Review of Developments in Combating COVID-19 by Vaccines, Inhibitors, Radiations, and Nonthermal Plasma
Abstract
:1. Introduction
1.1. Classifications
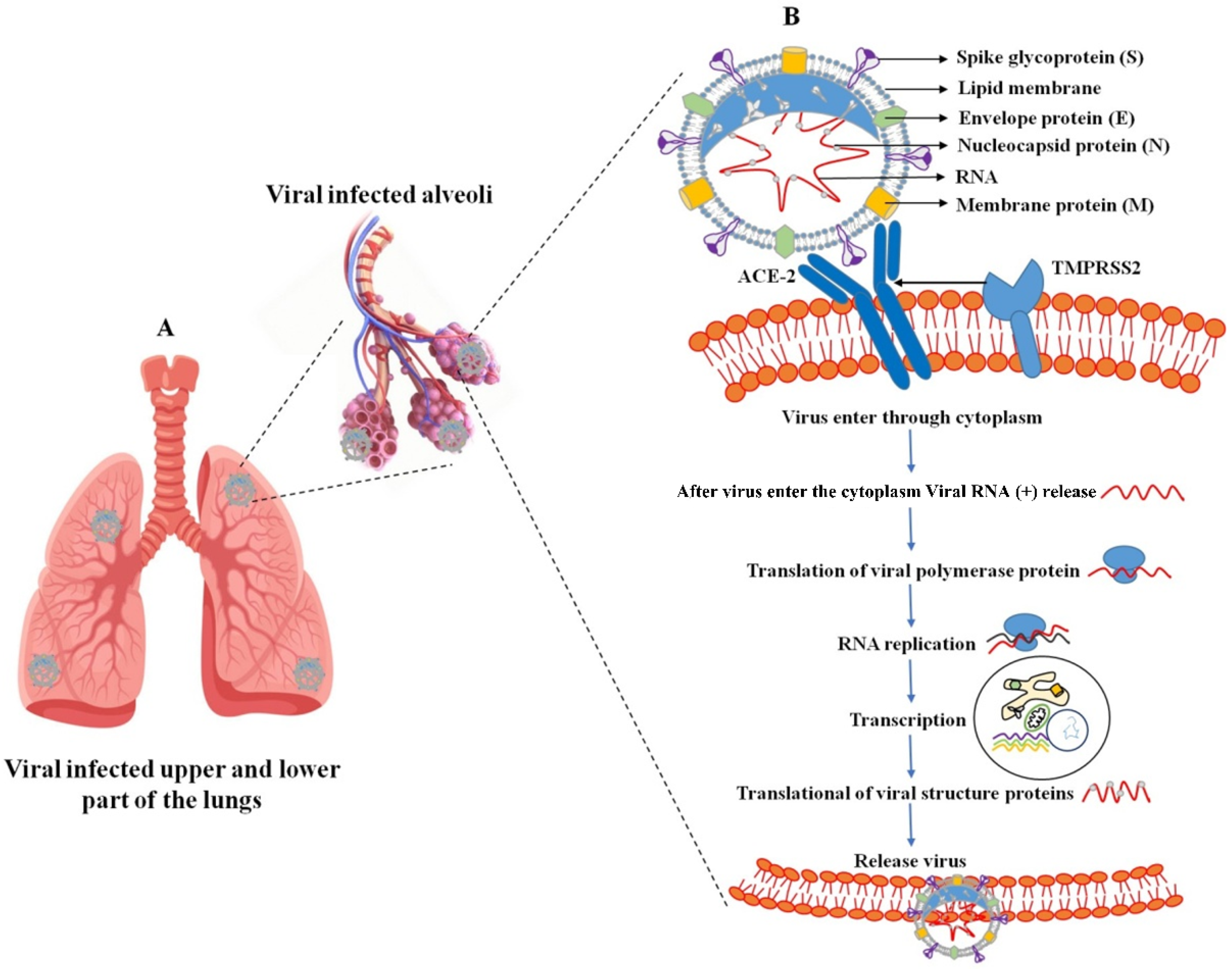
1.2. Virion Structure
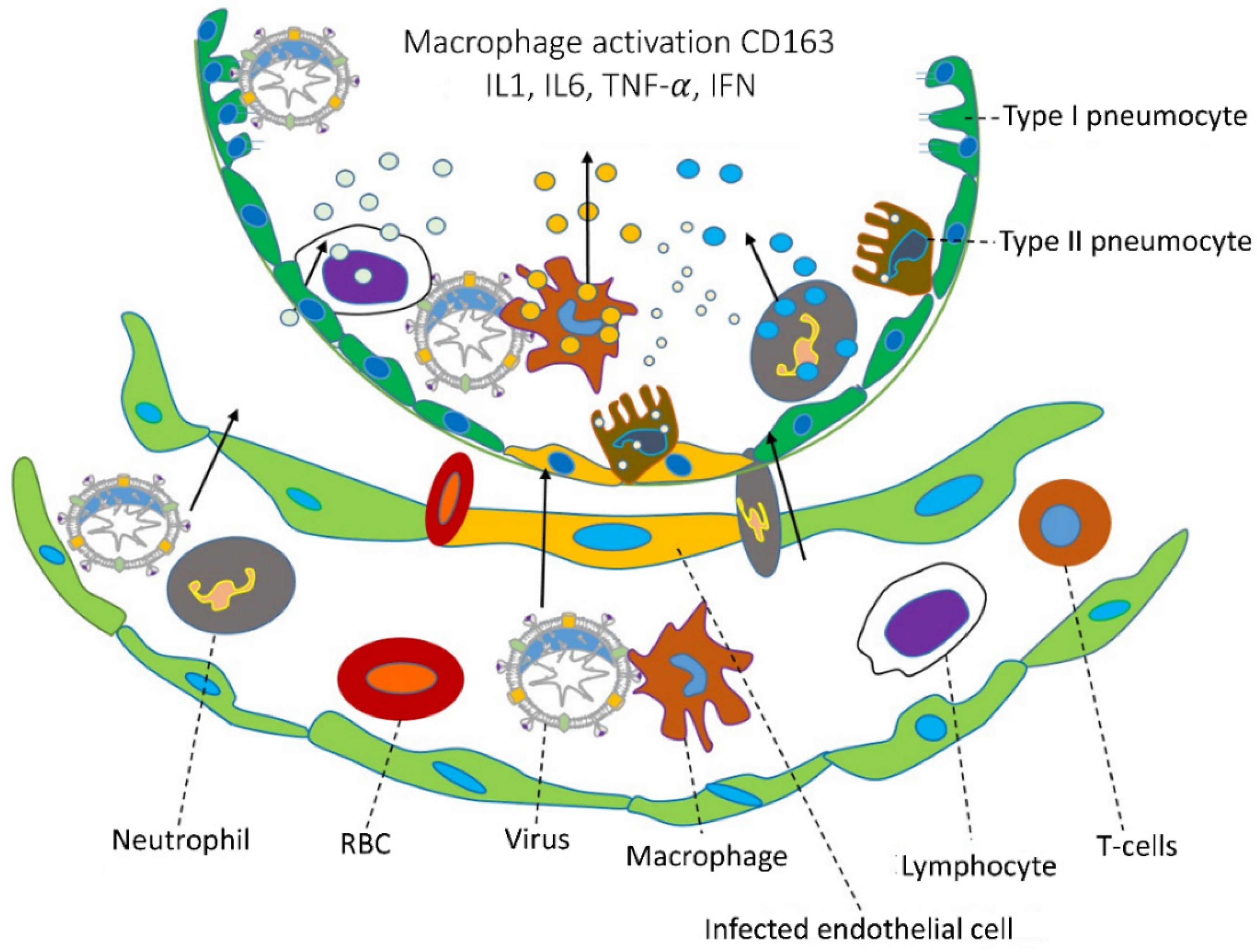
1.3. Viral Inflammation
2. FDA-Approved and Emergency-Approved Drugs against SARS-CoV-2
3. Vaccine
3.1. Protein Subunit Vaccines
3.2. Viral-Vector-Based Vaccines
3.3. Nucleic Acid-Based Vaccine
3.4. Live-Attenuated Virus Vaccines
4. Inhibitors
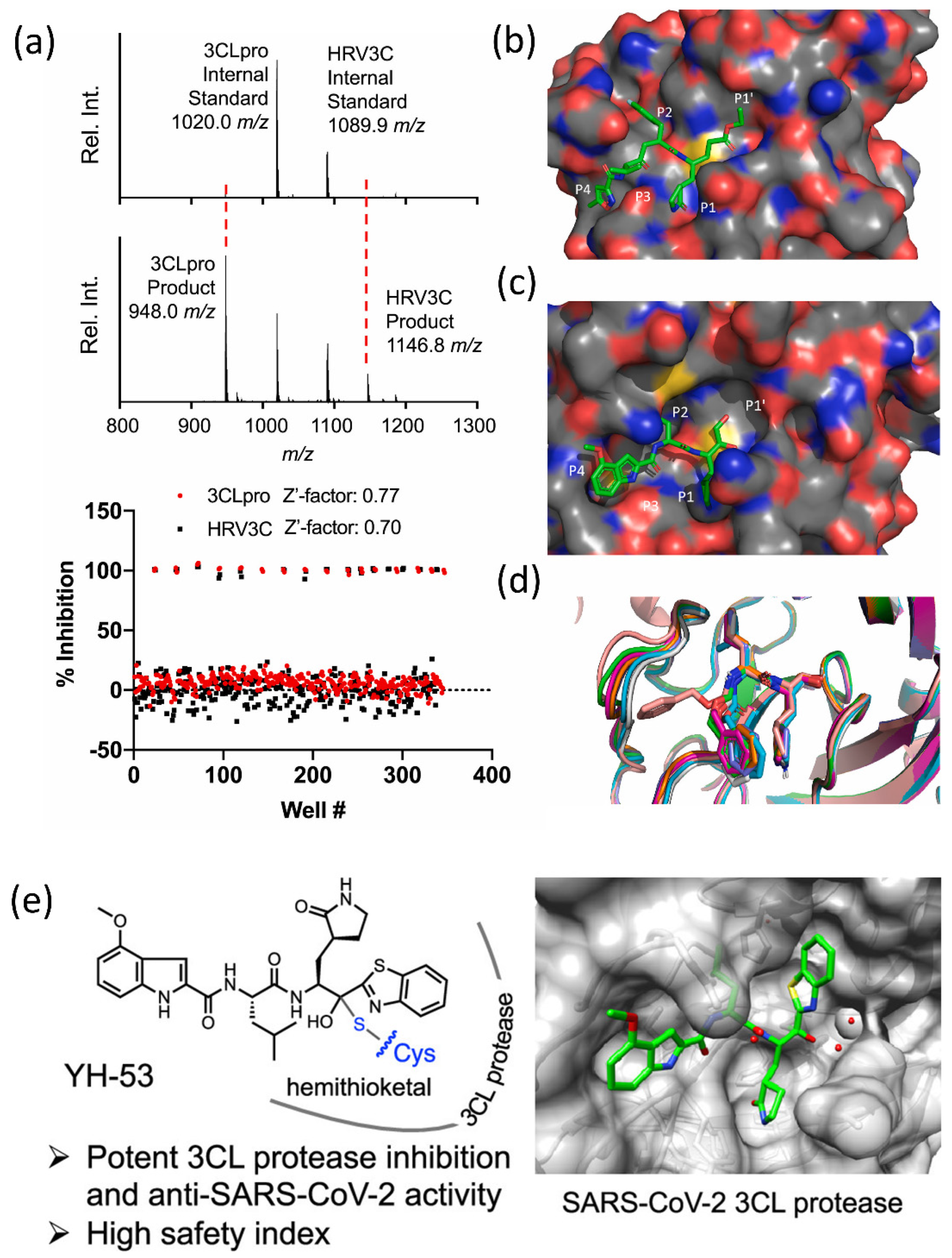
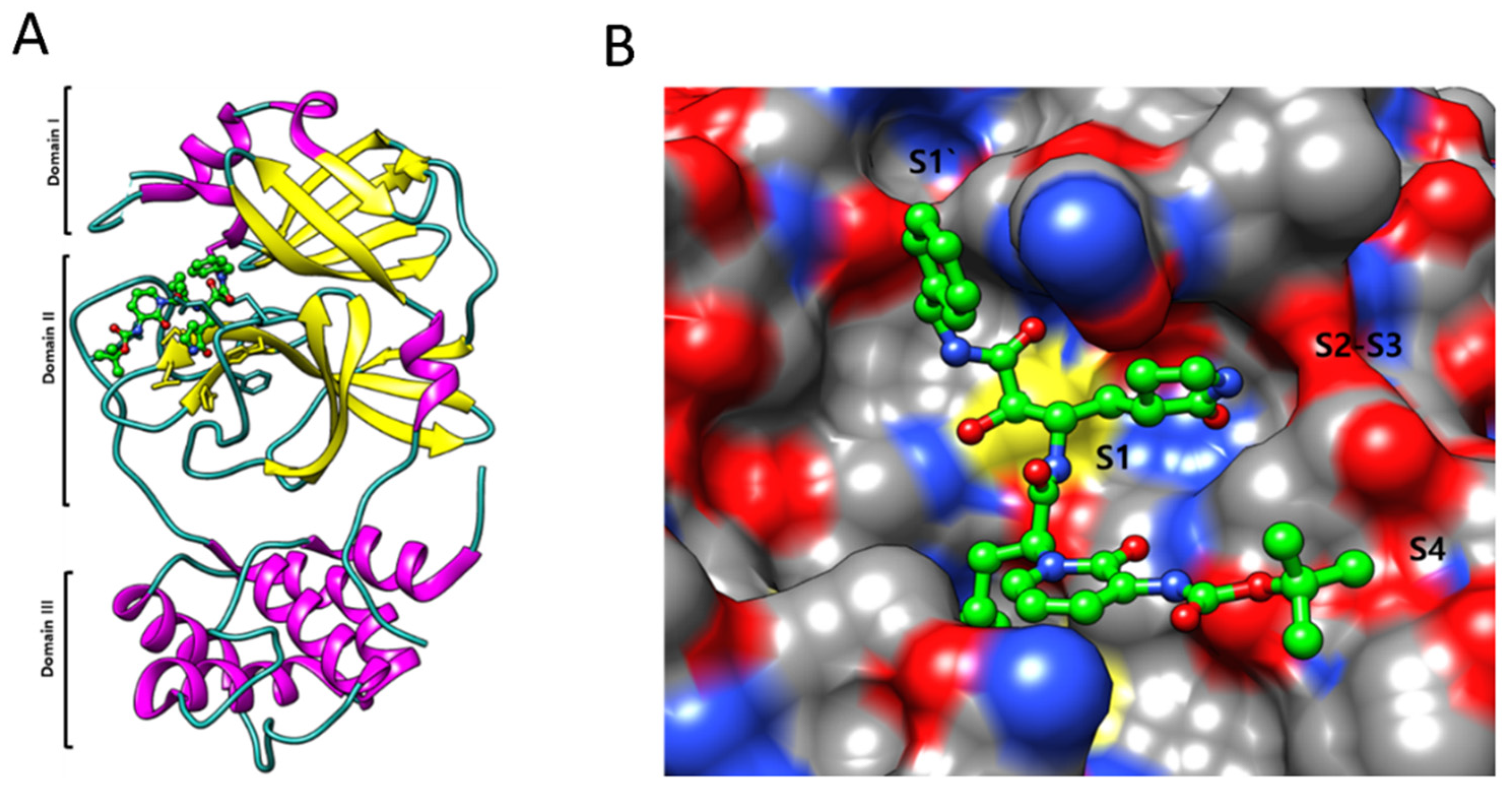
4.1. SARS-CoV-2 3CLpro Protease Inhibitors
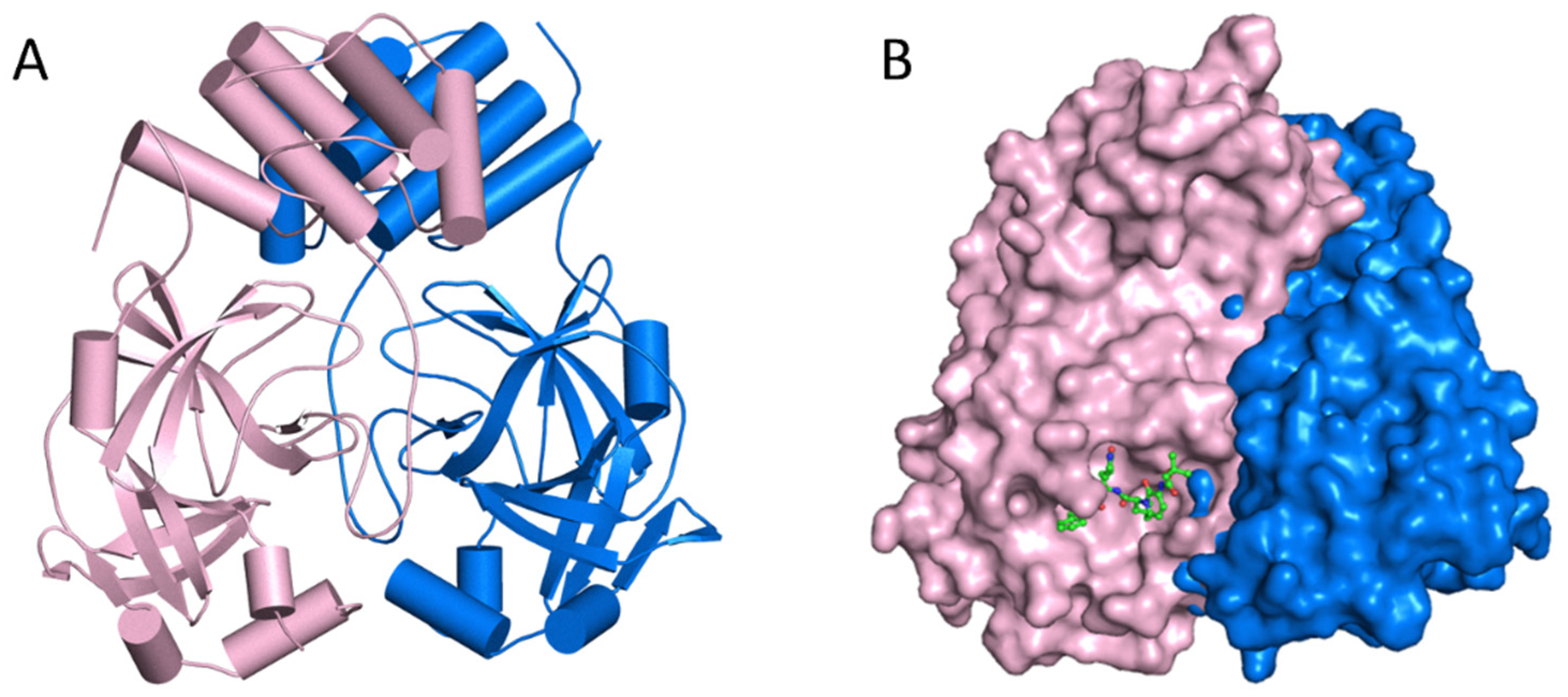
4.2. Mpro Inhibitors
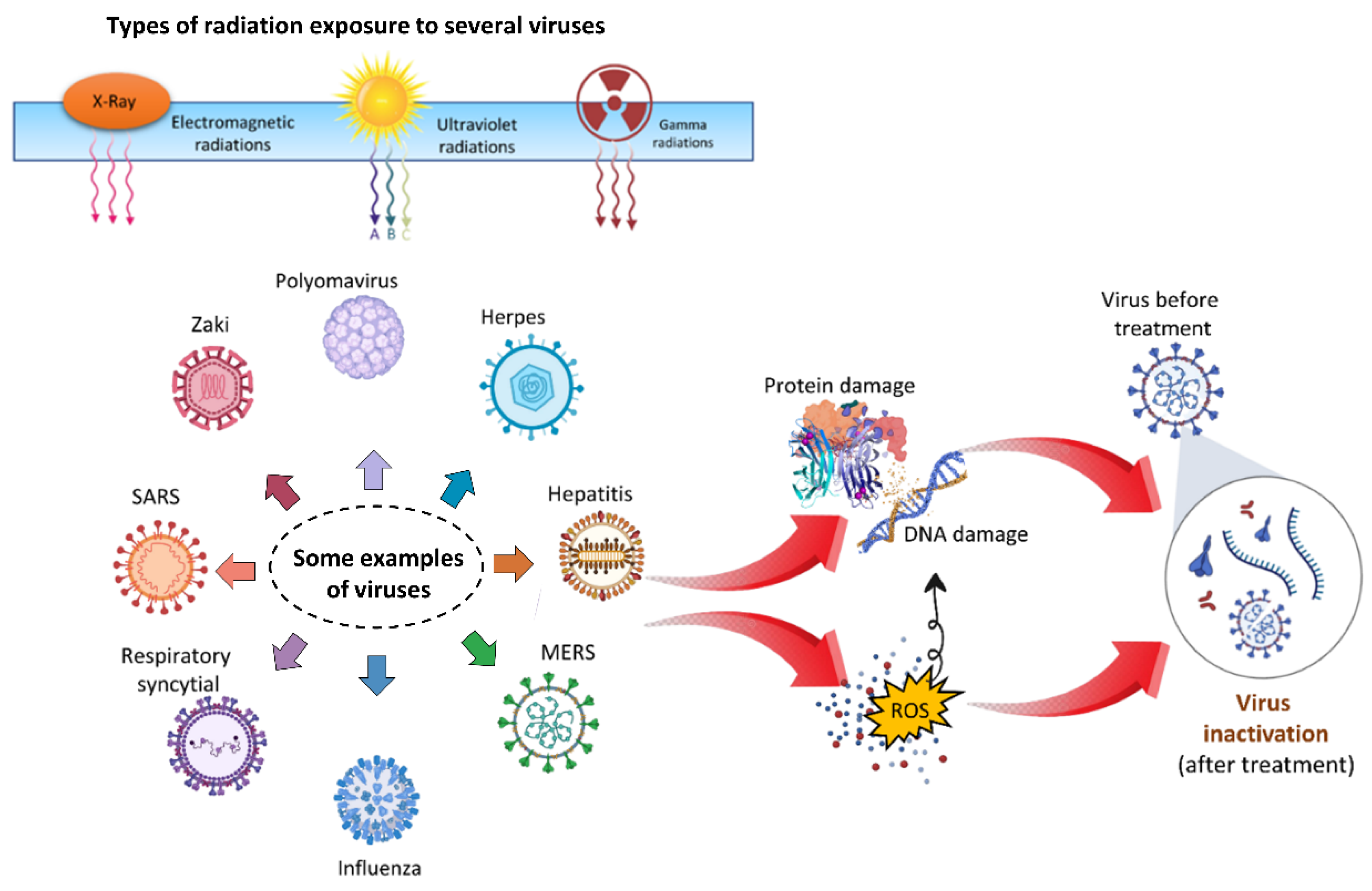
5. Virus Deactivation by Radiation
5.1. Ultraviolet Radiations
5.2. Gamma Radiations
5.3. X-rays
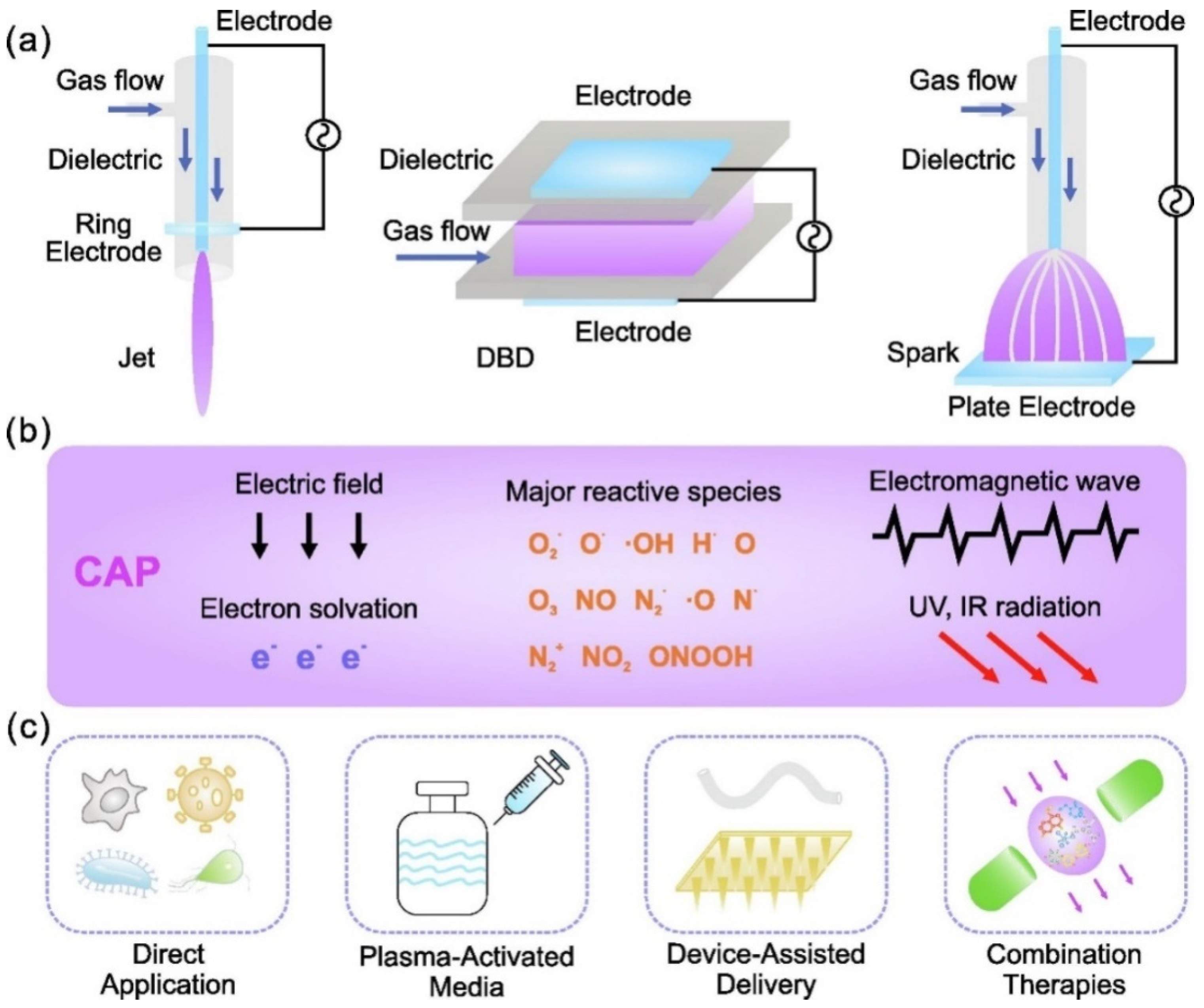
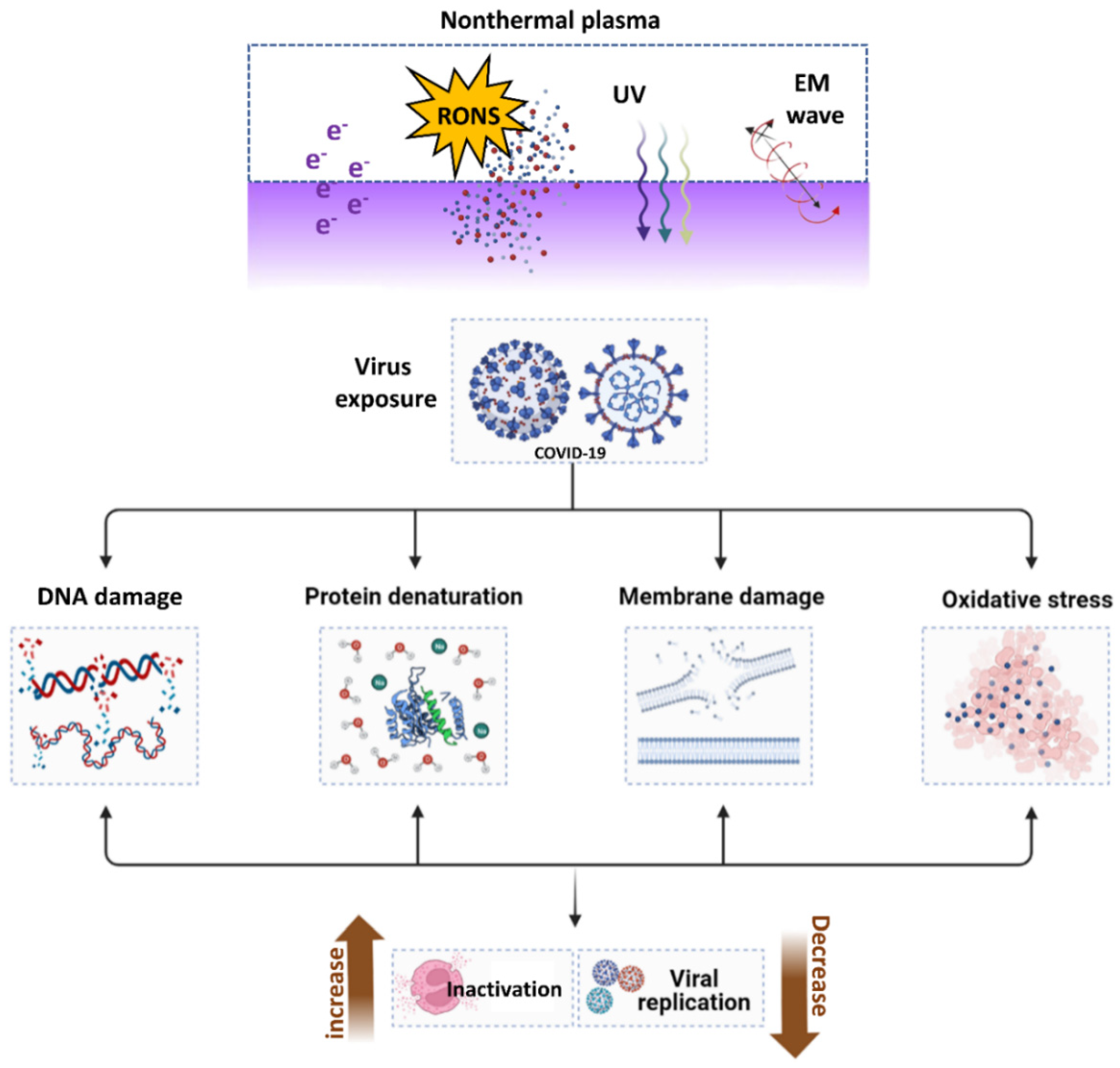
6. Virus Deactivation by Nonthermal Atmospheric Plasma (NTAP)
6.1. The Introduction of NTAP
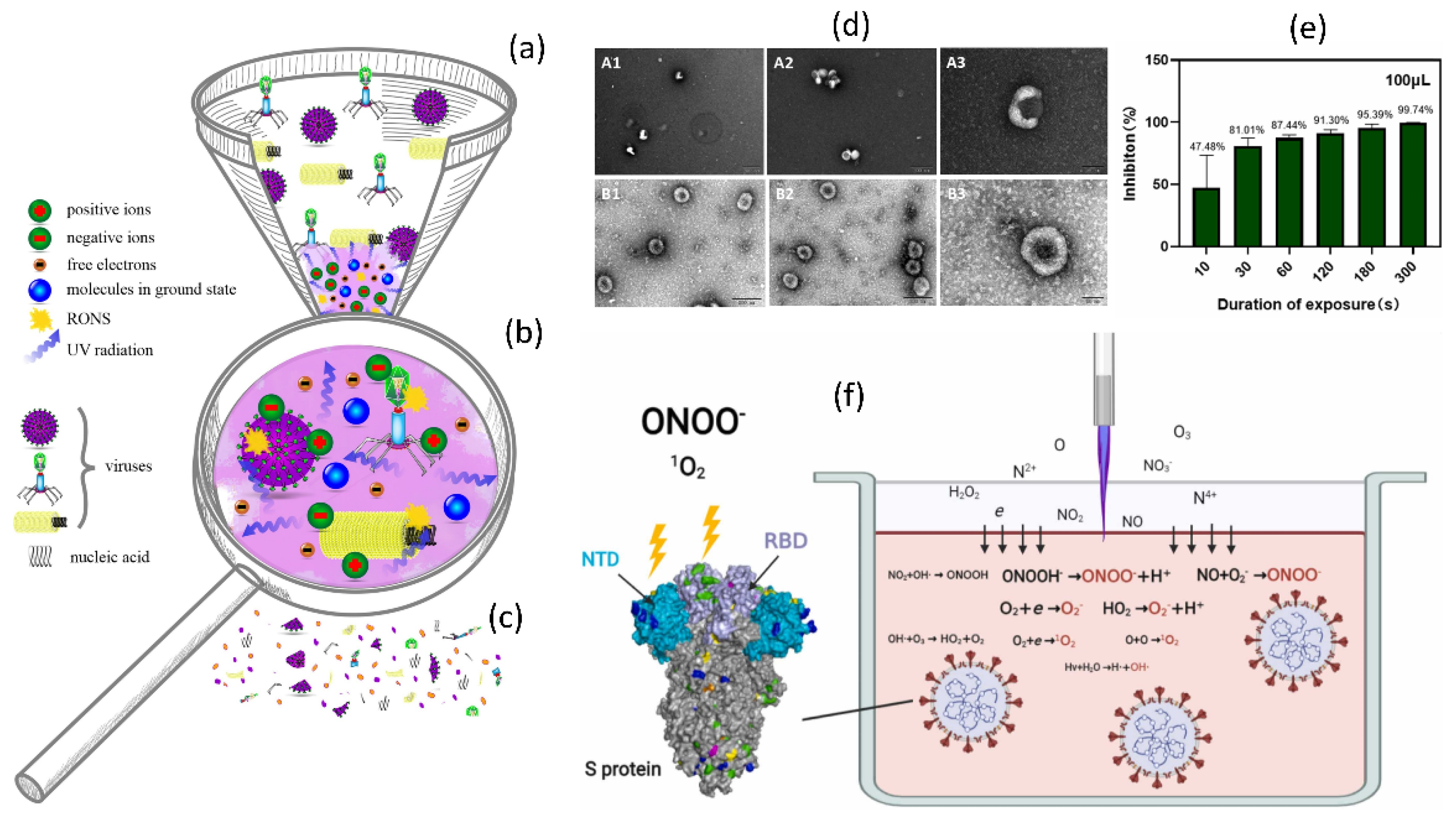
6.2. ROS Production by NTAP and Their Importance in Virus Inactivation
6.3. Applications and Mechanism of NTAP for Virus Deactivation
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamre, D.; Procknow, J.J. A New Virus Isolated from the Human Respiratory Tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Dees, J.H.; Becker, W.B.; Kapikian, A.Z.; Chanock, R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. USA 1967, 57, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Hoek, L.; Pyrc, K.; Berkhout, B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol. Rev. 2006, 30, 760–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, P.C.; Lau, S.K.; Chu, C.M.; Chan, K.H.; Tsoi, H.W.; Huang, Y.; Wong, B.H.; Poon, R.W.; Cai, J.J.; Luk, W.K.; et al. Characterization and Complete Genome Sequence of a Novel Coronavirus, Coronavirus HKU1, from Patients with Pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Chapter Eight—Hosts and Sources of Endemic Human Coronaviruses. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J.B.T.-A., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 100, pp. 163–188. ISBN 0065-3527. [Google Scholar]
- Brian, D.A.; Baric, R.S. Coronavirus Genome Structure and Replication BT—Coronavirus Replication and Reverse Genetics; Enjuanes, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–30. ISBN 978-3-540-26765-2. [Google Scholar]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Molecular Evolution of Human Coronavirus Genomes. Trends Microbiol. 2017, 25, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Al-Qaaneh, A.M.; Alshammari, T.; Aldahhan, R.; Aldossary, H.; Alkhalifah, Z.A.; Borgio, J.F. Genome composition and genetic characterization of SARS-CoV-2. Saudi J. Biol. Sci. 2021, 28, 1978–1989. [Google Scholar] [CrossRef]
- Nelson, C.W.; Ardern, Z.; Goldberg, T.L.; Meng, C.; Kuo, C.-H.; Ludwig, C.; Kolokotronis, S.-O.; Wei, X. Dynamically evolving novel overlapping gene as a factor in the SARS-CoV-2 pandemic. Elife 2020, 9, e59633. [Google Scholar] [CrossRef] [PubMed]
- Renhong, Y.; Yuanyuan, Z.; Yaning, L.; Lu, X.; Yingying, G.; Qiang, Z. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Maier, H.J.; Hawes, P.C.; Cottam, E.M.; Mantell, J.; Verkade, P.; Monaghan, P.; Wileman, T.; Britton, P. Infectious Bronchitis Virus Generates Spherules from Zippered Endoplasmic Reticulum Membranes. MBio 2022, 4, e00801-13. [Google Scholar] [CrossRef] [Green Version]
- Angelini, M.M.; Akhlaghpour, M.; Neuman, B.W.; Buchmeier, M.J. Severe Acute Respiratory Syndrome Coronavirus Nonstructural Proteins 3, 4, and 6 Induce Double-Membrane Vesicles. MBio 2022, 4, e00524-13. [Google Scholar] [CrossRef] [Green Version]
- Masters, P.S. The Molecular Biology of Coronaviruses. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2006; Volume 66, pp. 193–292. ISBN 0065-3527. [Google Scholar]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef]
- Wu, M.-L.; Liu, F.-L.; Sun, J.; Li, X.; He, X.-Y.; Zheng, H.-Y.; Zhou, Y.-H.; Yan, Q.; Chen, L.; Yu, G.-Y.; et al. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Bonica, R.; Cotugno, S.; Tulone, O.; Camporeale, M.; Smith, L.; Trott, M.; Bruyere, O.; Mirarchi, L.; Rizzo, G.; et al. Interventions for Improving Long COVID-19 Symptomatology: A Systematic Review. Viruses 2022, 14, 1863. [Google Scholar] [CrossRef] [PubMed]
- Huzair, F.; Sturdy, S. Biotechnology and the transformation of vaccine innovation: The case of the hepatitis B vaccines 1968–2000. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2017, 64, 11–21. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Qiang, G.; Linlin, B.; Haiyan, M.; Lin, W.; Kangwei, X.; Minnan, Y.; Yajing, L.; Ling, Z.; Nan, W.; Zhe, L.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug. Discov. 2020, 19, 305–306. [Google Scholar]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.M.; Iqbal, M.Z.; Mumtaz, S.; Akhtar, I. Multifunctional and high-performance GeSe/PdSe2 heterostructure device with a fast photoresponse. J. Mater. Chem. C 2020, 8, 4743–4753. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, Y.; Siddiqui, P.; Jiang, S. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem. Biophys. Res. Commun. 2004, 325, 445–452. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, S.; Wang, K.; Zhang, S.; Zhu, W.; Chen, Z. Elicitation of Immunity in Mice After Immunization with the S2 Subunit of the Severe Acute Respiratory Syndrome Coronavirus. DNA Cell Biol. 2005, 24, 510–515. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.; Du, L. Prospects for a MERS-CoV spike vaccine. Expert Rev. Vaccines 2018, 17, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Ertl, H.C.J. Viral vectors as vaccine carriers. Curr. Opin. Virol. 2016, 21, 1–8. [Google Scholar] [CrossRef]
- Bouard, D.; Alazard-Dany, N.; Cosset, F.-L. Viral vectors: From virology to transgene expression. Br. J. Pharmacol. 2009, 157, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Deng, Y.; Chen, H.; Lan, J.; Wang, W.; Zou, X.; Hung, T.; Lu, Z.; Tan, W. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology 2015, 145, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabry, C.M.S.; Rosa-Calatrava, M.; Conway, J.F.; Zubieta, C.; Cusack, S.; Ruigrok, R.W.H.; Schoehn, G. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005, 24, 1645–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef]
- Uludağ, H.; Parent, K.; Aliabadi, H.M.; Haddadi, A. Prospects for RNAi Therapy of COVID-19. Front. Bioeng. Biotechnol. 2020, 8, 916. [Google Scholar] [CrossRef]
- Stark, J.M.; Tibbitt, C.A.; Coquet, J.M. The Metabolic Requirements of Th2 Cell Differentiation. Front. Immunol. 2019, 10, 2318. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Yoshinaga, N.; Yanagihara, K.; Yuba, E.; Kataoka, K.; Itaka, K. Designing immunostimulatory double stranded messenger RNA with maintained translational activity through hybridization with poly A sequences for effective vaccination. Biomaterials 2018, 150, 162–170. [Google Scholar] [CrossRef]
- Lee, L.Y.Y.; Izzard, L.; Hurt, A.C. A Review of DNA Vaccines Against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef] [Green Version]
- Ton, A.-T.; Gentile, F.; Hsing, M.; Ban, F.; Cherkasov, A. Rapid Identification of Potential Inhibitors of SARS-CoV-2 Main Protease by Deep Docking of 1.3 Billion Compounds. Mol. Inform. 2020, 39, 2000028. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, A.M.; Isgrò, C.; Palese, L.L. SARS-CoV-2 Main Protease Active Site Ligands in the Human Metabolome. Molecules 2021, 26, 1409. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pearce, R.; Omenn, G.S.; Zhang, Y. Identification of 13 Guanidinobenzoyl- or Aminidinobenzoyl-Containing Drugs to Potentially Inhibit TMPRSS2 for COVID-19 Treatment. Int. J. Mol. Sci. 2021, 22, 7060. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Krüger, N.; Arora, P.; Sørensen, L.K.; Søgaard, O.S.; Hasselstrøm, J.B.; Winkler, M.; Hempel, T.; et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Singh, D.D.; Han, I.; Kumar, Y.; Choi, E.-H. Current Potential Therapeutic Approaches against SARS-CoV-2: A Review. Biomedicines 2021, 9, 1620. [Google Scholar] [CrossRef]
- Konno, S.; Kobayashi, K.; Senda, M.; Funai, Y.; Seki, Y.; Tamai, I.; Schäkel, L.; Sakata, K.; Pillaiyar, T.; Taguchi, A.; et al. 3CL Protease Inhibitors with an Electrophilic Arylketone Moiety as Anti-SARS-CoV-2 Agents. J. Med. Chem. 2022, 65, 2926–2939. [Google Scholar] [CrossRef]
- Liu, C.; Boland, S.; Scholle, M.D.; Bardiot, D.; Marchand, A.; Chaltin, P.; Blatt, L.M.; Beigelman, L.; Symons, J.A.; Raboisson, P.; et al. Dual inhibition of SARS-CoV-2 and human rhinovirus with protease inhibitors in clinical development. Antiviral Res. 2021, 187, 105020. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, H.; Patel, C.N. In silico prediction of potential inhibitors for the main protease of SARS-CoV-2 using molecular docking and dynamics simulation based drug-repurposing. J. Infect. Public Health 2020, 13, 1210–1223. [Google Scholar] [CrossRef]
- Ianevski, A.; Yao, R.; Biza, S.; Zusinaite, E.; Mannik, A.; Kivi, G.; Planken, A.; Kurg, K.; Tombak, E.-M.; Ustav, M.; et al. Identification and Tracking of Antiviral Drug Combinations. Viruses 2020, 12, 1178. [Google Scholar] [CrossRef]
- Arshan, N.; Gustavo, C.-A. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2022, 1, e1500527. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.W.; Suttle, C.A. Viruses and Nutrient Cycles in the Sea: Viruses play critical roles in the structure and function of aquatic food webs. Bioscience 1999, 49, 781–788. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Bedell, K.; Buchaklian, A.H.; Perlman, S. Efficacy of an Automated Multiple Emitter Whole-Room Ultraviolet-C Disinfection System Against Coronaviruses MHV and MERS-CoV. Infect. Control Hosp. Epidemiol. 2016, 37, 598–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, P.; Sahay, J.J.; Maidili, T.; Rajan, R.; Pooja, S. Breakthrough of COVID-19 using radiotherapy treatment modalities. Radiother. Oncol. 2020, 148, 225–226. [Google Scholar] [CrossRef]
- DeAngelis, H.E.; Grillet, A.M.; Nemer, M.B.; Wasiolek, M.A.; Hanson, D.J.; Omana, M.A.; Sanchez, A.L.; Vehar, D.W.; Thelen, P.M. Gamma radiation sterilization of N95 respirators leads to decreased respirator performance. PLoS ONE 2021, 16, e0248859. [Google Scholar] [CrossRef]
- Mumtaz, S.; Bhartiya, P.; Kaushik, N.; Adhikari, M.; Lamichhane, P.; Lee, S.-J.; Kaushik, N.K.; Choi, E.H. Pulsed high-power microwaves do not impair the functions of skin normal and cancer cells in vitro: A short-term biological evaluation. J. Adv. Res. 2020, 22, 47–55. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Mumtaz, S.; Lim, J.S.; Jang, J.H.; Kim, D.; Sahu, B.D.; Bogaerts, A.; Choi, E.H. Evaluation of non-thermal effect of microwave radiation and its mode of action in bacterial cell inactivation. Sci. Rep. 2021, 11, 14003. [Google Scholar] [CrossRef]
- Bhartiya, P.; Mumtaz, S.; Lim, J.S.; Kaushik, N.; Lamichhane, P.; Nguyen, L.N.; Jang, J.H.; Yoon, S.H.; Choi, J.J.; Kaushik, N.K.; et al. Pulsed 3.5 GHz high power microwaves irradiation on physiological solution and their biological evaluation on human cell lines. Sci. Rep. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Afzal, A.M.; Javed, Y.; Akhtar Shad, N.; Iqbal, M.Z.; Dastgeer, G.; Munir Sajid, M.; Mumtaz, S. Tunneling-based rectification and photoresponsivity in black phosphorus/hexagonal boron nitride/rhenium diselenide van der Waals heterojunction diode. Nanoscale 2020, 12, 3455–3468. [Google Scholar] [CrossRef]
- Afzal, A.M.; Javed, Y.; Hussain, S.; Ali, A.; Yaqoob, M.Z.; Mumtaz, S. Enhancement in photovoltaic properties of bismuth ferrite/zinc oxide heterostructure solar cell device with graphene/indium tin oxide hybrid electrodes. Ceram. Int. 2020, 46, 9161–9169. [Google Scholar] [CrossRef]
- Mumtaz, S.; Choi, E.H. An Efficient Vircator With High Output Power and Less Drifting Electron Loss by Forming Multivirtual Cathodes. IEEE Electron. Device Lett. 2022, 43, 1756–1759. [Google Scholar] [CrossRef]
- Mumtaz, S.; Nguyen, L.N.; Uhm, H.; Lamichhane, P.; Lee, S.W.; Choi, E.H. A novel approach to form second virtual cathode by installing a floating zone plate inside the drift tube. Results Phys. 2020, 17, 103052. [Google Scholar] [CrossRef]
- Afzal, A.M.; Mumtaz, S.; Iqbal, M.Z.; Iqbal, M.W.; Manzoor, A.; Dastgeer, G.; Iqbal, M.J.; Javed, Y.; Khan, R.; Shad, N.A.; et al. Fast and high photoresponsivity gallium telluride/hafnium selenide van der Waals heterostructure photodiode. J. Mater. Chem. C 2021, 9, 7110–7118. [Google Scholar] [CrossRef]
- Mumtaz, S.; Chandra Adhikari, B.; Minin, I.V.; Minin, O.V.; Lamichhane, P.; Paneru, R.; Ha Choi, E. Particle in cell simulation for the power enhancement by forming the second virtual cathode in an axial vircator. Results Phys. 2021, 24, 104126. [Google Scholar] [CrossRef]
- Mumtaz, S.; Uhm, H.; Lim, J.S.; Choi, E.H. Output-Power Enhancement of Vircator Based on Second Virtual Cathode Formed by Wall Charge on a Dielectric Reflector. IEEE Trans. Electron. Devices 2022, 69, 2043–2050. [Google Scholar] [CrossRef]
- Mumtaz, S.; Munnaf, S.A.; Choi, E.H. Numerical study on the formation of second virtual cathode by using different material floating zone plate inside drift tube region. In Proceedings of the 2021 22nd International Vacuum Electronics Conference (IVEC), Rotterdam, The Netherlands, 27–30 April 2021; pp. 1–2. [Google Scholar]
- Mumtaz, S.; Lamichhane, P.; Sup Lim, J.; Ho Yoon, S.; Hyun Jang, J.; DoyoungKim; Woo Lee, S.; Joo Choi, J.; Ha Choi, E. Enhancement in the power of microwaves by the interference with a cone-shaped reflector in an axial vircator. Results Phys. 2019, 15, 102611. [Google Scholar] [CrossRef]
- Yoon, S.; Jeong, K.; Mumtaz, S.; Choi, E.H. Electromagnetic pulse shielding effectiveness of circular multi-waveguides for fluids. Results Phys. 2020, 16, 102946. [Google Scholar] [CrossRef]
- Jang, J.H.; Mumtaz, S.; Lee, S.W.; Kim, D.-Y.; Lim, J.S.; Kaushik, N.K.; Choi, E.H. Focus of high-power microwaves with positive and negative zone plate to increase the receiving power in axial virtual cathode oscillator. Curr. Appl. Phys. 2021, 29, 89–96. [Google Scholar] [CrossRef]
- Mumtaz, S.; Lim, J.S.; Ghimire, B.; Lee, S.W.; Choi, J.J.; Choi, E.H. Enhancing the power of high power microwaves by using zone plate and investigations for the position of virtual cathode inside the drift tube. Phys. Plasmas 2018, 25, 103113. [Google Scholar] [CrossRef]
- Afzal, A.M.; Iqbal, M.Z.; Dastgeer, G.; Nazir, G.; Mumtaz, S.; Usman, M.; Eom, J. WS2/GeSe/WS2 Bipolar Transistor-Based Chemical Sensor with Fast Response and Recovery Times. ACS Appl. Mater. Interfaces 2020, 12, 39524–39532. [Google Scholar] [CrossRef]
- Brooks, A.L.; Hoel, D.G.; Preston, R.J. The role of dose rate in radiation cancer risk: Evaluating the effect of dose rate at the molecular, cellular and tissue levels using key events in critical pathways following exposure to low LET radiation. Int. J. Radiat. Biol. 2016, 92, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.K.; Singh, H.; Deep, A.; Khatri, M.; Bhaumik, J.; Kim, K.-H.; Bhardwaj, N. UVC-based photoinactivation as an efficient tool to control the transmission of coronaviruses. Sci. Total Environ. 2021, 792, 148548. [Google Scholar] [CrossRef]
- David, L.C.; Jose-Luis, S. Predicted Inactivation of Viruses of Relevance to Biodefense by Solar Radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef] [Green Version]
- Pendyala, B.; Patras, A.; Pokharel, B.; D’Souza, D. Genomic Modeling as an Approach to Identify Surrogates for Use in Experimental Validation of SARS-CoV-2 and HuNoV Inactivation by UV-C Treatment. Front. Microbiol. 2020, 11, 572331. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [Green Version]
- Rauth, A.M. The Physical State of Viral Nucleic Acid and the Sensitivity of Viruses to Ultraviolet Light. Biophys. J. 1965, 5, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Bartolini, D.; Stabile, A.M.; Monari, C.; Galli, F.; Rende, M.; et al. SARS-CoV-2 Survival on Surfaces and the Effect of UV-C Light. Viruses 2021, 13, 408. [Google Scholar] [CrossRef]
- Kitagawa, H.; Nomura, T.; Nazmul, T.; Omori, K.; Shigemoto, N.; Sakaguchi, T.; Ohge, H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control 2021, 49, 299–301. [Google Scholar] [CrossRef]
- Darnell, M.E.R.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef]
- Sabino, C.P.; Sellera, F.P.; Sales-Medina, D.F.; Machado, R.R.G.; Durigon, E.L.; Freitas-Junior, L.H.; Ribeiro, M.S. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2020, 32, 101995. [Google Scholar] [CrossRef]
- Criscuolo, E.; Diotti, R.A.; Ferrarese, R.; Alippi, C.; Viscardi, G.; Signorelli, C.; Mancini, N.; Clementi, M.; Clementi, N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021, 10, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Sarkis-Onofre, R.; Borges, R.D.C.; Demarco, G.; Dotto, L.; Schwendicke, F.; Demarco, F.F. Decontamination of N95 respirators against SARS-CoV-2: A scoping review. J. Dent. 2021, 104, 103534. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Fassolitis, A.C.; Larkin, E.P.; Read, R.B.; Peeler, J.T. Inactivation of Thirty Viruses by Gamma Radiation. Appl. Microbiol. 1971, 22, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hume, A.J.; Ames, J.; Rennick, L.J.; Duprex, W.P.; Marzi, A.; Tonkiss, J.; Mühlberger, E. Inactivation of RNA Viruses by Gamma Irradiation: A Study on Mitigating Factors. Viruses 2016, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donya, M.; Radford, M.; ElGuindy, A.; Firmin, D.; Yacoub, M.H. Radiation in medicine: Origins, risks and aspirations. Glob. Cardiol. Sci. Pract. 2015, 2014, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, F.; Shupert, W.L.; Haddock, E.; Twardoski, B.; Feldmann, H. Gamma Irradiation as an Effective Method for Inactivation of Emerging Viral Pathogens. Am. J. Trop. Med. Hyg. 2019, 100, 1275–1277. [Google Scholar] [CrossRef]
- Libertin, C.R.; Panozzo, J.; Groh, K.R.; Chang-Liu, C.-M.; Schreck, S.; Woloschak, G.E. Effects of Gamma Rays, Ultraviolet Radiation, Sunlight, Microwaves and Electromagnetic Fields on Gene Expression Mediated by Human Immunodeficiency Virus Promoter. Radiat. Res. 1994, 140, 91–96. [Google Scholar] [CrossRef]
- Farkas, J. Irradiation as a method for decontaminating food: A review. Int. J. Food Microbiol. 1998, 44, 189–204. [Google Scholar] [CrossRef]
- Jordan, R.T.; Kempe, L.L. Inactivation of Some Animal Viruses with Gamma Radiation from Cobalt-60. Proc. Soc. Exp. Biol. Med. 1956, 91, 212–215. [Google Scholar] [CrossRef]
- Latarjet, R.; Cramer, R.; Montagnier, L. Inactivation, by UV-, X-, and γ-radiations, of the infecting and transforming capacities of polyoma virus. Virology 1967, 33, 104–111. [Google Scholar] [CrossRef]
- Decker, C.; Guir, J.; Kirn, A. Infectivity and Capacity for DNA Replication of Vaccinia Virus Irradiated by γ-Rays. J. Gen. Virol. 1969, 4, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Zargar, M.; Ghaemi, A. Inactivation methods for whole influenza vaccine production. Rev. Med. Virol. 2019, 29, e2074. [Google Scholar] [CrossRef] [PubMed]
- Bidawid, S.; Farber, J.M.; Sattar, S.A. Inactivation of hepatitis A virus (HAV) in fruits and vegetables by gamma irradiation. Int. J. Food Microbiol. 2000, 57, 91–97. [Google Scholar] [CrossRef]
- Abolaban, F.A.; Djouider, F.M. Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development. Open Life Sci. 2021, 16, 558–570. [Google Scholar] [CrossRef]
- Haddock, E.; Feldmann, F.; Shupert, W.L.; Feldmann, H. Inactivation of SARS-CoV-2 Laboratory Specimens. Am. J. Trop. Med. Hyg. 2021, 104, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.F.; Anderson, R.S. The effects of roentgen rays on cell-virus associations: Findings with virus-induced rabbit papillomas and fibromas. J. Exp. Med. 1943, 78, 285–304. [Google Scholar] [CrossRef]
- Jinia, A.J.; Sunbul, N.B.; Meert, C.A.; Miller, C.A.; Clarke, S.D.; Kearfott, K.J.; Matuszak, M.M.; Pozzi, S.A. Review of Sterilization Techniques for Medical and Personal Protective Equipment Contaminated With SARS-CoV-2. IEEE Access 2020, 8, 111347–111354. [Google Scholar] [CrossRef]
- Tauxe, R.V. Food safety and irradiation: Protecting the public from foodborne infections. Emerg. Infect. Dis. 2001, 7, 516–521. [Google Scholar] [CrossRef]
- Afrough, B.; Eakins, J.; Durley-White, S.; Dowall, S.; Findlay-Wilson, S.; Graham, V.; Lewandowski, K.; Carter, D.P.; Hewson, R. X-ray inactivation of RNA viruses without loss of biological characteristics. Sci. Rep. 2020, 10, 21431. [Google Scholar] [CrossRef]
- Buzzell, A.; Brandon, F.B.; Lauffer, M.A. Effects of x-radiation on influenza virus. Arch. Biochem. Biophys. 1955, 58, 318–331. [Google Scholar] [CrossRef]
- Kroeger, A.V.; Kempf, J.E. Inactivation of the influenza virus by low voltage roentgen rays. J. Bacteriol. 1959, 77, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 33001. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef] [Green Version]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Ghimire, B.; Lee, G.J.; Mumtaz, S.; Choi, E.H. Scavenging effects of ascorbic acid and mannitol on hydroxyl radicals generated inside water by an atmospheric pressure plasma jet. AIP Adv. 2018, 8, 75021. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Kaushik, N.; Lamichhane, P.; Mumtaz, S.; Paneru, R.; Bhartiya, P.; Kwon, J.S.; Mishra, Y.K.; Nguyen, L.Q.; Kaushik, N.K.; et al. In situ plasma-assisted synthesis of polydopamine-functionalized gold nanoparticles for biomedical applications. Green Chem. 2020, 22, 6588–6599. [Google Scholar] [CrossRef]
- Lamichhane, P.; Ghimire, B.; Mumtaz, S.; Paneru, R.; Ki, S.H.; Choi, E.H. Control of hydrogen peroxide production in plasma activated water by utilizing nitrification. J. Phys. D Appl. Phys. 2019, 52, 265206. [Google Scholar] [CrossRef]
- Han, I.; Rana, J.N.; Kim, J.-H.; Choi, E.H.; Kim, Y. A Non-thermal Biocompatible Plasma-Modified Chitosan Scaffold Enhances Osteogenic Differentiation in Bone Marrow Stem Cells. Pharmaceutics 2022, 14, 465. [Google Scholar] [CrossRef]
- Li, Y.; Ho Kang, M.; Sup Uhm, H.; Joon Lee, G.; Ha Choi, E.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-package cold plasma technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Jenns, K.; Sassi, H.P.; Zhou, R.; Cullen, P.J.; Carter, D.; Mai-Prochnow, A. Inactivation of foodborne viruses: Opportunities for cold atmospheric plasma. Trends Food Sci. Technol. 2022, 124, 323–333. [Google Scholar] [CrossRef]
- Palma, D.; Richard, C.; Minella, M. State of the art and perspectives about non-thermal plasma applications for the removal of PFAS in water. Chem. Eng. J. Adv. 2022, 10, 100253. [Google Scholar] [CrossRef]
- He, Y.; Sang, W.; Lu, W.; Zhang, W.; Zhan, C.; Jia, D. Recent Advances of Emerging Organic Pollutants Degradation in Environment by Non-Thermal Plasma Technology: A Review. Water 2022, 14, 1351. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Sherman, J.H. Cold Plasma Cancer Therapy; 2053–2571; Morgan & Claypool Publishers: San Rafael, CA, USA, 2019; ISBN 978-1-64327-434-8. [Google Scholar]
- Trimukhe, A.M.; Pandiyaraj, K.N.; Patekar, M.; Miller, V.; Deshmukh, R.R. Perspectives and Advances of Nonthermal Plasma Technology in Cancers. IEEE Trans. Plasma Sci. 2022, 50, 2489–2515. [Google Scholar] [CrossRef]
- Machala, Z.; Pavlovich, M.J. A New Phase in Applied Biology. Trends Biotechnol. 2018, 36, 577–578. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360. [Google Scholar] [CrossRef]
- Lamichhane, P.; Adhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 45026. [Google Scholar] [CrossRef]
- Paneru, R.; Ki, S.H.; Lamichhane, P.; Nguyen, L.N.; Adhikari, B.C.; Jeong, I.J.; Mumtaz, S.; Choi, J.; Kwon, J.S.; Choi, E.H. Enhancement of antibacterial and wettability performances of polyvinyl alcohol/chitosan film using non-thermal atmospheric pressure plasma. Appl. Surf. Sci. 2020, 532, 147339. [Google Scholar] [CrossRef]
- Lim, J.S.; Hong, Y.J.; Ghimire, B.; Choi, J.; Mumtaz, S.; Choi, E.H. Measurement of electron density in transient spark discharge by simple interferometry. Results Phys. 2021, 20, 103693. [Google Scholar] [CrossRef]
- Lamichhane, P.; Paneru, R.; Nguyen, L.N.; Lim, J.S.; Bhartiya, P.; Adhikari, B.C.; Mumtaz, S.; Choi, E.H. Plasma-assisted nitrogen fixation in water with various metals. React. Chem. Eng. 2020, 5, 2053–2057. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.J.; Wang, S.B.; Choi, E.H.; Han, I. Non-Thermal Bio-Compatible Plasma Induces Osteogenic Differentiation of Human Mesenchymal Stem/Stromal Cells With ROS-Induced Activation of MAPK. IEEE Access 2020, 8, 36652–36663. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Peterhans, E. Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Elem. Res. 1997, 56, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, A.R.; Tiwari, U.; Ezhilarasi, P.N.; Rajauria, G. Application of cold plasma on food matrices: A review on current and future prospects. J. Food Process. Preserv. 2021, 45, e15070. [Google Scholar] [CrossRef]
- Van Gaens, W.; Bogaerts, A. Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. J. Phys. D Appl. Phys. 2013, 46, 275201. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Qiu, H.; He, S.-T.; Hong, B.; Liu, K.; Lou, F.; Li, M.; Hu, P.; Kong, X.; Song, Y.; et al. Efficient disinfection of SARS-CoV-2-like coronavirus, pseudotyped SARS-CoV-2 and other coronaviruses using cold plasma induces spike protein damage. J. Hazard. Mater. 2022, 430, 128414. [Google Scholar] [CrossRef] [PubMed]
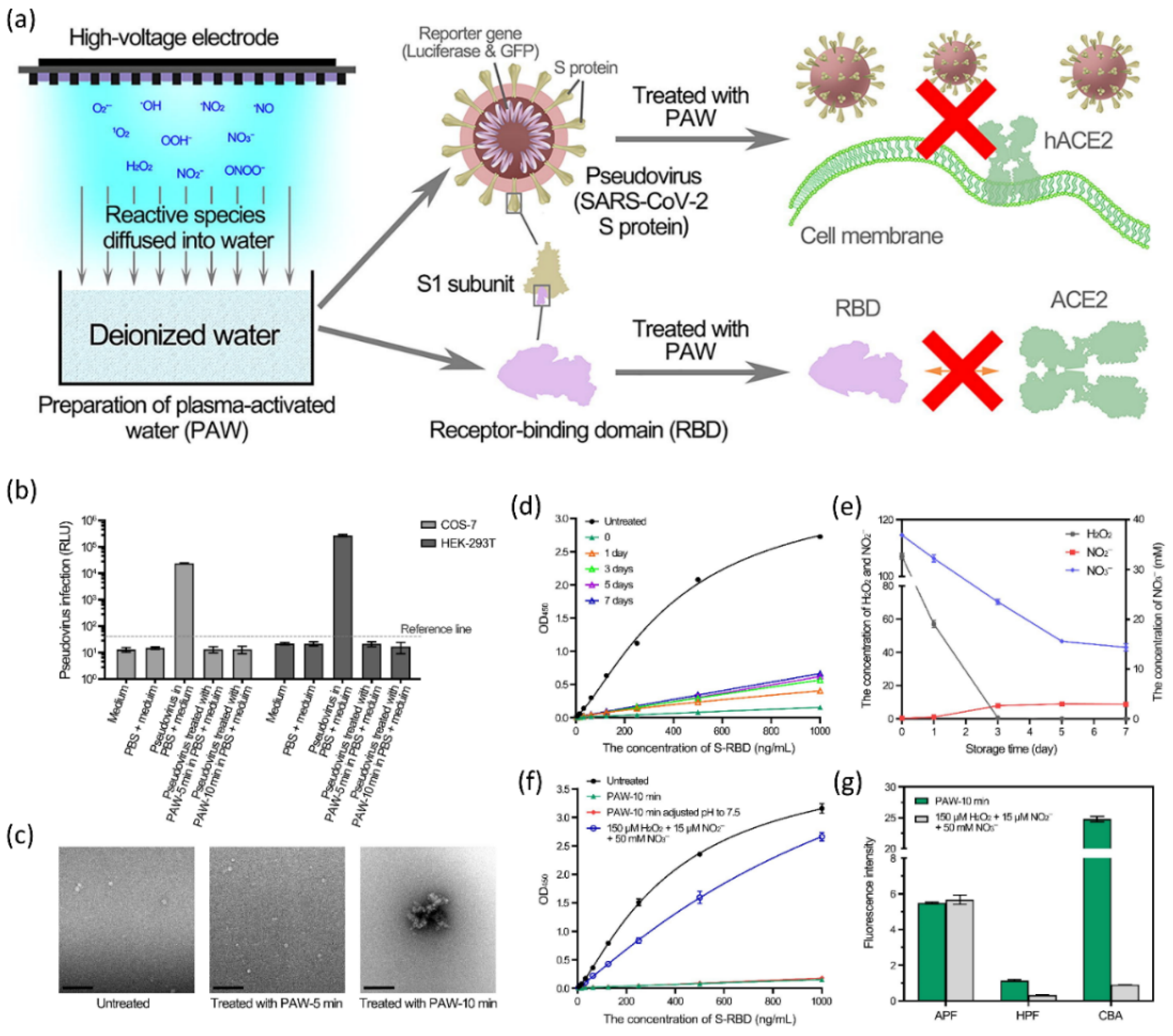
- Guo, L.; Yao, Z.; Yang, L.; Zhang, H.; Qi, Y.; Gou, L.; Xi, W.; Liu, D.; Zhang, L.; Cheng, Y.; et al. Plasma-activated water: An alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem. Eng. J. 2021, 421, 127742. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Platform | Total Vaccine | Vaccine Type |
|---|---|---|
| Protein subunit (PS) | 55 | Maximum spike protein-based and less combined with nanoparticle (4 no) |
| Viral vector non-replicating (VVnr) | 23 | Adenovirus, lentiviral and Sendai viral vector-based |
| DNA | 16 | DNA plasmid encoding RDB, S and others |
| Inactivated virus (IV) | 22 | Inactivated whole virus or CpG1018 |
| RNA | 40 | mRNA |
| Viral vector replicating (VVr) | 4 | Based on the influenza A virus |
| Virus-like particle (VLP) | 6 | expressing S, E, M, and N |
| VVr + antigen-presenting cell (VVr + APC) | 2 | - |
| Live attenuated virus (LAV) | 2 | Codon deoptimized live attenuated virus |
| VVnr + antigen-presentingcell (VVnr + APC) | 1 | Mesenchymal stem cell expressing with S and N |
| Bacterial antigen-spore expression vector (BacAg-SpV) | 1 | Oral salmonella enteritidis (3934 vac) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, I.; Mumtaz, S.; Ashokkumar, S.; Yadav, D.K.; Choi, E.H. Review of Developments in Combating COVID-19 by Vaccines, Inhibitors, Radiations, and Nonthermal Plasma. Curr. Issues Mol. Biol. 2022, 44, 5666-5690. https://doi.org/10.3390/cimb44110384
Han I, Mumtaz S, Ashokkumar S, Yadav DK, Choi EH. Review of Developments in Combating COVID-19 by Vaccines, Inhibitors, Radiations, and Nonthermal Plasma. Current Issues in Molecular Biology. 2022; 44(11):5666-5690. https://doi.org/10.3390/cimb44110384
Chicago/Turabian StyleHan, Ihn, Sohail Mumtaz, Sekar Ashokkumar, Dharmendra Kumar Yadav, and Eun Ha Choi. 2022. "Review of Developments in Combating COVID-19 by Vaccines, Inhibitors, Radiations, and Nonthermal Plasma" Current Issues in Molecular Biology 44, no. 11: 5666-5690. https://doi.org/10.3390/cimb44110384










