Using Odontoblasts Derived from Dog Endometrial Stem Cells Encapsulated in Fibrin Gel Associated with BMP-2 in a Rat Pulp-Capping Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation, Culture, and Identification
2.2. Differentiation of C-EnSCs into Adipose, Bone, and Nerve Cells
2.3. Flow Cytometric Analysis
2.4. Differentiation of C-EnSCs into Odontoblasts
2.5. cDNA Synthesis and RT-PCR Analysis
2.6. Preparation of Hydrogel-Cell Complex
2.7. Scanning Electron Microscopy Analysis
2.8. Evaluation of Cell Survival by Acridine Orange/Ethidium Bromide (AO/EB) Staining
2.9. BMP-2 Loading and Release Behavior in Fibrin Gel
2.10. MTT (2,5-Diphenyl-2H-tetrazolium Bromide) Assay
2.11. Preparation of the Teeth and Design of Models
2.12. Transplantation of Stem Cells and Grouping
2.13. Analyzing Histomorphometric Data
2.14. Cell Tracker Staining
2.15. Statistical Analysis
3. Results
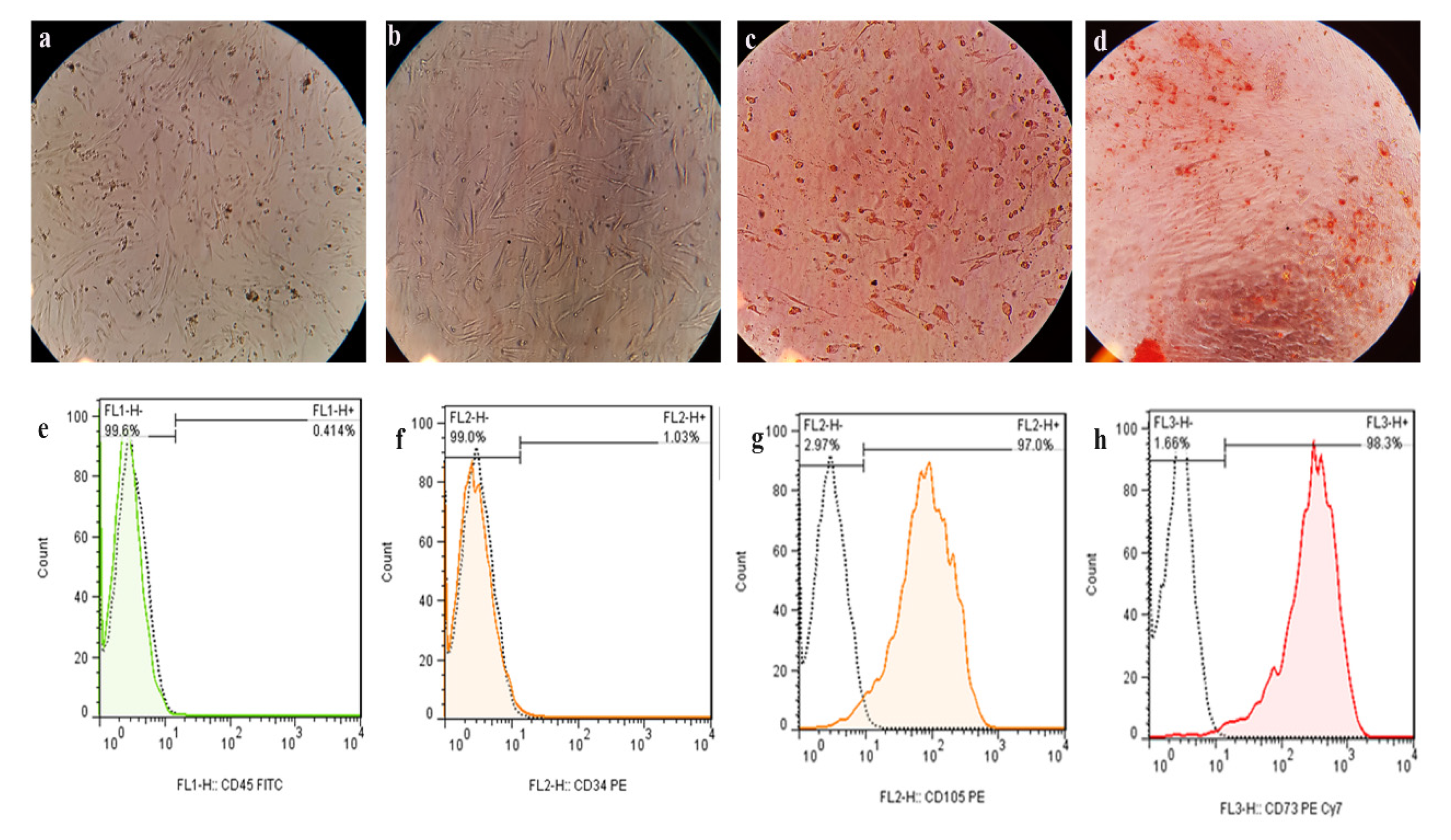
3.1. Isolation, Morphology, and Identification of C-EnSCs
3.2. Flow Cytometry Results
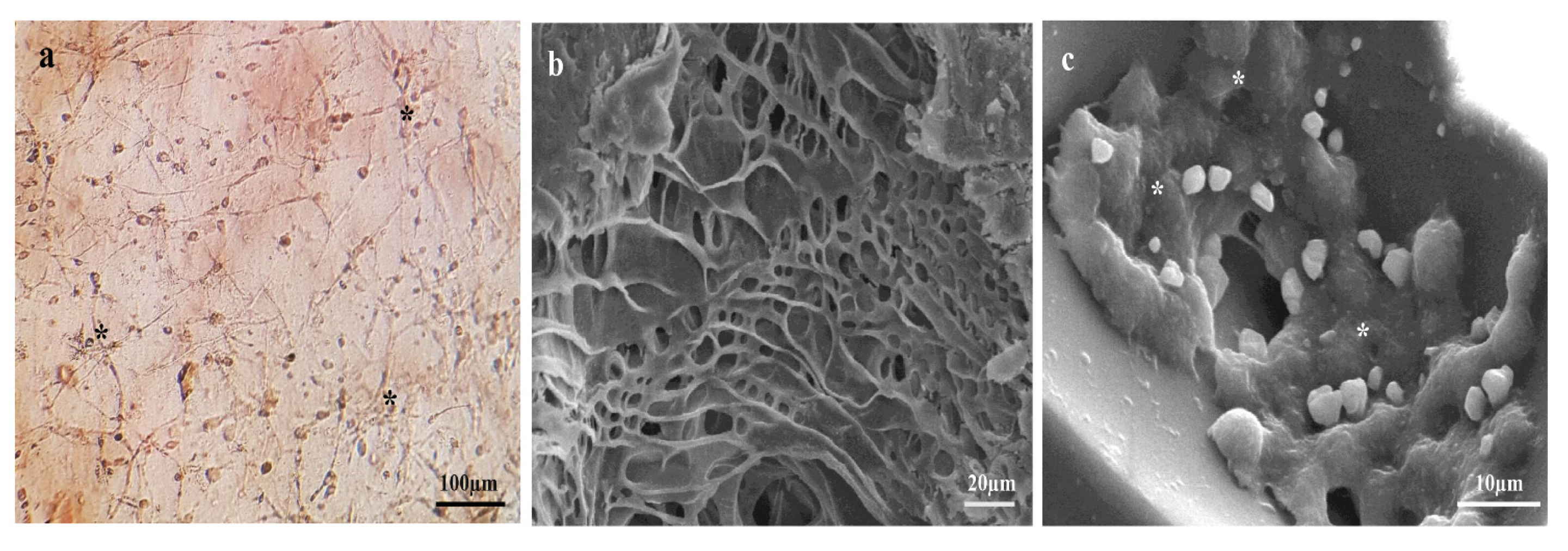
3.3. Odontogenic Differentiation Analysis Using Alizarin Red Staining and qRT-PCR
3.4. SEM Observations of Fibrin Gel
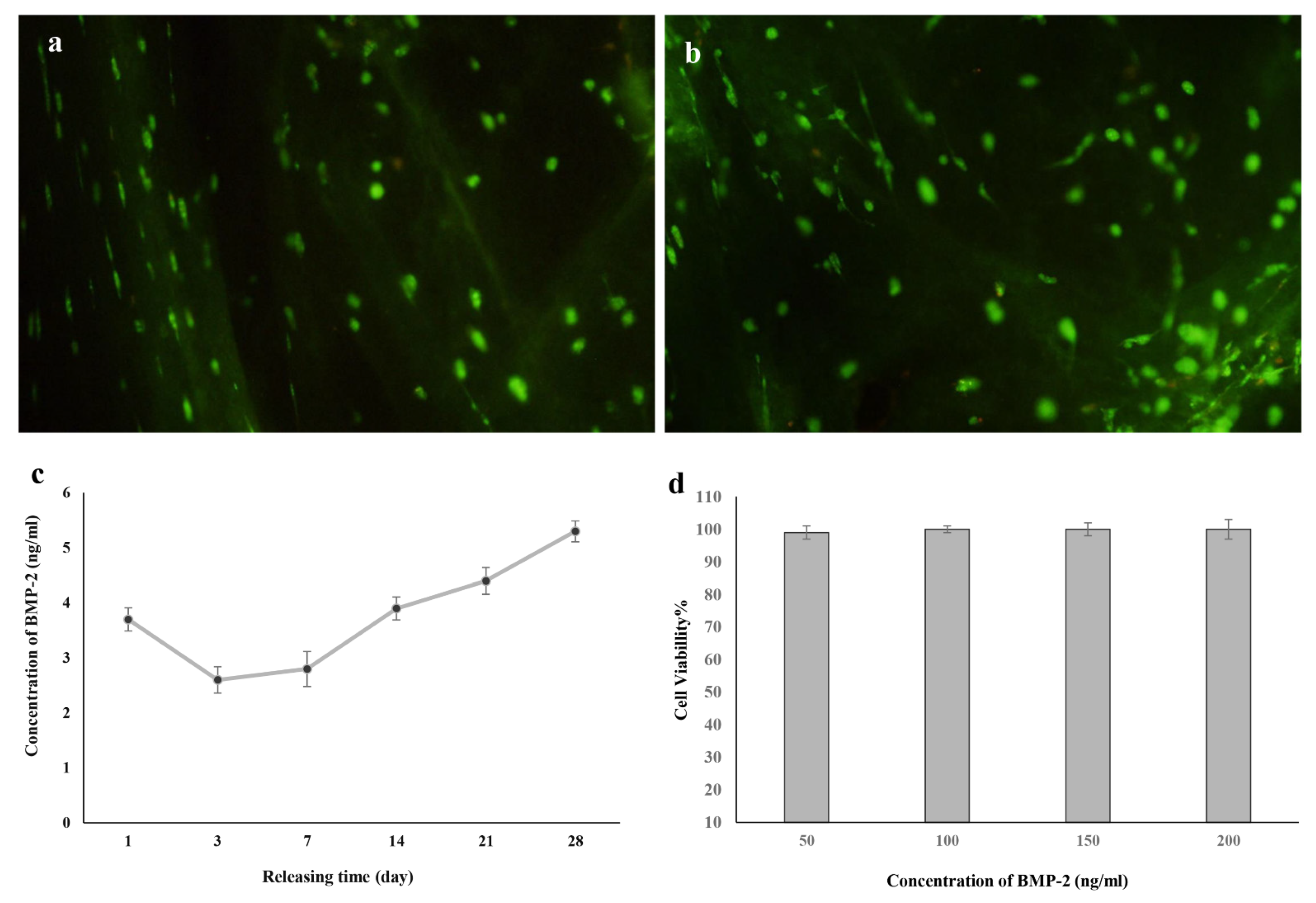
3.5. Viability Assessment
3.6. Release Behavior of BMP-2
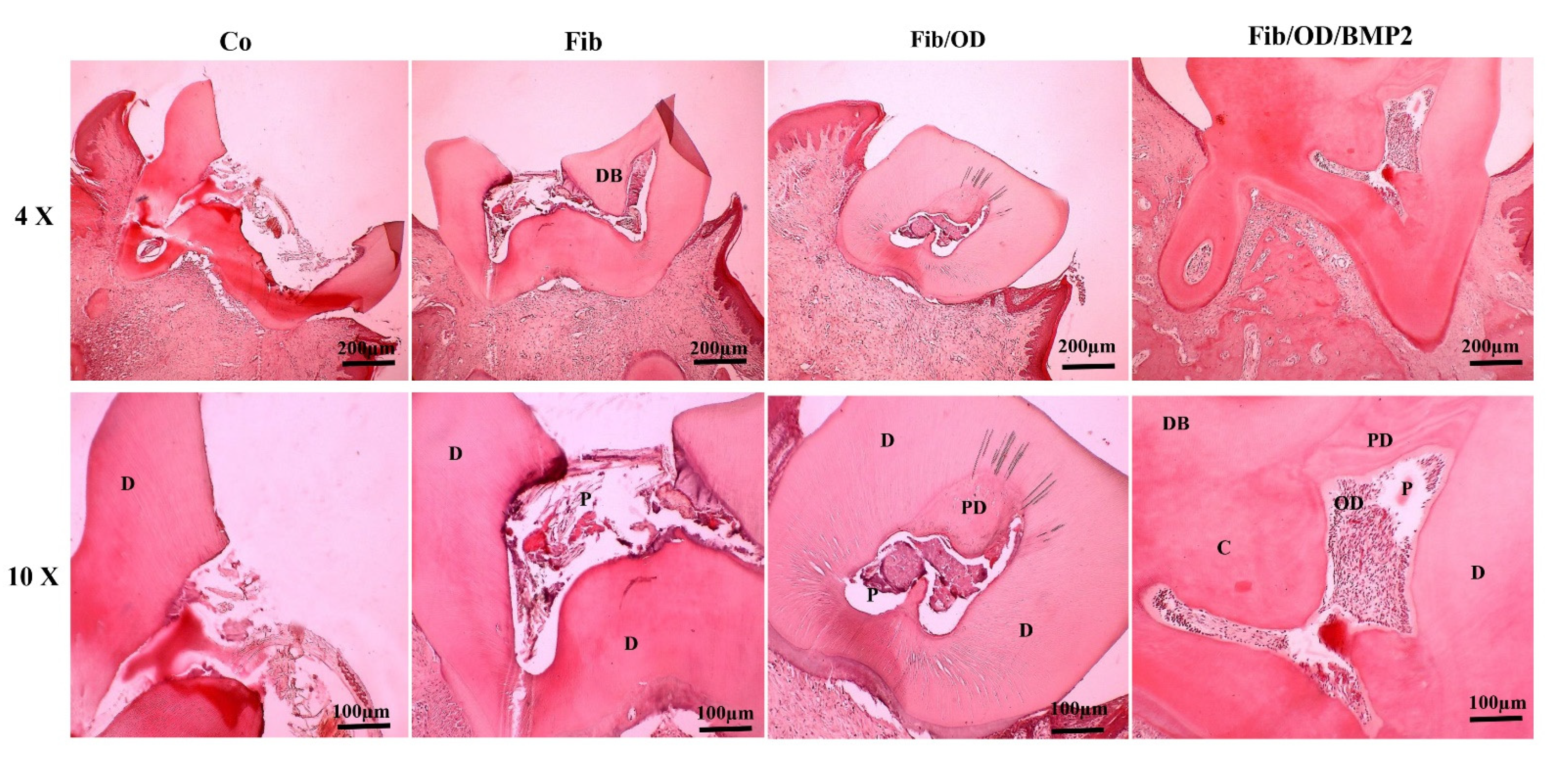
3.7. Results of Histomorphology and Histomorphometry Using H&E Staining
3.8. The Thickness of the Dentin Bridge
3.9. Cell Tracking
4. Discussion
4.1. The Endometrium Contains a Vascular Stroma with High Regeneration Capability
4.2. Differentiation of C-EnSCs
4.3. Role of Scaffolds in Tissue Repairments
4.4. Growth Factors Role in Differentiation and Tissue Regeneration
4.5. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, K.; Liao, X.; Liang, L.; Li, Y.; Huang, Z.; Li, X.; Zhou, W.; Zuo, Q. Clinical application of oral Chinese Patent Medicines in ophthalmology: A scoping review protocol. BMJ Open 2022, 12, e059571. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emami, E.; de Souza, R.F.; Kabawat, M.; Feine, J.S. The impact of edentulism on oral and general health. Int. J. Dent. 2013, 2013, 498305. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dang, M.; Zhang, Z.; Hu, J.; Eyster, T.W.; Ni, L.; Ma, P.X. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016, 36, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. A review on the use of computational methods to characterize, design, and optimize tissue engineering scaffolds, with a potential in 3D printing fabrication. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1329–1351. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Azizi, R.; Aghebati-Maleki, L.; Nouri, M.; Marofi, F.; Negargar, S.; Yousefi, M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed. Pharmacother. 2018, 102, 333–343. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Das, J.K.; Nayak, S. Isolation, culture, characterization, and osteogenic differentiation of canine endometrial mesenchymal stem cell. Vet. World 2017, 10, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Jongwattanapisan, P.; Terajima, M.; Miguez, P.A.; Querido, W.; Nagaoka, H.; Sumida, N.; Gurysh, E.G.; Ainslie, K.M.; Pleshko, N.; Perera, L.; et al. Identification of the effector domain of biglycan that facilitates BMP-2 osteogenic function. Sci. Rep. 2018, 8, 7022. [Google Scholar] [CrossRef] [Green Version]
- Shamszadeh, S.; Asgary, S.; Torabzadeh, H.; Hosseinzadeh, S.; Nosrat, A. Cytokine co-stimulation effect on odontogenic differentiation of stem cells. Clin. Oral Investig. 2022, 26, 4789–4796. [Google Scholar] [CrossRef]
- Chakka, L.R.J.; Vislisel, J.; Vidal, C.M.P.; Biz, M.T.; Salem, A.K.; Cavalcanti, B.N. Application of BMP-2/FGF-2 gene-activated scaffolds for dental pulp capping. Clin. Oral Investig. 2020, 24, 4427–4437. [Google Scholar] [CrossRef]
- Hoveizi, E.; Tavakol, S. Therapeutic potential of human mesenchymal stem cells derived beta cell precursors on a nanofibrous scaffold: An approach to treat diabetes mellitus. J. Cell Physiol. 2019, 234, 10196–10204. [Google Scholar] [CrossRef]
- Ebrahimi-Barough, S.; Hoveizi, E.; Norouzi Javidan, A.; Ai, J. Investigating the neuroglial differentiation effect of neuroblastoma conditioned medium in human endometrial stem cells cultured on 3D nanofibrous scaffold. J. Biomed. Mater. Res. A 2015, 3, 2621–2627. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Dammaschke, T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab. Anim. 2010, 44, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tzanetakis, G.N.; Tsiouma, O.; Mougiou, E.; Koletsi, D. Factors Related to Pulp Survival After Complicated Crown Fracture Following Vital Pulp Therapy: A Systematic Review and Meta-analysis. J. Endod. 2022, 48, 457–478.e4. [Google Scholar] [CrossRef]
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental pulp stem cells response on the nanotopography of scaffold to regenerate dentin-pulp complex tissue. Regen. Ther. 2020, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Mancini, L.; Torge, D.; Cristiano, L.; Mattei, A.; Varvara, G.; Macchiarelli, G.; Marchetti, E.; Bernardi, S. Bio-Morphological Reaction of Human Periodontal Ligament Fibroblasts to Different Types of Dentinal Derivates: In Vitro Study. Int. J. Mol. Sci. 2021, 22, 8681. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Mazidi, S.A.; Mohammadi Asl, S.; Ellini, M.R.; Moshiri, A.; Nekoofar, M.H.; Dummer, P.M.H. The role of stem cell therapy in regeneration of dentine-pulp complex: A systematic review. Prog. Biomater. 2018, 7, 249–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoveizi, E.; Mohammadi, T. Differentiation of endometrial stem cells into insulin-producing cells using signaling molecules and zinc oxide nanoparticles, and three-dimensional culture on nanofibrous scaffolds. J. Mater. Sci. Mater. Med. 2019, 30, 101. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Liu, B.; Liu, G.; Zhang, W.; Cen, L.; Sun, J.; Yin, S.; Liu, W.; Cao, Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 2007, 28, 5477–5486. [Google Scholar] [CrossRef]
- Verdi, J.; Tan, A.; Shoae-Hassani, A.; Seifalian, A.M. Endometrial stem cells in regenerative medicine. J. Biol. Eng. 2014, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, D.; Edwards, S.L.; White, J.F.; Supit, T.; Ramshaw, J.A.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. A preclinical evaluation of alternative synthetic biomaterials for fascial defect repair using a rat abdominal hernia model. PLoS ONE 2012, 7, e50044. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi-Barough, S.; Hoveizi, E.; Yazdankhah, M.; Ai, J.; Khakbiz, M.; Faghihi, F.; Tajerian, R.; Bayat, N. Inhibitor of PI3K/Akt Signaling Pathway Small Molecule Promotes Motor Neuron Differentiation of Human Endometrial Stem Cells Cultured on Electrospun Biocomposite Polycaprolactone/Collagen Scaffolds. Mol. Neurobiol. 2017, 54, 2547–2554. [Google Scholar] [CrossRef]
- Shoae-Hassani, A.; Sharif, S.; Seifalian, A.M.; Mortazavi-Tabatabaei, S.A.; Rezaie, S.; Verdi, J. Endometrial stem cell differentiation into smooth muscle cell: A novel approach for bladder tissue engineering in women. BJU Int. 2013, 112, 854–863. [Google Scholar] [CrossRef]
- Tziafas, D. Characterization of Odontoblast-like Cell Phenotype and Reparative Dentin Formation In Vivo: A Comprehensive Literature Review. J. Endod. 2019, 45, 241–249. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Li, Y.; Ren, L.; Deng, H.; Yin, X.; Gao, X.; Pan, S.; Niu, Y. Human Umbilical Cord Mesenchymal Stem Cell Differentiation Into Odontoblast-Like Cells and Endothelial Cells: A Potential Cell Source for Dental Pulp Tissue Engineering. Front. Physiol. 2020, 11, 593. [Google Scholar] [CrossRef]
- Kimura, M.; Mochizuki, H.; Satou, R.; Iwasaki, M.; Kokubu, E.; Kono, K.; Nomura, S.; Sakurai, T.; Kuroda, H.; Shibukawa, Y. Plasma Membrane Ca2+–ATPase in Rat and Human Odontoblasts Mediates Dentin Mineralization. Biomolecules 2021, 11, 1010. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, M.R.; Ansari-Asl, Z.; Hoveizi, E.; Kiasat, A.R. Polyacrylonitrile/Fe (III) metal-organic framework fibrous nanocomposites designed for tissue engineering applications. Mater. Chem. Phys. 2019, 229, 242–250. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.-C.; Verron, E. Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Park, S.S.; Kim, K.; Kim, S.J.; Lee, J.H.; Yoon, S.S.; Mun, Y.C.; Lee, J.J.; Eom, H.S.; Kim, J.S.; Min, C.K.; et al. A Phase I/II, Open-Label, Prospective, Multicenter Study to Evaluate the Efficacy and Safety of Lower Doses of Bortezomib Plus Busulfan and Melphalan as a Conditioning Regimen in Patients with Multiple Myeloma Undergoing Autologous Peripheral Blood Stem Cell Transplantation: The KMM103 Study. Biol. Blood Marrow Transplant. 2019, 25, 1312–1319. [Google Scholar]
- Xie, J.; Willerth, S.M.; Li, X.; Macewan, M.R.; Rader, A.; Sakiyama-Elbert, S.E.; Xia, Y. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 2009, 30, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Aksel, H.; Öztürk, Ş.; Serper, A. Ulubayram K 2018 VEGF/BMP-2 loaded three-dimensional model for enhanced angiogenic and odontogenic potential of dental pulp stem cells. Int. Endod. J. 2018, 51, 420–430. [Google Scholar] [CrossRef]
- Casagrande, L.; Demarco, F.F.; Zhang, Z.; Araujo, F.B.; Shi, S.; Nör, J.E. Dentin-derived BMP-2 and odontoblast differentiation. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef]
- Xiao, M.; Qiu, J.; Kuang, R.; Zhang, B.; Wang, W.; Yu, Q. Synergistic effects of stromal cell-derived factor-1α and bone morphogenetic protein-2 treatment on odontogenic differentiation of human stem cells from apical papilla cultured in the VitroGel 3D system. Cell Tissue Res. 2019, 378, 207–220. [Google Scholar] [CrossRef] [PubMed]


| Name | Primer Sequence (5′→3′) | Accession |
|---|---|---|
| ACTB (R) | TCGTCCCAGTTGGTGACGAT | NM_001101.5 |
| ACTB (F) | GCATGGGTCAGAAGGATTCCT | |
| DSPP (R) | TTGCTTTGAGGAACTGGAAT | NM_014208.3 |
| DSPP (F) | ATGCAAAAGTCCAGGACAG | |
| DMP1(R) | TTGATACCTGGTTACTGGGA | NM_004407.4 |
| DMP1(F) | TTCTTTGTGAACTACGGAGG |
| Case | Control | Fibrin | Fibrin/ODs | Fibrin/ODs/BMP-2 |
|---|---|---|---|---|
| 1 | 0.25 | 0.38 | 0.99 | 0.97 |
| 2 | 0.20 | 0.42 | 0.95 | 1.19 |
| 3 | 0.33 | 0.39 | 0.96 | 1.11 |
| 4 | 0.19 | 0.33 | 0.97 | 1.25 |
| 5 | 0.22 | 0.43 | 0.99 | 1.29 |
| Mean ± DS | 0.24 ± 0.14 | 0.39 ± 0.10 | 0.97 ± 0.04 | 1.16 ± 0.32 |
| Case | Control | Fibrin | Fibrin/ODs | Fibrin/ODs/BMP-2 |
|---|---|---|---|---|
| 1 | 35 | 87 | 250 | 297 |
| 2 | 41 | 94 | 255 | 295 |
| 3 | 43 | 83 | 266 | 290 |
| 4 | 30 | 83 | 262 | 294 |
| 5 | 53 | 90 | 258 | 289 |
| Mean ± DS | 40 ± 13 | 87 ± 7 | 258 ± 16 | 293 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoveizi, E.; Naddaf, H.; Ahmadianfar, S.; Bernardi, S. Using Odontoblasts Derived from Dog Endometrial Stem Cells Encapsulated in Fibrin Gel Associated with BMP-2 in a Rat Pulp-Capping Model. Curr. Issues Mol. Biol. 2023, 45, 2984-2999. https://doi.org/10.3390/cimb45040196
Hoveizi E, Naddaf H, Ahmadianfar S, Bernardi S. Using Odontoblasts Derived from Dog Endometrial Stem Cells Encapsulated in Fibrin Gel Associated with BMP-2 in a Rat Pulp-Capping Model. Current Issues in Molecular Biology. 2023; 45(4):2984-2999. https://doi.org/10.3390/cimb45040196
Chicago/Turabian StyleHoveizi, Elham, Hadi Naddaf, Sina Ahmadianfar, and Sara Bernardi. 2023. "Using Odontoblasts Derived from Dog Endometrial Stem Cells Encapsulated in Fibrin Gel Associated with BMP-2 in a Rat Pulp-Capping Model" Current Issues in Molecular Biology 45, no. 4: 2984-2999. https://doi.org/10.3390/cimb45040196






