Revealing Potential Bioactive Compounds and Mechanisms of Lithospermum erythrorhizon against COVID-19 via Network Pharmacology Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selective Compounds’ Construction and Drug-Likeness Evaluation
2.2. Targets Associated with Selected Compounds or COVID-19
2.3. The Analysis of the Protein–Protein Interaction (PPI) Networks
2.4. The Construction of a Bubble Chart
2.5. The Assembly of Signaling Pathways–Targets–Bioactive Compounds (STB) Networks
2.6. The Preparation of the Bioactive Compounds and Targets for Molecular Docking Test (MDT)
2.7. The MDT on a Key Signaling Pathway
3. Results
3.1. Potential Bioactive Compounds from LE
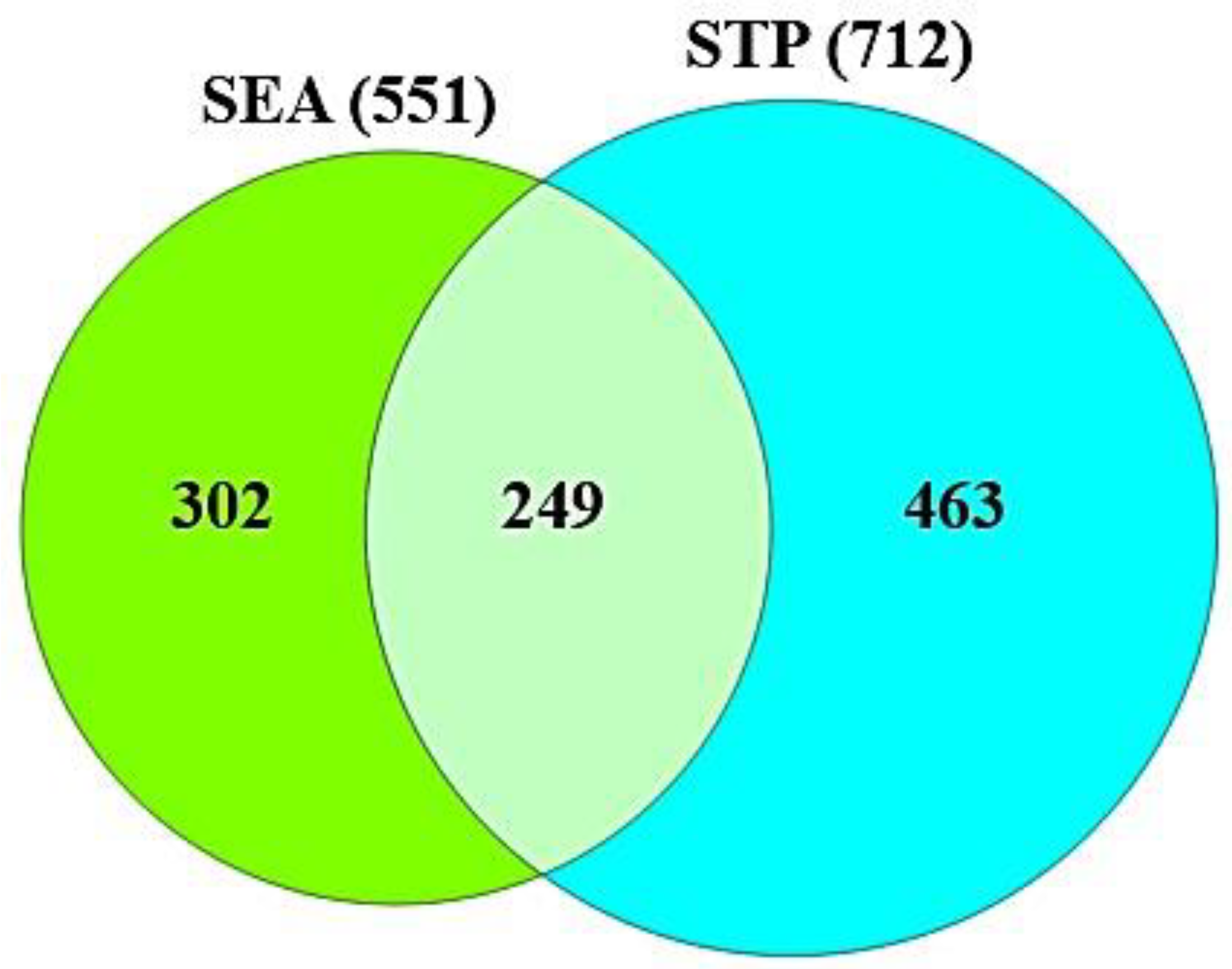
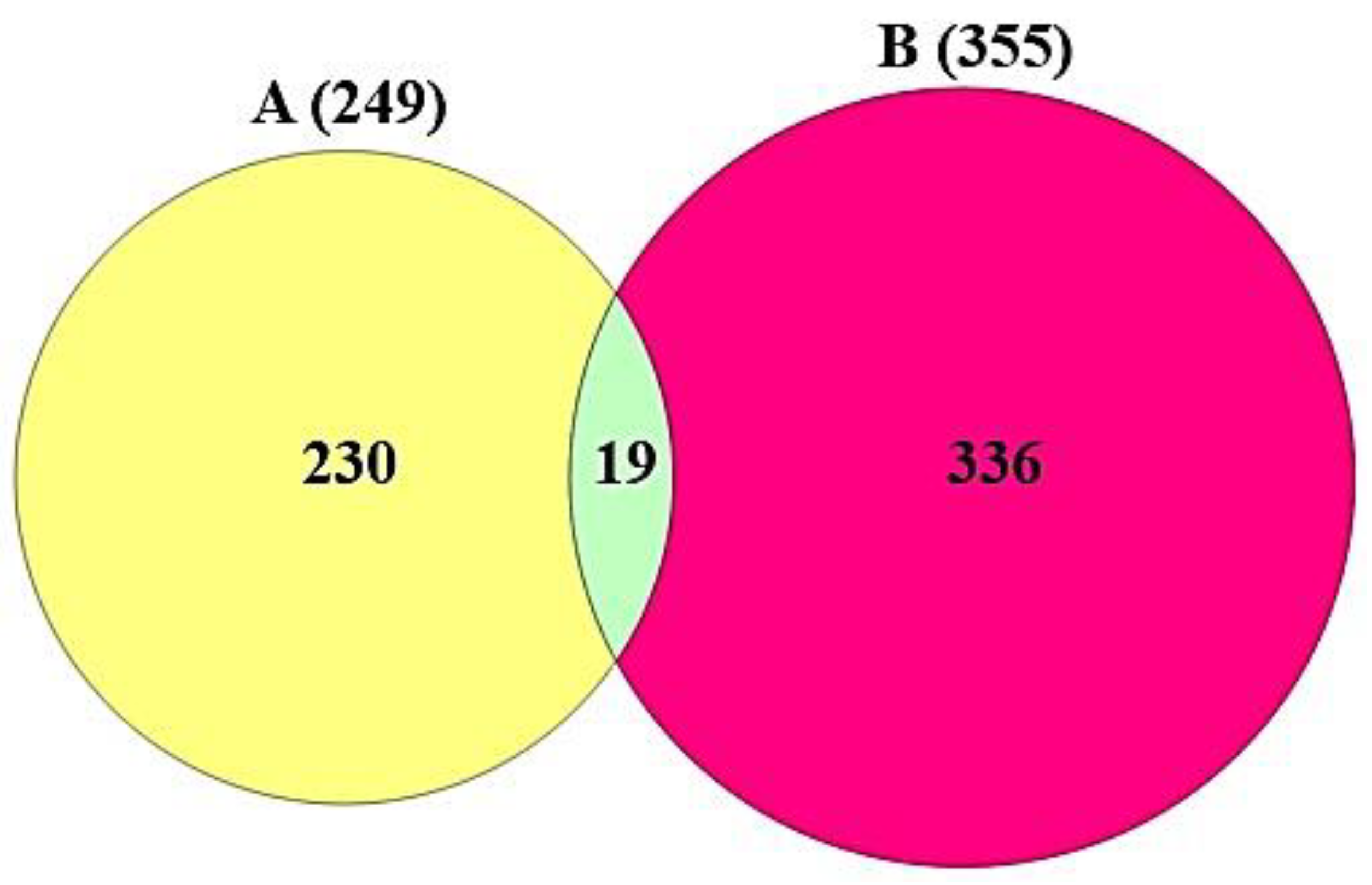
3.2. Targets Associated with the 82 Compounds or COVID-19
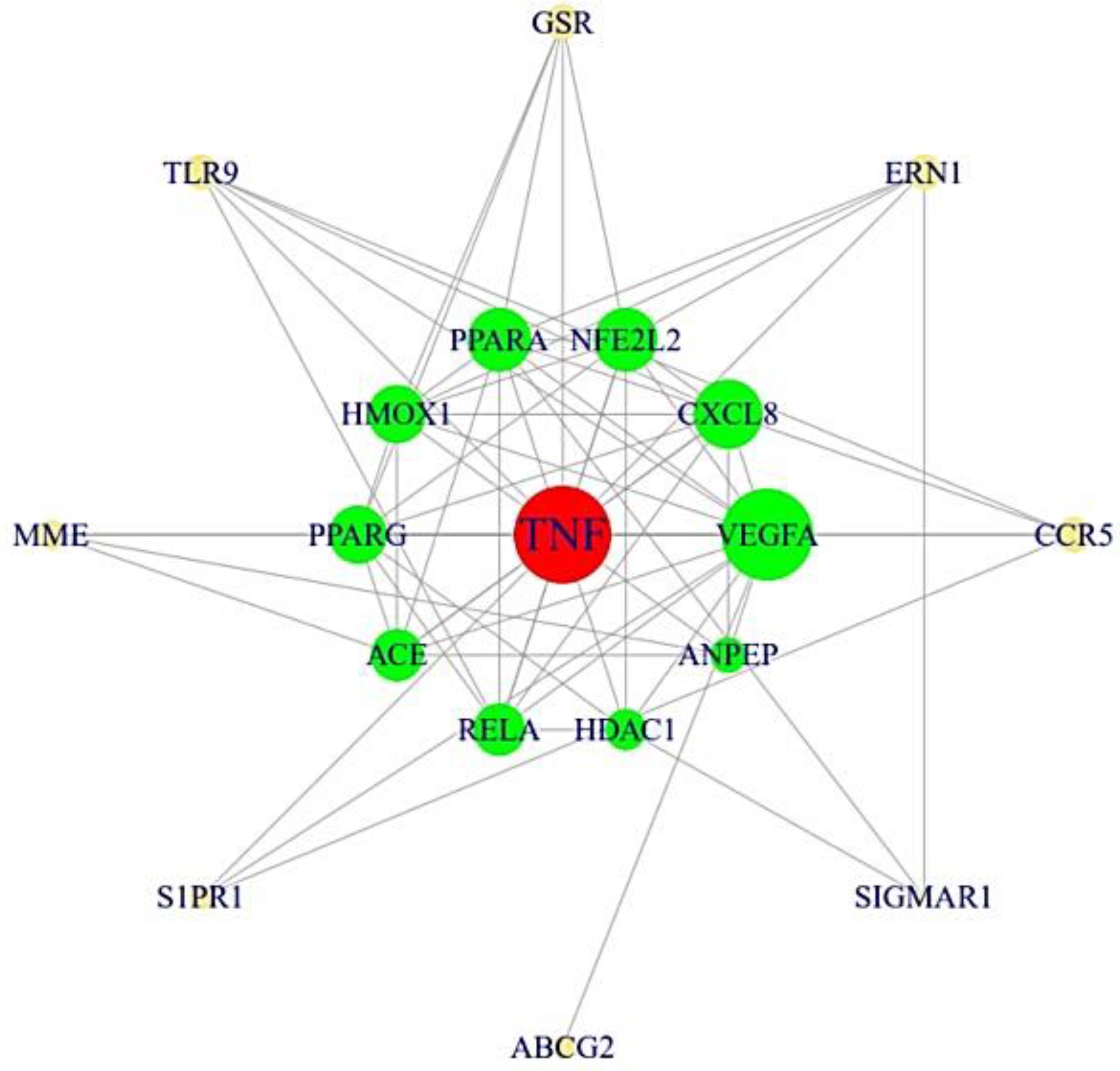
3.3. The Protein–Protein Interaction (PPI) Networks from 19 Targets
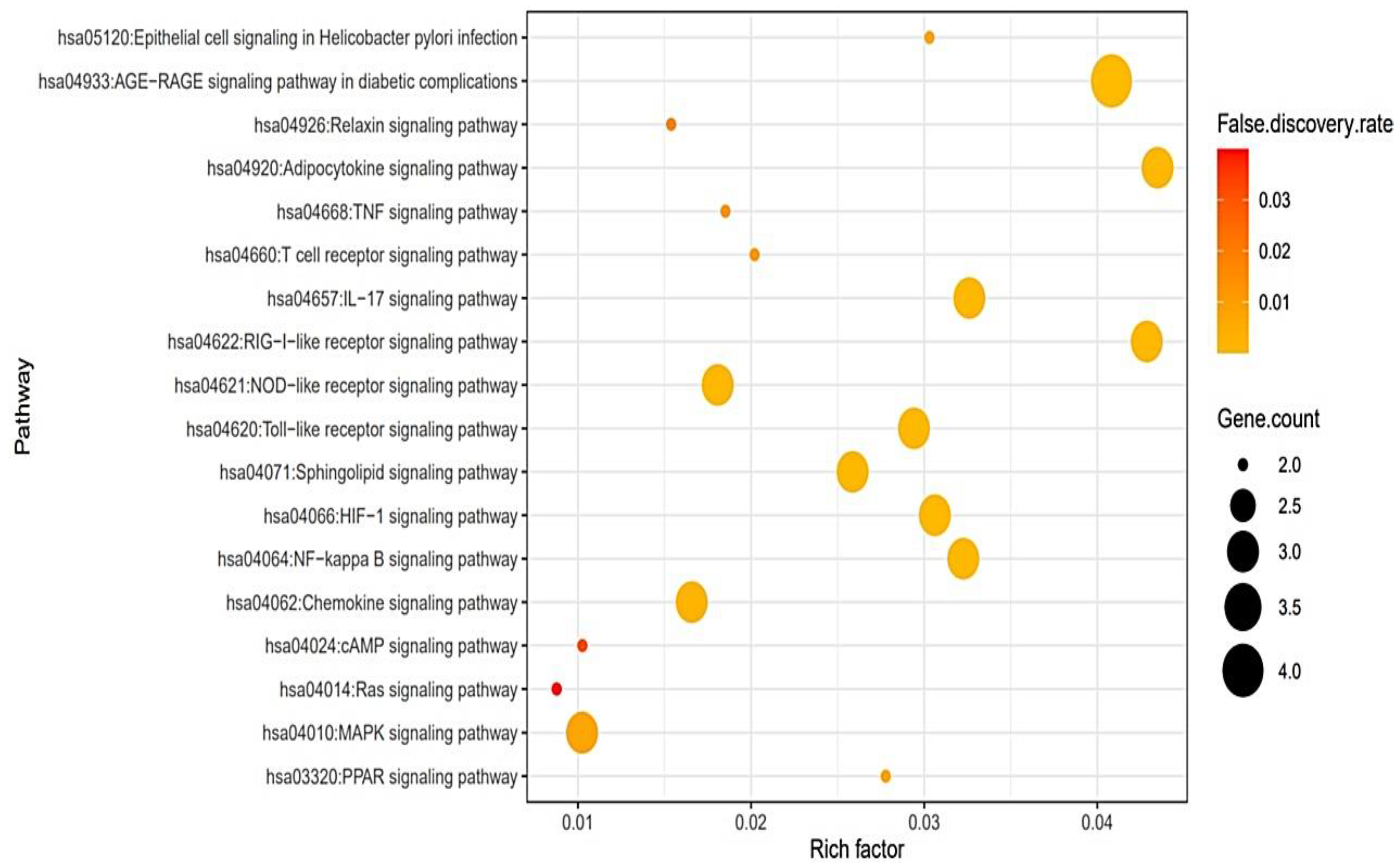
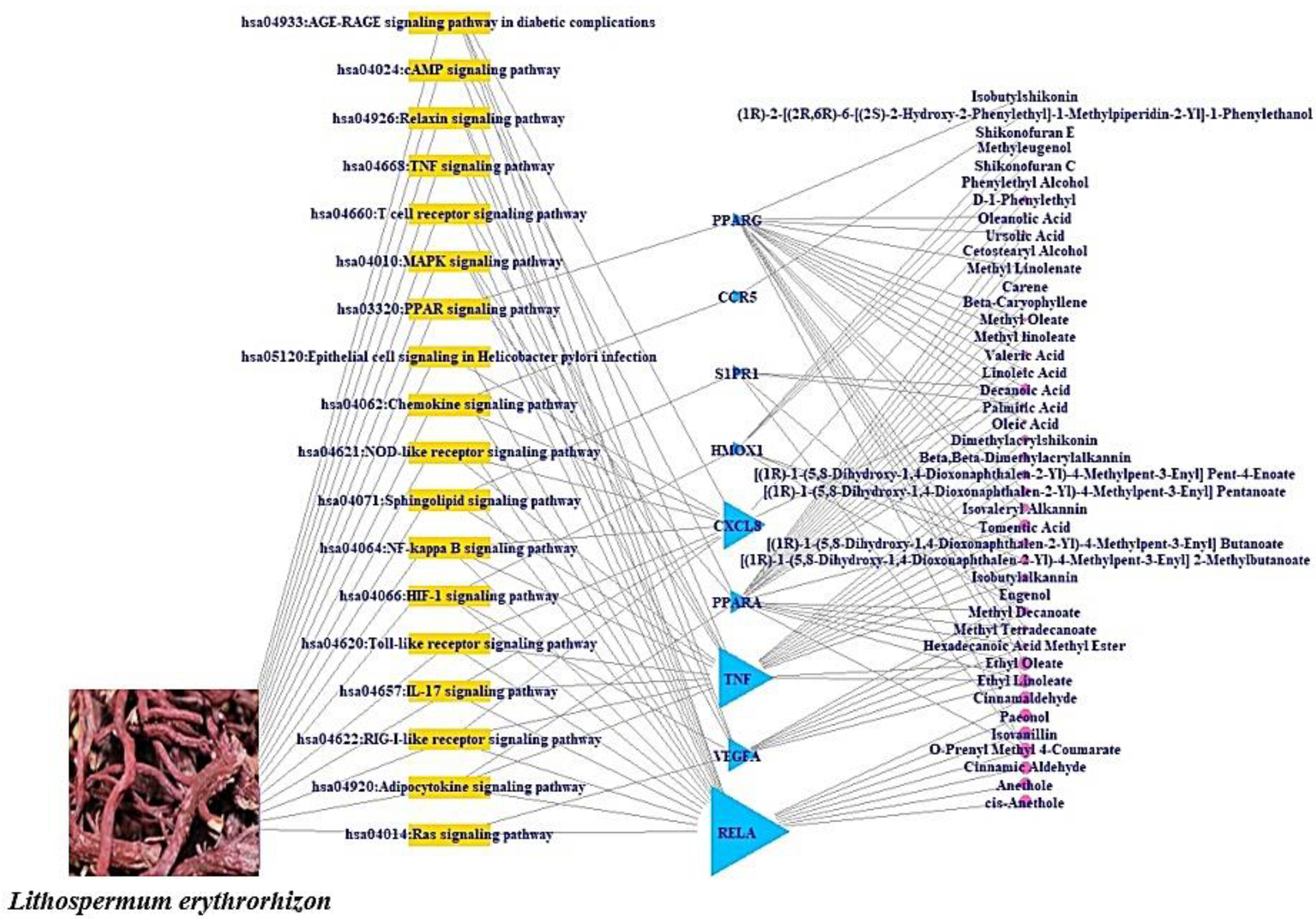
3.4. A Bubble Plot and Signaling Pathways–Targets–Bioactive Compounds (STB) Networks
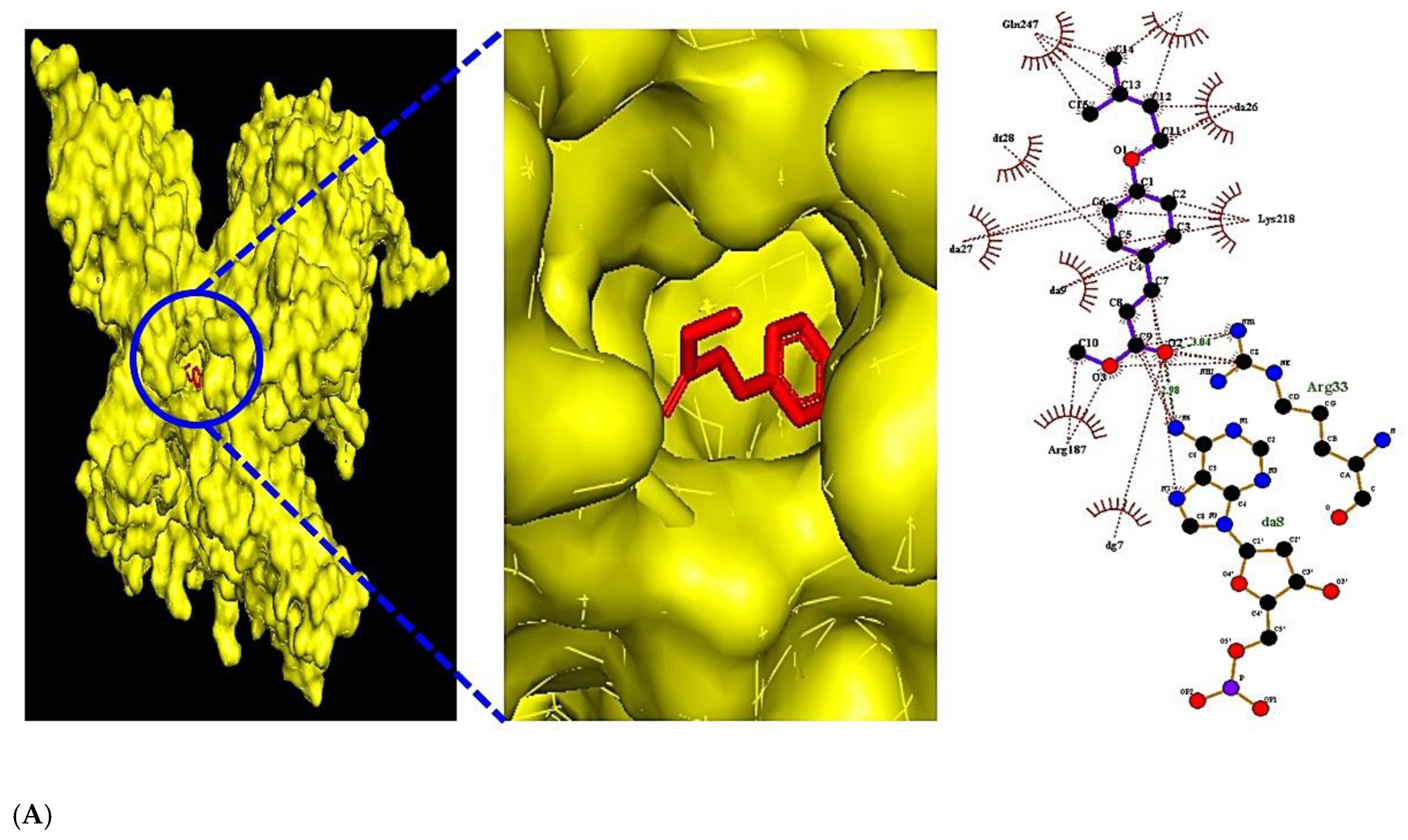
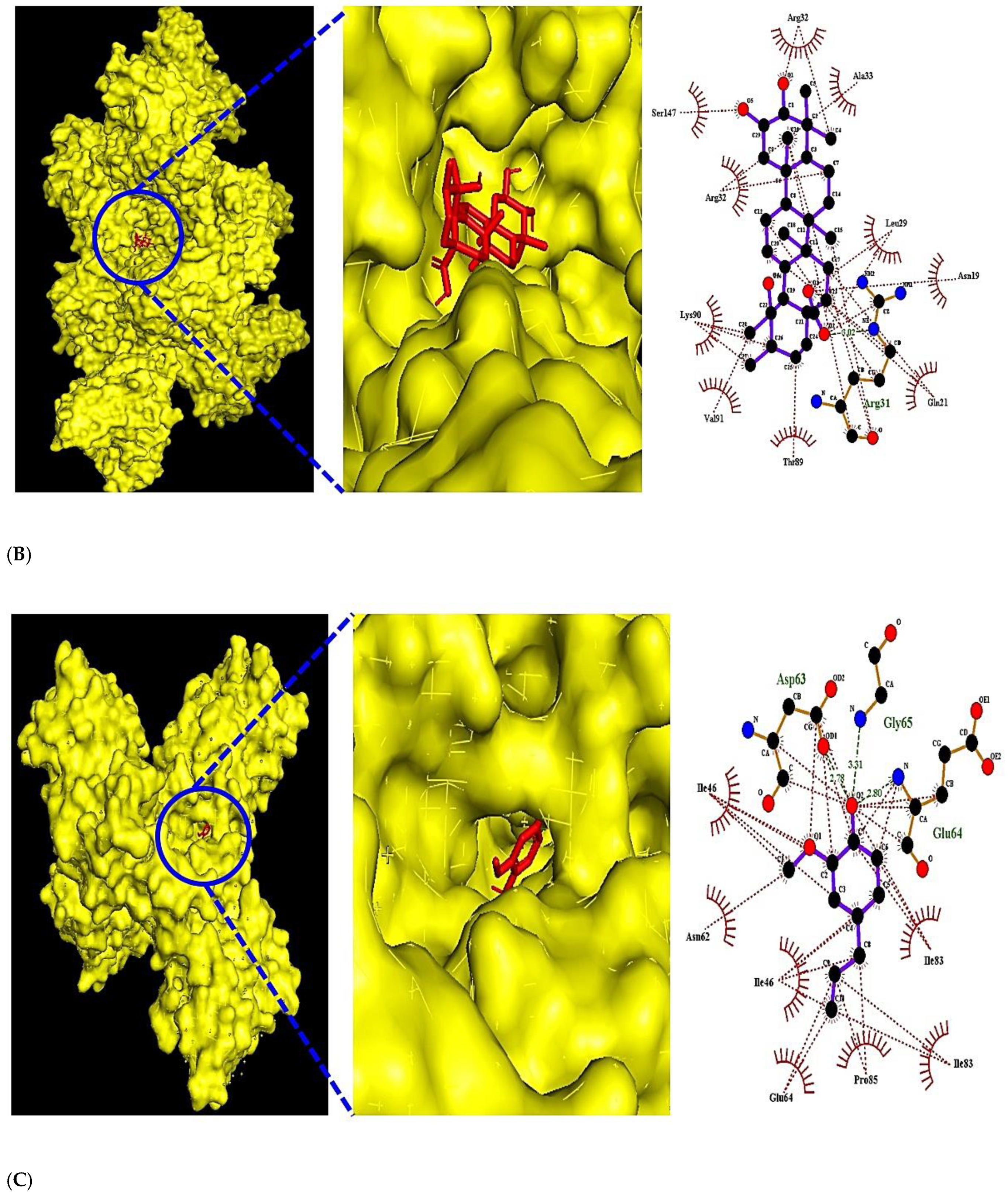
3.5. The Molecular Docking Test on MAPK Signaling Pathway against COVID-19
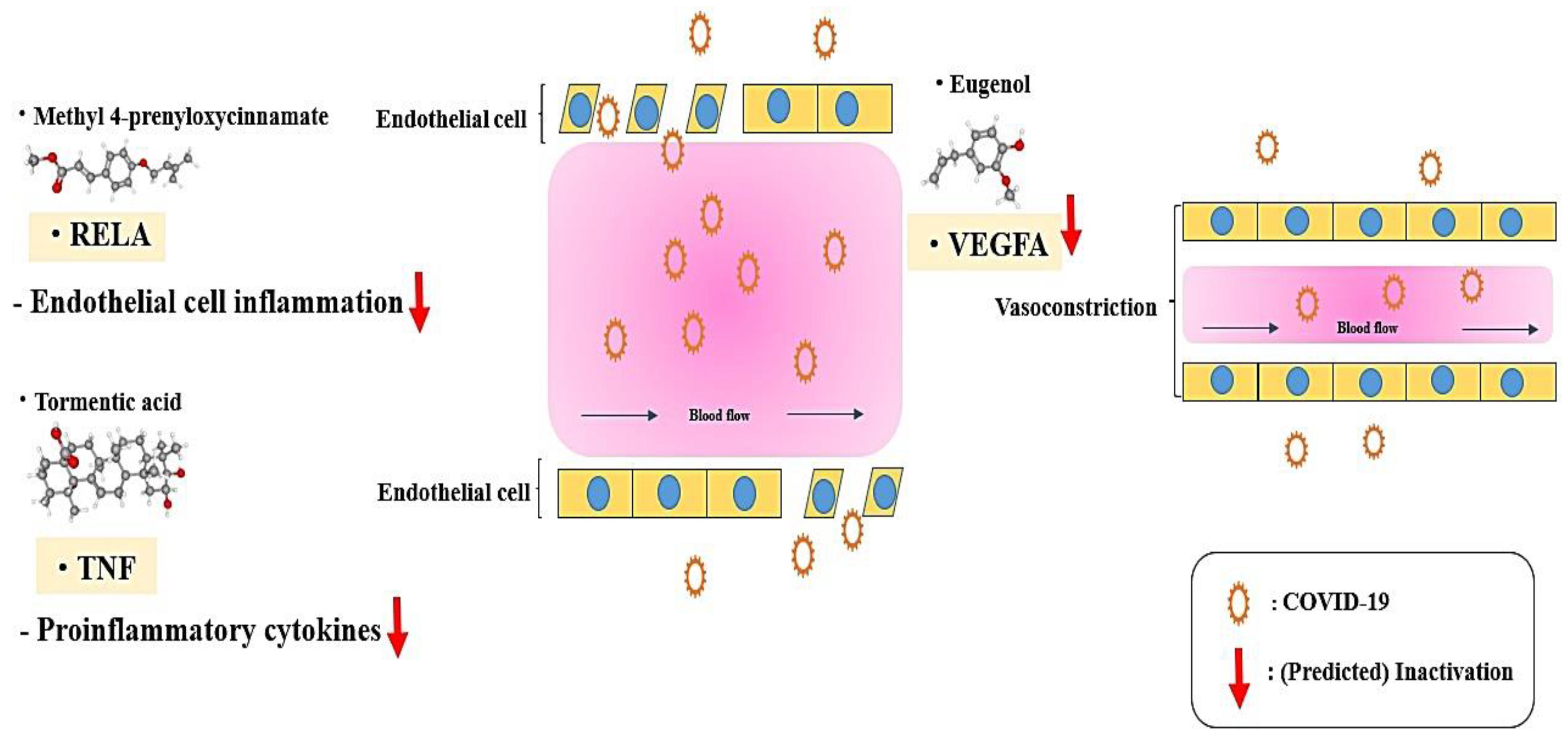
4. Discussion
- AGE-RAGE signaling pathway in diabetic complications: Activation of the binding of AGE to its receptor RAGE can stimulate cytokine production, can cause tissue damage, and the suppression of AGE-RAGE can effectively reduce inflammation [28].
- RIG-I-like receptor (RLR) signaling pathway: The RIG-I-like receptors (RLRs), RIG-I, MDA5, and LGP2, play an important role in pathogen recognition of RNA virus infections that instigate and regulate antiviral immunity [31].
- IL-17 signaling pathway: IL-17 can play vital roles in responding to pathogenicity in diverse tissues, as well as being important for inflammation balance and tissue cohesion during viral attacks [32].
- Toll-like receptor (TLR) signaling pathway: Nucleic acids originating from bacteria and viruses can be recognized by the intracellular Toll-like receptor (TLR) and they are also sensitive to self-nucleic acids in disease conditions such as autoimmunity [33].
- HIF-1 signaling pathway: The dysfunction of HIF-1α develops influenza A virus (IAV) replication by triggering autophagy in alveolar epithelial cells [27].
- NF-κb (Nuclear Factor kappa-light-chain-enhancer of activated B cells) signaling pathway: Viruses have evolved to exploit NF-κb-driven cellular functions, and the understanding of molecular mechanisms might be a new strategy against viral diseases [34].
- Sphingolipid signaling pathway: Sphingolipid metabolites, such as ceramide and sphingosine-1-phosphate, are signaling messengers that tune a wide range of cellular processes and are essential for immunity, inflammation, and inflammatory disorders [35].
- NOD-like receptor (NLR) signaling pathway: NLRs have been linked to human diseases, including infections, inflammatory disorders, and even chronic inflammation [36].
- Chemokine signaling pathway: In COVID-19 patients, inhibiting the secretion of cytokines and chemokines dulled the cytokine storm that represented the severity of the disease and was a negative side effect [37].
- PPAR (Peroxisome Proliferator-Activated Receptor) signaling pathway: The regulation of PPAR-α (Peroxisome Proliferator-Activated Receptor-alpha) with agonists enhanced herpesvirus replication and reactive oxygen species (ROS) production [38].
- MAPK (Mitogen-Activated Protein Kinase) signaling pathway: it was reported that the virus’s existence in hosts could activate the MAPK signaling pathway; some viral specific proteins can maintain the persistent activation of the MAPK signaling pathway [39].
- T cell receptor (TCR) signaling pathway: The TCR recognizes pathogens on major histocompatibility complex molecules with the cooperation of CD4 (Cluster of Differentiation 4) or CD8 (Cluster of Differentiation 8) co-receptors and produces cytokines [40].
- TNF (Tumor necrosis factor-alpha) signaling pathway: TNF-α boosters influenza A virus-induced production of antiviral cytokines by activating RIG-I (Retinoic acid-inducible gene I) gene expression [41].
- Relaxin signaling pathway: Relaxin receptor abnormality enhances vascular inflammation and damages external remodeling in arteriovenous fistulas [42].
- cAMP (Cyclic Adenosine MonoPhosphate) signaling pathway: cAMP stimulates interleukin-10 production as the anti-inflammatory cytokine [43].
- RAS (Renin-Angiotensin System) signaling pathway: The use of RAS antagonists might increase the risk of developing a SARS-CoV-2 infection. However, it is not sufficient evidence for discontinuing RAS blockers in patients with hypertension [44].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
International Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin Converting Enzyme 2 |
| AGE-RAGE | Advanced Glycation Endproduct–Receptor for Advanced Glycation Endproduct |
| cAMP | Cyclic Adenosine MonoPhosphate |
| CD4 | Cluster of Differentiation 4 |
| CD8 | Cluster of Differentiation 8 |
| COVID-19 | Coronavirus disease 2019 |
| HIF-1 | Hypoxia-inducible factor 1 |
| HIF-1α | Hypoxia-inducible factor 1α |
| IL-17 | Interleukin 17 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LE | Lithospermum erythrorhizon |
| LGP2 | Laboratory of Genetics and Physiology 2 |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA5 | Melanoma Differentiation-Associated protein 5 |
| NF-κb | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRs | NOD-like receptors |
| NPASS | Natural Product Activity and Species Source |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PPAR-α | Peroxisome Proliferator Activated Receptor Alpha |
| RAS | Renin-Angiotensin System |
| RELA | v-rel avian reticuloendotheliosis viral oncogene homolog A |
| RIG-I | Retinoic acid-inducible gene I |
| RLRs | RIG-I-like receptors |
| RNA | Ribonucleic acid |
| ROS | Reactive Oxygen Species |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SEA | Similarity Ensemble Approach |
| SMILES | Simplified Molecular Input Line Entry System |
| STP | Swiss Target Prediction |
| TCR | T Cell Receptor |
| TLR | Toll-like Receptor |
| TNF | Tumor Necrosis Factor |
| TNF-α | Tumor Necrosis Factor Alpha |
| VEGFA | Vascular Endothelial Growth Factor A |
References
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Outbreak. Emergencies-Disease; WHO: Geneve, Switzerland, 2020.
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Worldometers. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 25 March 2022).
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural products and their de-rivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef]
- Desborough, M.J.R.; Keeling, D.M. The aspirin story-from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Patra, F.; Shah, N.P.; Shukla, D. Biological control of mycotoxins by probiotic lactic acid bacteria. Dynamism Dairy Ind. Consum. Demands 2017, 2015, 2–4. [Google Scholar] [CrossRef]
- Nam, C.; Hwang, J.-S.; Kim, M.-J.; Choi, Y.W.; Han, K.-G.; Kang, J.-K. Single- and Repeat-dose Oral Toxicity Studies of Lithospermum erythrorhizon extract in Dogs. Toxicol. Res. 2015, 31, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Zhang, P.; He, W.; Qin, C.; Chen, S.; Tao, L.; Wang, Y.; Tan, Y.; Gao, D.; Wang, B.; et al. NPASS: Natural product activity and species source database for natural product research, discovery and tool development. Nucleic Acids Res. 2018, 46, D1217–D1222. [Google Scholar] [CrossRef] [Green Version]
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Cheminform. 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J. Network Pharmacology Strategies Toward Multi-Target Anticancer Therapies: From Computational Models to Experimental Design Principles. Curr. Pharm. Des. 2014, 20, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, U.; Mehendale, N.; Patil, S.; Chaguturu, R.; Patwardhan, B. Network Pharmacology. In Innovative Approaches in Drug Discovery: Ethnopharmacology, Systems Biology and Holistic Targeting; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 127–164. ISBN 9780128018224. [Google Scholar]
- Xu, M.; Shi, J.; Min, Z.; Zhu, H.; Sun, W. A Network Pharmacology Approach to Uncover the Molecular Mechanisms of Herbal Formula Kang-Bai-Ling for Treatment of Vitiligo. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Ihlenfeldt, W.D.; Bolton, E.; Bryant, S.H. The PubChem chemical structure sketcher. J. Cheminform. 2009, 1, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Keiser, M.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sun, Y.; Gao, X.; Qi, R. Integrated bioinformatic analysis of differentially expressed genes and signaling pathways in plaque psoriasis. Mol. Med. Rep. 2019, 20, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Matsson, P.; Kihlberg, J. How Big Is Too Big for Cell Permeability? J. Med. Chem. 2017, 60, 1662–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AGE-RAGE Signaling Pathway in Diabetic Complications-Cusabio. Available online: https://www.cusabio.com/pathway/AGE-RAGE-signaling-pathway-in-diabetic-complications.html (accessed on 7 August 2020).
- Ouchi, N.; Ohashi, K.; Shibata, R.; Murohara, T. Adipocytokines and obesity-linked disorders. Nagoya J. Med. Sci. 2012, 74, 19–30. [Google Scholar] [PubMed]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; La Rocca, C.; Formisano, L.; Matarese, G. Role of adipokines signaling in the modulation of T cells function. Front. Immunol. 2013, 4, 332. [Google Scholar] [CrossRef] [Green Version]
- Loo, Y.-M.; Gale, M., Jr. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.-T.; Yao, X.-T.; Peng, Q.; Chen, D.-K. The protective and pathogenic roles of IL-17 in viral infections: Friend or foe? Open Biol. 2019, 9, 190109. [Google Scholar] [CrossRef] [Green Version]
- Toll-like Receptor Signaling Pathway-Creative Diagnostics. Available online: https://www.creative-diagnostics.com/Toll-like-Receptor-Signaling-Pathway.htm (accessed on 7 August 2020).
- Santoro, M.; Rossi, A.; Amici, C. New EMBO Member’s Review: NF-kappaB and virus infection: Who controls whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catanzaro, M.; Fagiani, F.; Racchi, M.; Corsini, E.; Govoni, S.; Lanni, C. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Dryden, P.; Lowe, A.; Wang, G.; Dozmorov, I.; Chang, T.; Reese, T.A. WY14643 Increases Herpesvirus Replication Independently of PPARα Expression and Inhibits IFNβ Production. bioRxiv 2021. bioRxiv:2021.10.29.466551. [Google Scholar] [CrossRef]
- Kumar, R.; Khandelwal, N.; Thachamvally, R.; Tripathi, B.N.; Barua, S.; Kashyap, S.K.; Maherchandani, S.; Kumar, N. Role of MAPK/MNK1 signaling in virus replication. Virus Res. 2018, 253, 48–61. [Google Scholar] [CrossRef]
- Hwang, J.-R.; Byeon, Y.; Kim, D.; Park, S.-G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef]
- Matikainen, S.; Sireén, J.; Tissari, J.; Veckman, V.; Pirhonen, J.; Severa, M.; Sun, Q.; Lin, R.; Meri, S.; Uzeé, G.; et al. Tumor Necrosis Factor Alpha Enhances Influenza A Virus-Induced Expression of Antiviral Cytokines by Activating RIG-I Gene Expression. J. Virol. 2006, 80, 3515–3522. [Google Scholar] [CrossRef] [Green Version]
- Bezhaeva, T.; de Vries, M.R.; Geelhoed, W.J.; van der Veer, E.P.; Versteeg, S.; van Alem, C.M.A.; Voorzaat, B.M.; Eijkelkamp, N.; van der Bogt, K.E.; Agoulnik, A.I.; et al. Relaxin receptor deficiency promotes vascular inflammation and impairs outward remodeling in arteriovenous fistulas. FASEB J. 2018, 32, 6293–6304. [Google Scholar] [CrossRef] [Green Version]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef]
- Shibata, S.; Arima, H.; Asayama, K.; Hoshide, S.; Ichihara, A.; Ishimitsu, T.; Kario, K.; Kishi, T.; Mogi, M.; Nishiyama, A.; et al. Hypertension and related diseases in the era of COVID-19: A report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens. Res. 2020, 43, 1–19. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.A.; Paiva, J.G.; Pimentel, F.N.; Guimarães, R.S.; Moreira, M.R. COVID-19 in patients with rheumatological diseases treated with anti-TNF. Ann. Rheum. Dis. 2020, 80, e62. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Vasile, E.; Feng, D.; Sundberg, C.; Brown, L.F.; Detmar, M.J.; Lawitts, J.A.; Benjamin, L.; Tan, X.; Manseau, E.J.; et al. Vascular Permeability Factor/Vascular Endothelial Growth Factor Induces Lymphangiogenesis as well as Angiogenesis. J. Exp. Med. 2002, 196, 1497–1506. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Lipinski Rules | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PubChem ID | MW | HBA | HBD | MLogP | Lipinski’s Violations | Bioavailability Score | TPSA | ||
| No. | <500 | <10 | ≤5 | ≤4.15 | ≤1 | >0.1 | <140 Ų | ||
| 1 | (S)-1-Phenylethanol | 443135 | 122.16 | 1 | 1 | 1.87 | 0 | 0.55 | 20.23 |
| 2 | 3-Methylbutanoic acid | 10430 | 102.13 | 2 | 1 | 0.89 | 0 | 0.85 | 37.30 |
| 3 | cis-Caffeic acid | 1549111 | 180.16 | 4 | 3 | 0.70 | 0 | 0.55 | 77.76 |
| 4 | Phenylethyl alcohol | 6054 | 122.16 | 1 | 1 | 1.87 | 0 | 0.55 | 20.23 |
| 5 | Thiophene | 8030 | 84.14 | 0 | 0 | 1.12 | 0 | 0.55 | 28.24 |
| 6 | Caryophyllene | 5281515 | 204.35 | 0 | 0 | 4.63 | 1 | 0.55 | 0.00 |
| 7 | Alkannin | 72521 | 288.30 | 5 | 3 | 0.42 | 0 | 0.55 | 94.83 |
| 8 | b-b-Dimethylacrylalkannin | 442720 | 370.40 | 6 | 2 | 1.43 | 0 | 0.55 | 100.90 |
| 9 | Shikonofuran C | 5321288 | 358.43 | 5 | 2 | 2.36 | 0 | 0.55 | 79.90 |
| 10 | Isovanillin | 12127 | 152.15 | 3 | 1 | 0.51 | 0 | 0.55 | 46.53 |
| 11 | (R)-2-methylbutanoate | 6950479 | 102.13 | 2 | 1 | 0.89 | 0 | 0.85 | 37.30 |
| 12 | Ethyl oleate | 5363269 | 310.51 | 2 | 0 | 5.03 | 1 | 0.55 | 26.30 |
| 13 | Camphor | 2537 | 152.23 | 1 | 0 | 2.30 | 0 | 0.55 | 17.07 |
| 14 | (−)-Caryophyllene oxide | 1742210 | 220.35 | 1 | 0 | 3.67 | 0 | 0.55 | 3.67 |
| 15 | Methyl linolenate | 5319706 | 292.46 | 2 | 0 | 4.61 | 1 | 0.55 | 26.30 |
| 16 | Totarol | 92783 | 286.45 | 1 | 1 | 4.92 | 1 | 0.55 | 20.23 |
| 17 | Oleanolic acid | 10494 | 456.70 | 3 | 2 | 5.82 | 1 | 0.85 | 57.53 |
| 18 | (S)-2-methylbutanoate | 6950480 | 101.12 | 2 | 0 | 0.89 | 0 | 0.85 | 40.13 |
| 19 | Hexadecanoic acid methyl ester | 8181 | 270.45 | 2 | 0 | 4.44 | 1 | 0.55 | 26.30 |
| 20 | (−)-Borneol | 1201518 | 154.25 | 1 | 1 | 2.45 | 0 | 0.55 | 20.23 |
| 21 | Cinnamic aldehyde | 637511 | 132.16 | 1 | 0 | 2.01 | 0 | 0.55 | 17.07 |
| 22 | Valeric acid | 7991 | 102.13 | 2 | 1 | 0.89 | 0 | 0.85 | 37.30 |
| 23 | Methyl 4-prenyloxycinnmate | 14414116 | 246.30 | 3 | 0 | 3.48 | 0 | 0.55 | 35.53 |
| 24 | (3S,4S)-4,7,7-trimethylbicyclo[2.2.1]heptan-3-ol | 12242815 | 154.25 | 1 | 1 | 2.45 | 0 | 0.55 | 20.23 |
| 25 | Paeonol | 11092 | 166.17 | 3 | 1 | 0.83 | 0 | 0.55 | 46.53 |
| 26 | β-Selinene | 519361 | 204.35 | 0 | 0 | 4.63 | 1 | 0.55 | 0.00 |
| 27 | Docosanol | 12620 | 326.60 | 1 | 1 | 5.84 | 1 | 0.55 | 20.23 |
| 28 | Phenylacetaldehyde | 998 | 120.15 | 1 | 0 | 1.78 | 0 | 0.55 | 17.07 |
| 29 | 11-O-Acetylalkannin | 137628887 | 330.33 | 6 | 2 | 0.82 | 0 | 0.55 | 100.90 |
| 30 | palmitic acid | 985 | 256.42 | 2 | 1 | 4.19 | 1 | 0.85 | 37.30 |
| 31 | furfural | 7362 | 96.08 | 2 | 0 | −0.56 | 0 | 0.55 | 30.21 |
| 32 | isobutyric acid | 6590 | 88.11 | 2 | 1 | 0.49 | 0 | 0.85 | 37.30 |
| 33 | isobutylshikonin | 479500 | 358.39 | 6 | 2 | 1.28 | 0 | 0.55 | 100.90 |
| 34 | Shikalkin | 5208 | 288.30 | 5 | 3 | 0.42 | 0 | 0.55 | 94.83 |
| 35 | shikonin | 479503 | 288.30 | 5 | 3 | 0.42 | 0 | 0.55 | 94.83 |
| 36 | Propionylshikonin | 153984 | 344.36 | 6 | 2 | 1.06 | 0 | 0.55 | 100.90 |
| 37 | β-hydroxyisovalerylshikonin | 479502 | 388.41 | 7 | 3 | 0.71 | 0 | 0.55 | 121.13 |
| 38 | eugenol | 3314 | 164.20 | 2 | 1 | 2.01 | 0 | 0.55 | 29.46 |
| 39 | cis-Anethole | 1549040 | 148.20 | 1 | 0 | 2.67 | 0 | 0.55 | 9.23 |
| 40 | tormentic Acid | 73193 | 488.70 | 5 | 4 | 4.14 | 0 | 0.55 | 97.99 |
| 41 | oleic Acid | 445639 | 282.46 | 2 | 1 | 4.57 | 1 | 0.85 | 37.30 |
| 42 | 1-eicosanol | 12404 | 298.55 | 1 | 1 | 5.39 | 1 | 0.55 | 20.23 |
| 43 | decanoic acid | 2969 | 172.26 | 2 | 1 | 2.58 | 0 | 0.85 | 37.30 |
| 44 | borneol | 64685 | 154.25 | 1 | 1 | 2.45 | 0 | 0.55 | 20.23 |
| 45 | ethyl linoleate | 5282184 | 308.50 | 2 | 0 | 4.93 | 1 | 0.55 | 26.30 |
| 46 | cetostearyl alcohol | 62238 | 512.93 | 2 | 2 | 7.28 | 2 | 0.17 | 40.46 |
| 47 | 2-acetylpyrrole | 14079 | 109.13 | 1 | 1 | −0.18 | 0 | 0.55 | 32.86 |
| 48 | (1R)-2-[(2R,6R)-6-[(2S)-2-hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl]-1-phenylethanol | 6604328 | 339.47 | 3 | 2 | 3.03 | 0 | 0.55 | 43.70 |
| 49 | methyleugenol | 7127 | 178.23 | 2 | 0 | 2.30 | 0 | 0.55 | 18.46 |
| 50 | caffeic acid | 689043 | 180.16 | 4 | 3 | 0.70 | 0 | 0.55 | 77.76 |
| 51 | β-Ionone | 638014 | 192.30 | 1 | 0 | 2.94 | 0 | 0.55 | 17.07 |
| 52 | carene | 26049 | 136.23 | 0 | 0 | 4.29 | 1 | 0.55 | 0.00 |
| 53 | dimethylacrylshikonin | 479499 | 370.40 | 6 | 2 | 1.43 | 0 | 0.55 | 100.90 |
| 54 | acetylshikonin | 479501 | 330.33 | 6 | 2 | 0.82 | 0 | 0.55 | 100.90 |
| 55 | methyl tetradecanoate | 31284 | 242.40 | 2 | 0 | 3.94 | 0 | 0.55 | 26.30 |
| 56 | deoxyshikonin | 98914 | 272.30 | 4 | 2 | 1.25 | 0 | 0.55 | 74.60 |
| 57 | buthylshikonin | 10089766 | 358.39 | 6 | 2 | 1.28 | 0 | 0.55 | 100.90 |
| 58 | 3-methylbut-2-enoic Acid | 10931 | 100.12 | 2 | 1 | 0.79 | 0 | 0.85 | 37.30 |
| 59 | shikonofuran E | 5321290 | 356.41 | 5 | 2 | 2.28 | 0 | 0.55 | 79.90 |
| 60 | methyl oleate | 5364509 | 296.49 | 2 | 0 | 4.80 | 1 | 0.55 | 26.30 |
| 61 | Isovalerylshikonin | 479497 | 372.41 | 6 | 2 | 1.51 | 0 | 0.55 | 100.90 |
| 62 | α-methyl-butylshikonin | 479498 | 372.41 | 6 | 2 | 1.51 | 0 | 0.55 | 100.90 |
| 63 | isobutylalkannin | 137629300 | 358.39 | 6 | 2 | 1.28 | 0 | 0.55 | 100.90 |
| 64 | Methyl linoleate | 5284421 | 294.47 | 2 | 0 | 4.70 | 1 | 0.55 | 26.30 |
| 65 | (−)-camphor | 444294 | 152.23 | 1 | 0 | 2.30 | 0 | 0.55 | 17.07 |
| 66 | isovaleryl alkannin | 5318685 | 372.41 | 6 | 2 | 1.51 | 0 | 0.55 | 100.90 |
| 67 | 2-pentylfuran | 19602 | 138.21 | 1 | 0 | 1.84 | 0 | 0.55 | 13.14 |
| 68 | [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] (6Z,9Z)-octadeca-6,9-dienoate | 44438574 | 550.73 | 6 | 2 | 3.95 | 1 | 0.55 | 100.90 |
| 69 | [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] pent-4-enoate | 9999214 | 370.40 | 6 | 2 | 1.43 | 0 | 0.55 | 100.90 |
| 70 | [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] benzoate | 10475609 | 392.40 | 6 | 2 | 1.99 | 0 | 0.55 | 100.90 |
| 71 | [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] pentanoate | 145992534 | 476.61 | 6 | 2 | 2.15 | 0 | 0.55 | 151.50 |
| 72 | nonanal | 31289 | 142.24 | 1 | 0 | 2.39 | 0 | 0.55 | 17.07 |
| 73 | ursolic acid | 64945 | 456.70 | 3 | 2 | 5.82 | 1 | 0.85 | 57.53 |
| 74 | methyl decanoate | 8050 | 186.29 | 2 | 0 | 2.87 | 0 | 0.55 | 26.30 |
| 75 | hexanal | 6184 | 100.16 | 1 | 0 | 1.39 | 0 | 0.55 | 17.07 |
| 76 | 2-methylbutanoic acid | 8314 | 102.13 | 2 | 1 | 0.89 | 0 | 0.85 | 37.30 |
| 77 | shikonofuran D | 5321289 | 344.40 | 5 | 2 | 2.14 | 0 | 0.55 | 79.90 |
| 78 | linoleic acid | 5280450 | 280.45 | 2 | 1 | 4.47 | 1 | 0.85 | 37.30 |
| 79 | D-1-phenylethyl | 637516 | 122.16 | 1 | 1 | 1.87 | 0 | 0.55 | 20.23 |
| 80 | P-cymene | 7463 | 134.22 | 0 | 0 | 4.47 | 1 | 0.55 | 0.00 |
| 81 | phenanthrene | 995 | 178.23 | 0 | 0 | 5.17 | 1 | 0.55 | 0.00 |
| 82 | anethole | 637563 | 148.20 | 1 | 0 | 2.67 | 0 | 0.55 | 9.23 |
| No. | Target | Degree of Values |
|---|---|---|
| 1 | TNF | 16 |
| 2 | VEGFA | 15 |
| 3 | CXCL8 | 11 |
| 4 | NFE2L2 | 10 |
| 5 | PPARA | 10 |
| 6 | HMOX1 | 9 |
| 7 | PPARG | 9 |
| 8 | ACE | 8 |
| 9 | RELA | 8 |
| 10 | HDAC1 | 6 |
| 11 | ANPEP | 5 |
| 12 | CCR5 | 5 |
| 13 | ERN1 | 5 |
| 14 | GSR | 5 |
| 15 | TLR9 | 5 |
| 16 | MME | 4 |
| 17 | S1PR1 | 3 |
| 18 | SIGMAR1 | 3 |
| 19 | ABCG2 | 1 |
| KEGG ID | Description | Target Genes | False Discovery Rate |
|---|---|---|---|
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | RELA, TNF, CXCL8, VEGFA | 0.000067 |
| hsa04920 | Adipocytokine signaling pathway | RELA, TNF, PPARA | 0.000440 |
| hsa04622 | RIG-I-like receptor signaling pathway | RELA, TNF, CXCL8 | 0.000440 |
| hsa04657 | IL-17 signaling pathway | RELA, TNF, CXCL8 | 0.000760 |
| hsa04620 | Toll-like receptor signaling pathway | RELA, TNF, CXCL8 | 0.000760 |
| hsa04066 | HIF-1 signaling pathway | RELA, HMOX1, VEGFA | 0.000760 |
| hsa04064 | NF-kappa B signaling pathway | RELA, TNF, CXCL8 | 0.000760 |
| hsa04071 | Sphingolipid signaling pathway | RELA, TNF, S1PR1 | 0.000950 |
| hsa04621 | NOD-like receptor signaling pathway | RELA, TNF, CXCL8 | 0.000950 |
| hsa04062 | Chemokine signaling pathway | RELA, CXCL8, CCR5 | 0.002100 |
| hsa05120 | Epithelial cell signaling in Helicobacter pylori infection | RELA, CXCL8 | 0.005700 |
| hsa03320 | PPAR signaling pathway | PPARA, PPARG | 0.006400 |
| hsa04010 | MAPK signaling pathway | RELA, TNF, VEGFA | 0.007200 |
| hsa04660 | T cell receptor signaling pathway | RELA, TNF | 0.010200 |
| hsa04668 | TNF signaling pathway | RELA, TNF | 0.011800 |
| hsa04926 | Relaxin signaling pathway | RELA, VEGFA | 0.016500 |
| hsa04024 | cAMP signaling pathway | RELA, PPARA | 0.031200 |
| hsa04014 | Ras signaling pathway | RELA, VEGFA | 0.039900 |
| No. | Target | Degree of Values |
|---|---|---|
| 1 | RELA | 17 |
| 2 | TNF | 11 |
| 3 | CXCL8 | 8 |
| 4 | VEGFA | 5 |
| 5 | PPARA | 3 |
| 6 | PPARG | 2 |
| 7 | CCR5 | 2 |
| 8 | HMOX1 | 1 |
| 9 | S1PR1 | 1 |
| 10 | NFE2L2 | 0 |
| 11 | ACE | 0 |
| 12 | HDAC1 | 0 |
| 13 | ANPEP | 0 |
| 14 | ERN1 | 0 |
| 15 | GSR | 0 |
| 16 | TLR9 | 0 |
| 17 | MME | 0 |
| 18 | SIGMAR1 | 0 |
| 19 | ABCG2 | 0 |
| Grid Box | Hydrogen Bond Interactions | Hydrophobic Interactions | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Symbol | Binding Energy (kcal/mol) | Center | Dimension | Amino Acid Residue | Amino Acid Residue |
| RELA (PDB ID: 2O61) | Methyl 4-prenyloxycinnmate | 14414116 | R1 | −7.1 | x = 15.616 | size_x = 40 | Arg33 | Gln247, Lys218, Arg187 |
| y = −22.641 | size_y = 40 | |||||||
| z = −18.824 | size_z = 40 | |||||||
| Paeonol | 11092 | R2 | −6.2 | x = 15.616 | size_x = 40 | Arg246 | Lys272, Lys241 | |
| y = −22.641 | size_y = 40 | |||||||
| z = −18.824 | size_z = 40 | |||||||
| Isovanillin | 12127 | R3 | −5.7 | x = 15.616 | size_x = 40 | N/A | Arg33, Arg187, Lys218 | |
| y = −22.641 | size_y = 40 | |||||||
| z = −18.824 | size_z = 40 | |||||||
| Anethole | 637563 | R4 | −5.5 | x = 15.616 | size_x = 40 | Arg305 | Val248, Lys218, Arg246 | |
| y = −22.641 | size_y = 40 | Gln247, Phe307 | ||||||
| z = −18.824 | size_z = 40 | |||||||
| Cinnamic aldehyde | 637511 | R5 | −5.4 | x = 15.616 | size_x = 40 | N/A | Pro189, Asp185, Cys120 | |
| y = −22.641 | size_y = 40 | His88, Tyr36, Leu154 | ||||||
| z = −18.824 | size_z = 40 | Val121, Asn155, Ala188 | ||||||
| ` | Grid Box | Hydrogen Bond Interactions | Hydrophobic Interactions | |||||
|---|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Symbol | Binding Energy (kcal/mol) | Center | Dimension | Amino Acid Residue | Amino Acid Residue |
| TNF (PDB ID: 5YOY) | Tormentic acid | 73193 | T1 | −7.3 | x = 243.718 | size_x = 40 | Arg31 | Arg32, Ala33, Leu29 |
| y = −425.984 | size_y = 40 | Asn19, Gln21, Thr89 | ||||||
| z = 261.631 | size_z = 40 | Val91, Lys90, Arg32 | ||||||
| Ser147 | ||||||||
| [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] pent-4-enoate | 9999214 | T2 | −7.1 | x = 243.718 | size_x = 40 | Asn30 | Lys128, Arg31, Ala84, | |
| y = −425.984 | size_y = 40 | Leu29, Arg82, Tyr87 | ||||||
| z = 261.631 | size_z = 40 | Gln27, Trp28, Asn46 | ||||||
| Asp45, Leu43, Glu127 | ||||||||
| Dimethylacrylshikonin | 479499 | T3 | −6.6 | x = 243.718 | size_x = 40 | Trp94, Phe144 | Gln21, Ala145, Gly105 | |
| y = −425.984 | size_y = 40 | Lys65, Asp143, Pro20 | ||||||
| z = 261.631 | size_z = 40 | |||||||
| Isovalerylshikonin | 479497 | T4 | −6.5 | x = 243.718 | size_x = 40 | Asn93, Phe144 | Pro20, Gln21, Gly105 | |
| y = −425.984 | size_y = 40 | Lys65, Asp143. Ala145 | ||||||
| z = 261.631 | size_z = 40 | Trp94 | ||||||
| Isobutylalkannin | 137629300 | T5 | −6.4 | x = 243.718 | size_x = 40 | Thr69. Tyr60, Ser85 | Lys65, Gly66, Phe68 | |
| y = −425.984 | size_y = 40 | Arg67, Gly66, Lys58 | ||||||
| z = 261.631 | size_z = 40 | |||||||
| α-Methyl-butylshikonin | 479498 | T6 | −6.3 | x = 243.718 | size_x = 40 | Ala33, Ala145 | Val17, Arg32, Ala18 | |
| y = −425.984 | size_y = 40 | Pro20, Gln21, Glu146 | ||||||
| z = 261.631 | size_z = 40 | Arg31, Val91, Ser147 | ||||||
| Isobutylalkannin | 137629300 | T7 | −6.3 | x = 243.718 | size_x = 40 | Thr69, Tyr60, Ser85 | Lys65, Gly66, Phe68 | |
| y = −425.984 | size_y = 40 | Arg67, Lys58 | ||||||
| z = 261.631 | size_z = 40 | |||||||
| β-β-Dimethylacrylalkannin | 442720 | T8 | −6.3 | x = 243.718 | size_x = 40 | Ser56, Asp54, Tyr53 | His73, Leu75, Pro113 | |
| y = −425.984 | size_y = 40 | Gln67, Lys65 | Asn57,Tyr115 | |||||
| z = 261.631 | size_z = 40 | |||||||
| Buthylshikonin | 10089766 | T9 | −6.2 | x = 243.718 | size_x = 40 | Thr79, Ser95, Gln149 | Lys90, Asn92, Ser81 | |
| y = −425.984 | size_y = 40 | Glu146, Ile97, Thr77 | ||||||
| z = 261.631 | size_z = 40 | Asn137, Ile136, Glu135 | ||||||
| His78 | ||||||||
| [(1R)-1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] pentanoate | 145992534 | T10 | −6.1 | x = 243.718 | size_x = 40 | Thr69, Tyr60 | Gly66, Lys65, Lys58 | |
| y = −425.984 | size_y = 40 | Arg67, Ser85, Phe68 | ||||||
| z = 261.631 | size_z = 40 | |||||||
| Ethyl oleate | 5363269 | T11 | −5.6 | x = 243.718 | size_x = 40 | N/A | Glu127, Arg82, Leu36 | |
| y = −425.984 | size_y = 40 | Ala35, Leu36, Gln125 | ||||||
| z = 261.631 | size_z = 40 | Arg31, Asn34, Arg32 | ||||||
| Ala35, Gln125, Asn34 | ||||||||
| Ethyl linoleate | 5282184 | T12 | −5.0 | x = 243.718 | size_x = 40 | N/A | Gly101, Tyr53, Asn57 | |
| y = −425.984 | size_y = 40 | Ser56, His73, Leu75 | ||||||
| z = 261.631 | size_z = 40 | Pro113, Ala111, Ser52 | ||||||
| Ala33, Gln67 | ||||||||
| Grid Box | Hydrogen Bond Interactions | Hydrophobic Interactions | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Symbol | Binding Energy (kcal/mol) | Center | Dimension | Amino Acid Residue | Amino Acid Residue |
| VEGFA (PDB ID: 3P9W) | Eugenol | 3314 | V1 | −6.1 | x = −12.652 | x = 40 | Asp63, Gly65, Glu64 | Ile83, Pro85, Glu64 |
| y = 70.481 | y = 40 | Ile46, Asn62, Ile46 | ||||||
| z = −40.286 | z = 40 | |||||||
| Ethyl linoleate | 5282184 | V2 | −5.2 | x = −12.652 | x = 40 | N/A | Ile46, Pro85, His86 | |
| y = 70.481 | y = 40 | Phe36, Ser50, Cys60 | ||||||
| z = −40.286 | z = 40 | Asp34, Glu64 | ||||||
| Methyl tetradecanoate | 31284 | V3 | −4.7 | x = −12.652 | x = 40 | Glu64 | Ile46, Pro85, Asp63 | |
| y = 70.481 | y = 40 | His86, Phe36, Ser50 | ||||||
| z = −40.286 | z = 40 | Asn62, Phe47, Asp63 | ||||||
| Ile83 | ||||||||
| Ethyl oleate | 5363269 | V4 | −4.6 | x = −12.652 | x = 40 | N/A | Ile83, Pro85, Glu64 | |
| y = 70.481 | y = 40 | Asn62, Asp63, Glu64 | ||||||
| z = −40.286 | z = 40 | His86, Ile46, Asp63 | ||||||
| Ile83, Pro85 | ||||||||
| Hexadecanoic acid methyl ester | 8181 | V5 | −4.2 | x = −12.652 | x = 40 | N/A | Ile46, Ile83, Glu64 | |
| y = 70.481 | y = 40 | Phe36, His86, Ser50 | ||||||
| z = −40.286 | z = 40 | Cys61, Asn62, Pro85 | ||||||
| Methyl decanoate | 8050 | V6 | −3.7 | x = −12.652 | x = 40 | N/A | Cys68, Asp63, Phe47 | |
| y = 70.481 | y = 40 | Ile46, Ser50, Phe36 | ||||||
| z = −40.286 | z = 40 | Asp34,His86, Glu64 | ||||||
| Glu67 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, K.-K.; Adnan, M. Revealing Potential Bioactive Compounds and Mechanisms of Lithospermum erythrorhizon against COVID-19 via Network Pharmacology Study. Curr. Issues Mol. Biol. 2022, 44, 1788-1809. https://doi.org/10.3390/cimb44050123
Oh K-K, Adnan M. Revealing Potential Bioactive Compounds and Mechanisms of Lithospermum erythrorhizon against COVID-19 via Network Pharmacology Study. Current Issues in Molecular Biology. 2022; 44(5):1788-1809. https://doi.org/10.3390/cimb44050123
Chicago/Turabian StyleOh, Ki-Kwang, and Md. Adnan. 2022. "Revealing Potential Bioactive Compounds and Mechanisms of Lithospermum erythrorhizon against COVID-19 via Network Pharmacology Study" Current Issues in Molecular Biology 44, no. 5: 1788-1809. https://doi.org/10.3390/cimb44050123







