholar] [CrossRef]
- Su, J.-H.; Chen, B.-Y.; Hwang, T.-L.; Chen, Y.-H.; Huang, I.-C.; Lin, M.-R.; Chen, J.-J.; Fang, L.-S.; Wang, W.-H.; Li, J.-J.; et al. Excavatoids L-N, new 12-hydroxybriaranes from the cultured octocoral Briareum excavatum (Briareidae). Chem. Pharm. Bull. 2010, 58, 662–665. [Google Scholar]
- Sung, P.-J.; Lin, M.-R.; Chiang, M.Y.; Syu, S.-M.; Fang, L.-S.; Wang, W.-H.; Sheu, J.-H. Briarenolide D, a new hydroperoxybriarane diterpenoid from a cultured octocoral Briareum sp. Chem. Lett. 2010, 39, 1030–1032. [Google Scholar]
- Sung, P.-J.; Li, G.-Y.; Su, Y.-D.; Lin, M.-R.; Chang, Y.-C.; Kung, T.-H.; Lin, C.-S.; Chen, Y.-H.; Su, J.-H.; Lu, M.-C.; et al. Excavatoids O and P, new 12-hydroxybriaranes from the octocoral Briareum excavatum. Mar. Drugs 2010, 8, 2639–2646. [Google Scholar] [CrossRef]
- Hong, P.-H.; Su, Y.-D.; Lin, N.-C.; Chen, Y.-H.; Kuo, Y.-H.; Hwang, T.-L.; Wang, W.-H.; Chen, J.-J.; Sheu, J.-H.; Sung, P.-J. Briarenolide E: The first 2-ketobriarane diterpenoid from an octocoral Briareum sp. (Briareidae). Tetrahedron Lett. 2012, 53, 1710–1712. [Google Scholar]
- Yeh, T.-T.; Wang, S.-K.; Dai, C.-F.; Duh, C.-Y. Briacavatolides A-C, new briaranes from the Taiwanese octocoral Briareum excavatum. Mar. Drugs 2012, 10, 1019–1026. [Google Scholar]
- Chang, Y.-C.; Huang, I.-C.; Chiang, M.Y.; Hwang, T.-L.; Kung, T.-H.; Lin, C.-S.; Sheu, J.-H.; Sung, P.-J. Briaviodiol A, a new cembranoid from a soft coral Briareum violacea. Chem. Pharm. Bull. 2010, 58, 1666–1668. [Google Scholar]
- Sung, P.-J.; Chen, B.-Y.; Chen, Y.-H.; Chiang, M.Y.; Lin, M.-R. Loliolide: Occurrence of a carotenoid metabolite in the octocoral Briareum excavatum (Briareidae). Biochem. Syst. Ecol. 2010, 38, 116–118. [Google Scholar] [CrossRef]
- Aoki, S.; Okano, M.; Matsui, K.; Itoh, T.; Satari, R.; Akiyama, S.-I.; Kobayashi, M. Brianthein A, a novel briarane-type diterpene reversing multidrug resistance in human carcinoma cell line, from the gorgonian Briareum excavatum. Tetrahedron 2001, 57, 8951–8957. [Google Scholar]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Guerriero, A.; D’Ambrosio, M.; Pietra, F. Slowly interconverting conformers of the briarane diterpenoids verecynarmin B, C, and D, isolated from the nudibranch mollusc Armina maculata and the Pennatulacean octocoral Veretillum cynomorium of East Pyrenean waters. Helv. Chim. Acta 1988, 71, 472–485. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, N.S.K. Juncins G and H: New briarane diterpenoids of the Indian Ocean gorgonian Junceella juncea Pallas. J. Chem. Soc. Perkin Trans. 1 1997, 959–962. [Google Scholar] [CrossRef]
- Sung, P.-J.; Lin, M.-R.; Chen, W.-C.; Fang, L.-S.; Lu, C.-K.; Sheu, J.-H. Fragilide A, a novel diterpenoid from Junceella fragilis. Bull. Chem. Soc. Jpn. 2004, 77, 1229–1230. [Google Scholar] [CrossRef]
- Ospina, C.A.; Rodríguez, A.D. Bioactive compounds from the gorgonian Briareum polyanthes. Correction of the structures of four asbestinane-type diterpenes. J. Nat. Prod. 2006, 69, 1721–1727. [Google Scholar] [CrossRef]
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Wash. Soc. 1981, 94, 902–947. [Google Scholar]
- Benayahu, Y. Soft corals (Octocorallia: Alcyonacea) of the southern Ryukyu Archipelago: The families Tubiporidae, Clavulariidae, Alcyoniidae and Briareidae. Galaxea 2002, 4, 11–32. [Google Scholar] [CrossRef]
- Benayahu, Y.; Jeng, M.-S.; Perkol-Finkel, S.; Dai, C.-F. Soft corals (Octocorallia: Alcyonacea) from Southern Taiwan. II. Species diversity and distributional patterns. Zool. Stud. 2004, 43, 548–560. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 154–157. [Google Scholar]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar]
- Dookran, R.; Maharaj, D.; Mootoo, B.S.; Ramsewak, R.; McLean, S.; Reynolds, W.F.; Tinto, W.F. Briarane and asbestinane diterpenes from Briareum asbestinum. Tetrahedron 1994, 50, 1983–1992. [Google Scholar]
- Guerriero, A.; D’Ambrosio, M.; Pietra, F. Bis-allylic reactivity of the funicolides, 5,8(17)-diunsaturated briarane diterpenes of the sea pen Funiculina quadrangularis from the Tuscan Archipelago, leading to 16-nortaxane derivatives. Helv. Chim. Acta 1995, 78, 1465–1478. [Google Scholar] [CrossRef]
- Xu, L.; Patrick, B.O.; Roberge, M.; Allen, T.; van Ofwegen, L.; Andersen, R.J. New diterpenoids from the octocoral Pachyclavularia violacea collected in Papua New Guinea. Tetrahedron 2000, 56, 9031–9037. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hong, P.-H.; Su, Y.-D.; Su, J.-H.; Chen, Y.-H.; Hwang, T.-L.; Weng, C.-F.; Lee, C.-H.; Wen, Z.-H.; Sheu, J.-H.; Lin, N.-C.; et al. Briarenolides F and G, New Briarane Diterpenoids from a Briareum sp. Octocoral. Mar. Drugs 2012, 10, 1156-1168. https://doi.org/10.3390/md10051156
Hong P-H, Su Y-D, Su J-H, Chen Y-H, Hwang T-L, Weng C-F, Lee C-H, Wen Z-H, Sheu J-H, Lin N-C, et al. Briarenolides F and G, New Briarane Diterpenoids from a Briareum sp. Octocoral. Marine Drugs. 2012; 10(5):1156-1168. https://doi.org/10.3390/md10051156
Chicago/Turabian StyleHong, Pei-Han, Yin-Di Su, Jui-Hsin Su, Yung-Husan Chen, Tsong-Long Hwang, Ching-Feng Weng, Chia-Hung Lee, Zhi-Hong Wen, Jyh-Horng Sheu, Nai-Cheng Lin, and et al. 2012. "Briarenolides F and G, New Briarane Diterpenoids from a Briareum sp. Octocoral" Marine Drugs 10, no. 5: 1156-1168. https://doi.org/10.3390/md10051156







