Secondary Metabolites from an Algicolous Aspergillus versicolor Strain
Abstract
:1. Introduction
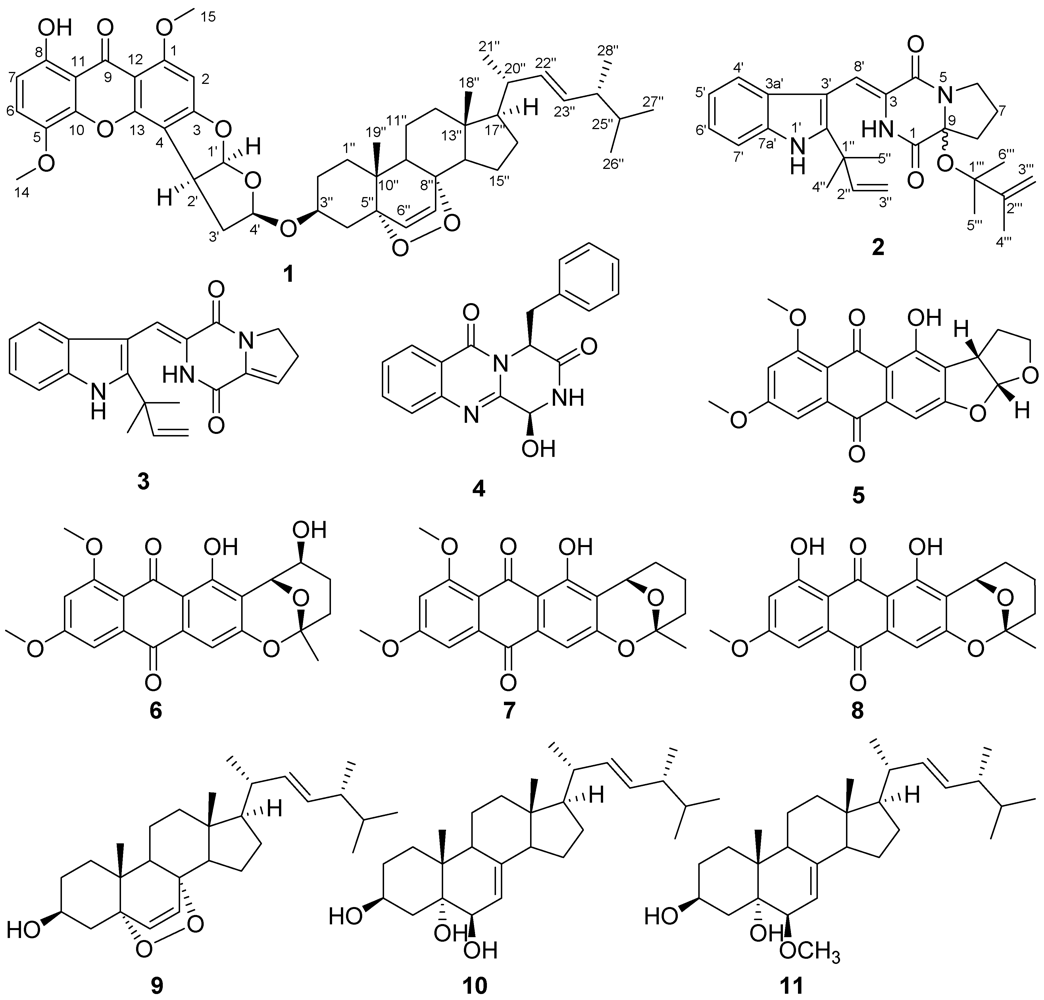
2. Results and Discussion
| Position | δH (J in Hz) | δC, mult. | Position | δH(J in Hz) | δC, mult. |
|---|---|---|---|---|---|
| 1 | 163.3, C | 6" | 6.11, d (8.5) | 135.3, CH | |
| 2 | 6.34, s | 90.1, CH | 7" | 6.44, d (8.5) | 130.7, CH |
| 3 | 165.3, C | 8" | 79.4, C | ||
| 4 | 108.2, C | 9" | 1.44, m | 51.1, CH | |
| 5 | 139.4, C | 10" | 37.1, C | ||
| 6 | 7.18, d (9.0) | 120.4, CH | 11"a | 1.36, m | 20.6, CH2 |
| 7 | 6.68, d (9.0) | 109.3, CH | 11"b | 1.56, m | |
| 8 | 155.3, C | 12"a | 1.20, m | 39.3, CH2 | |
| 9 | 181.6, C | 12"b | 1.92, m | ||
| 10 | 144.9, C | 13" | 44.5, C | ||
| 11 | 109.6, C | 14" | 1.52, m | 51.6, CH | |
| 12 | 105.7, C | 15"a | 1.16, m | 23.3, CH2 | |
| 13 | 153.9, C | 15"b | 1.45, m | ||
| 14 | 3.91, s | 57.8, CH3 | 16"a | 1.33, m | 28.6, CH2 |
| 15 | 3.99, s | 56.8, CH3 | 16"b | 1.73, m | |
| 1' | 6.51, d (6.0) | 113.5, CH | 17" | 1.20, m | 56.2, CH |
| 2' | 4.21, dd (9.2, 6.0) | 43.0, CH | 18" | 0.78, s | 12.8, CH3 |
| 3'a | 2.34, ddd (13.2, 9.2, 4.9) | 36.9, CH2 | 19" | 0.72, s | 18.0, CH3 |
| 3'a | 2.47, d (13.2) | 20" | 2.00, m | 39.7, CH | |
| 4' | 5.39, d (4.9) | 104.2, CH | 21" | 0.98, d (6.6) | 20.9, CH3 |
| 1"a | 1.57, m | 34.6, CH2 | 22" | 5.13, dd (15.3, 8.3) | 135.2, CH |
| 1"b | 1.86, m | 23" | 5.21, dd (15.3, 7.6) | 132.3, CH | |
| 2"a | 1.13, m | 27.6, CH2 | 24" | 1.84, m | 42.8, CH |
| 2"b | 1.73, m | 25" | 1.45, m | 33.1, CH | |
| 3" | 3.79, m | 72.1, CH | 26" | 0.81, d (6.8) | 19.6, CH3 |
| 4"a | 1.54, m | 33.6, CH2 | 27" | 0.83, d (6.8) | 20.0, CH3 |
| 4"b | 1.91, m | 28" | 0.90, d (6.8) | 17.6, CH3 | |
| 5" | 81.8, C | OH | 12.73, s |
| Position | δH (J in Hz) | δC, mult. | Position | δH(J in Hz) | δC, mult. |
|---|---|---|---|---|---|
| 1 | 162.3, C | 6' | 7.19, dd (7.5, 8.0) | 122.3, CH | |
| 2 | 7.48 br, s | 7' | 7.35, d (8.0) | 111.1, CH | |
| 3 | 126.2, C | 7a' | 134.2, C | ||
| 4 | 159.4, C | 8' | 7.29, s | 111.6, CH | |
| 5 | 1" | 39.3, C | |||
| 6a | 3.75, m | 45.2, CH2 | 2" | 6.08, dd (17.4, 10.6) | 144.3, CH |
| 6b | 3.95, m | 3"a | 5.21, d (17.4) | 113.4, CH2 | |
| 7a | 2.00, m | 19.8, CH2 | 3"b | 5.24, d (10.6) | |
| 7b | 2.10, m | 4" | 1.54, s | 27.3, CH3 | |
| 8a | 2.25, m | 33.5, CH2 | 5" | 1.54, s | 27.7, CH3 |
| 8b | 2.37, m | 1"' | 84.6, C | ||
| 9 | 94.3, C | 2"' | 148.1, C | ||
| 1' | 8.29 br, s | 3"'a | 4.89, s | 111.7, CH2 | |
| 2' | 144.1, C | 3"'b | 4.96, s | ||
| 3' | 103.5, C | 4"' | 1.83, s | 18.5, CH3 | |
| 3a' | 126.2, C | 5"' | 1.40, s | 24.0, CH3 | |
| 4' | 7.47, d (7.5) | 119.9, CH | 6"' | 1.32, s | 25.0, CH3 |
| 5' | 7.13, dd (7.5, 7.5) | 120.9, CH |
| Compounds | Inhibition Zone (mm) | Lethal Rates (%) | |
|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | Artemia salina | |
| 1 | 7 | 7 | 1.8 |
| 2 | 7 | 7 | 43.2 |
| 3 | 7 | 7 | 30.9 |
| 4 | 11 | 10 | 47.6 |
| 5 | 6 | 6 | 17.5 |
| 6 | 7 | 7 | 29.1 |
| 7 | 10 | 10 | 100.0 |
| 8 | 10 | 10 | 38.5 |
| chloramphenicol | 32 | 31 | |
3. Experimental Section
3.1. General
3.2. Microorganism and Fermentation
3.3. Extraction and Isolation
3.4. Antimicrobial Assay
3.5. Brine Shrimp Lethality Assay
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
Supplementary Files
References
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar]
- Osterhage, C.; König, G.M.; Höller, U.; Wright, A.D. Rare sesquiterpenes from the algicolous fungus Drechslera dematioidea. J. Nat. Prod. 2002, 65, 306–313. [Google Scholar]
- Li, G.Y.; Yang, T.; Luo, Y.G.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org. Lett. 2009, 11, 3714–3717. [Google Scholar]
- Shao, C.; She, Z.; Guo, Z.; Peng, H.; Cai, X.; Zhou, S.; Gu, Y.; Lin, Y. Spectral assignments and reference data. Magn. Reson. Chem. 2007, 45, 434–438. [Google Scholar]
- Kingston, D.G.I.; Chen, P.N.; Vercellotti, J.R. Metabolites of Aspergillus versicolor. 6,8-di-Ο-methylnidurufin, griseofulvin, dechlorogriseofluvin, and 3,8-dihydroxy-6-methoxy-1-methylxanthone. Phytochemistry 1976, 15, 1037–1039. [Google Scholar]
- Ren, H.; Gu, Q.Q.; Cui, C.B. Anthraquinone derivatives produced by marine-derived Penicillium flavidorsum SHK1-27 and their antitumor activities. Chin. J. Med. Chem. 2007, 17, 148–154. [Google Scholar]
- Steyn, P.S.; Vleggaar, R.; Wessels, P.S.; Cole, R.J.; Scott, D.B. Structure and carbon-13 nuclear magnetic resonance assignments of versiconal acetate, versiconol acetate, and versiconol, metabolites from cultures of Aspergillus parasiticus treated with ditchlorvos. J. Chem. Soc. Perkin Trans. I 1979, 451–459. [Google Scholar]
- Greca, M.D.; Mangoni, L.; Malinaro, A.; Monaco, P.; Previtera, L. 5β,8β-Epidioxyergosta-6,22-dien-3β-ol from Typha latifolia. Gazz. Chim. Ital. 1990, 120, 391–392. [Google Scholar]
- Kawagishi, H.; Katsumi, R.; Sazawa, T.; Mizuno, T.; Hagiwara, T.; Nakamura, T. Cytotoxic steroids from the mushroom Agaricus blazei. Phytochemistry 1988, 27, 2777–2779. [Google Scholar]
- Li, G.Y.; Li, L.M.; Yang, T.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Four new alkaloids, brevianamides O–R, from the fungus Aspergillus versicolor. Helv. Chim. Acta 2010, 93, 2075–2080. [Google Scholar]
- Schulz, B.; Sucker, J.; Aust, H.J.; Krohn, K.; Ludewig, K.; Jones, P.G.; Döring, D. Biologically active secondary metabolites of endophytic Pezicula species. Mycol. Res. 1995, 99, 1007–1015. [Google Scholar]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miao, F.-P.; Li, X.-D.; Liu, X.-H.; Cichewicz, R.H.; Ji, N.-Y. Secondary Metabolites from an Algicolous Aspergillus versicolor Strain. Mar. Drugs 2012, 10, 131-139. https://doi.org/10.3390/md10010131
Miao F-P, Li X-D, Liu X-H, Cichewicz RH, Ji N-Y. Secondary Metabolites from an Algicolous Aspergillus versicolor Strain. Marine Drugs. 2012; 10(1):131-139. https://doi.org/10.3390/md10010131
Chicago/Turabian StyleMiao, Feng-Ping, Xiao-Dong Li, Xiang-Hong Liu, Robert H. Cichewicz, and Nai-Yun Ji. 2012. "Secondary Metabolites from an Algicolous Aspergillus versicolor Strain" Marine Drugs 10, no. 1: 131-139. https://doi.org/10.3390/md10010131





